Chapter 12 Lesson 2 Matter and Its Changes








- Slides: 12

Chapter 12 Lesson 2: Matter and Its Changes Ms. Amanda and Ms. Diana

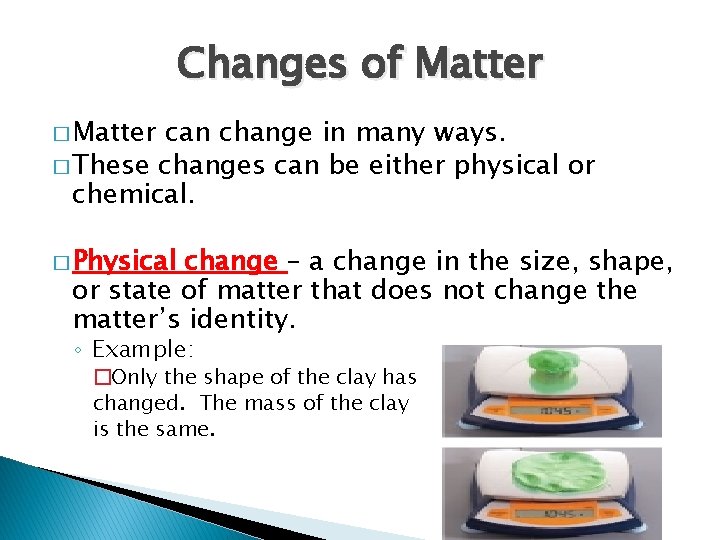
Changes of Matter � Matter can change in many ways. � These changes can be either physical or chemical. � Physical change – a change in the size, shape, or state of matter that does not change the matter’s identity. ◦ Example: �Only the shape of the clay has changed. The mass of the clay is the same.

Dissolving � Remember that solubility is the ability of a substance to dissolve, or mix evenly. � Dissolving is a physical change. � When the substances mix, their identities do not change.

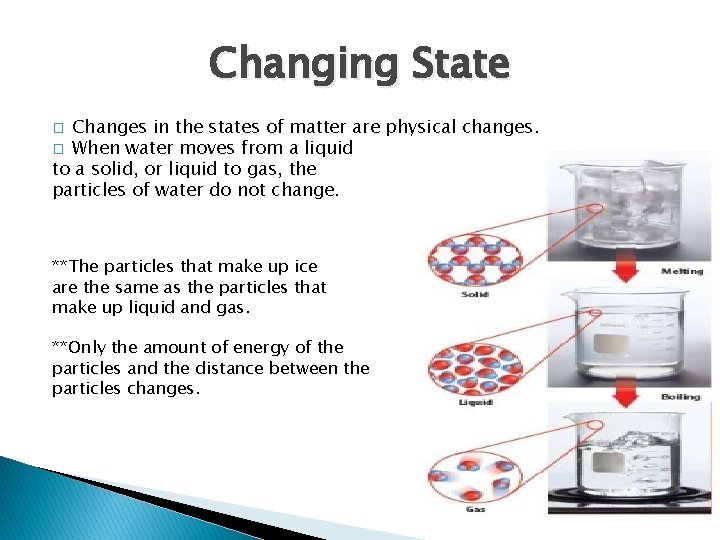
Changing State Changes in the states of matter are physical changes. � When water moves from a liquid to a solid, or liquid to gas, the particles of water do not change. � **The particles that make up ice are the same as the particles that make up liquid and gas. **Only the amount of energy of the particles and the distance between the particles changes.

Energy and Change in State � Changes in energy cause changes in the state of matter. � Energy must be added to a substance to change it from a solid to a liquid or from a liquid to a gas. � Adding energy to a substance can increase its temperature. � When the temperature increases to the boiling point, then the liquid changes to a gas. � When the temperature increases to the melting point, the solid changes to a liquid.

What are Chemical Changes? � Chemical change – a change in matter in which the substances that make up the matter change into other substances with different chemical and physical properties.

Signs of a Chemical Change � How can you tell that a chemical change has taken place? � Signs a chemical change have taken place: ◦ Formation of Gas ◦ Formation of a precipitate (a solid) ◦ Color change

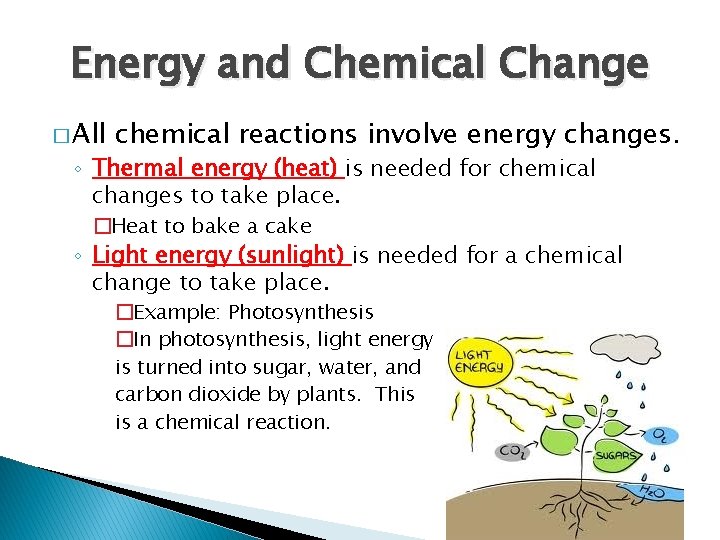
Energy and Chemical Change � All chemical reactions involve energy changes. ◦ Thermal energy (heat) is needed for chemical changes to take place. �Heat to bake a cake ◦ Light energy (sunlight) is needed for a chemical change to take place. �Example: Photosynthesis �In photosynthesis, light energy is turned into sugar, water, and carbon dioxide by plants. This is a chemical reaction.

Can changes be reversed? � Some changes can be reversed and others cannot. � When a chemical change has taken place you cannot reverse the process because the substances have changed. ◦ Example: Baking a cake �You cannot reverse the process and get back the ingredients of the cake. � Some physical changes can be reversed. ◦ Example: changing the shape of clay.

Conservation of Mass � Physical changes do not affect the mass of a substance. ◦ Example: �The mass of a piece of ice after it has melted into water is the same as the mass of the water. Mass is conserved (unchanged) during a physical and chemical change. Law of conservation of mass – states that the total mass before a chemical reaction is the same as the total mass after the chemical reaction.

Comparing Physical and Chemical Changes

Questions