Chapter 12 Intermolecular Attractions and the Properties of













- Slides: 102

Chapter 12: Intermolecular Attractions and the Properties of Liquids and Solids Chemistry: The Molecular Nature of Matter, 6 E Jespersen/Brady/Hyslop

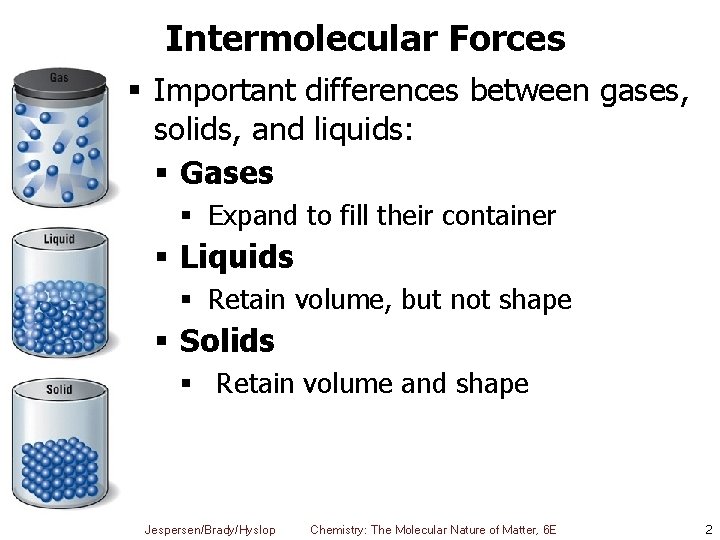
Intermolecular Forces § Important differences between gases, solids, and liquids: § Gases § Expand to fill their container § Liquids § Retain volume, but not shape § Solids § Retain volume and shape Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 2

Intermolecular Forces § Physical state of molecule depends on § Average kinetic energy of particles § Recall KE Tave § Intermolecular Forces § Energy of Inter-particle attraction § Physical properties of gases, liquids and solids determined by § How tightly molecules are packed together § Strength of attractions between molecules Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 3

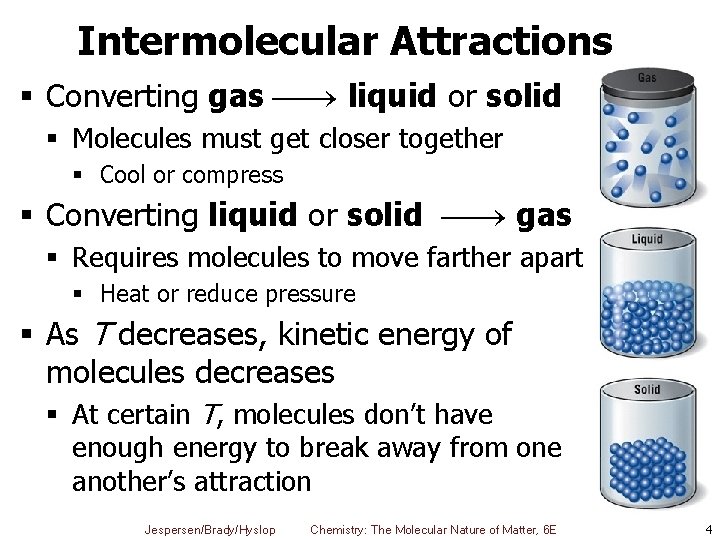
Intermolecular Attractions § Converting gas liquid or solid § Molecules must get closer together § Cool or compress § Converting liquid or solid gas § Requires molecules to move farther apart § Heat or reduce pressure § As T decreases, kinetic energy of molecules decreases § At certain T, molecules don’t have enough energy to break away from one another’s attraction Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 4

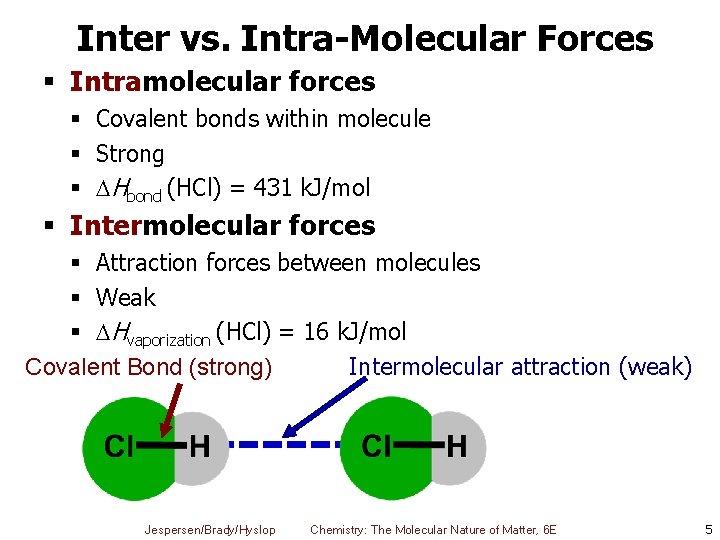
Inter vs. Intra-Molecular Forces § Intramolecular forces § Covalent bonds within molecule § Strong § Hbond (HCl) = 431 k. J/mol § Intermolecular forces § Attraction forces between molecules § Weak § Hvaporization (HCl) = 16 k. J/mol Covalent Bond (strong) Intermolecular attraction (weak) Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 5

Electronegativity Review Electronegativity: Measure of attractive force that one atom in a covalent bond has for electrons of the bond Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 6

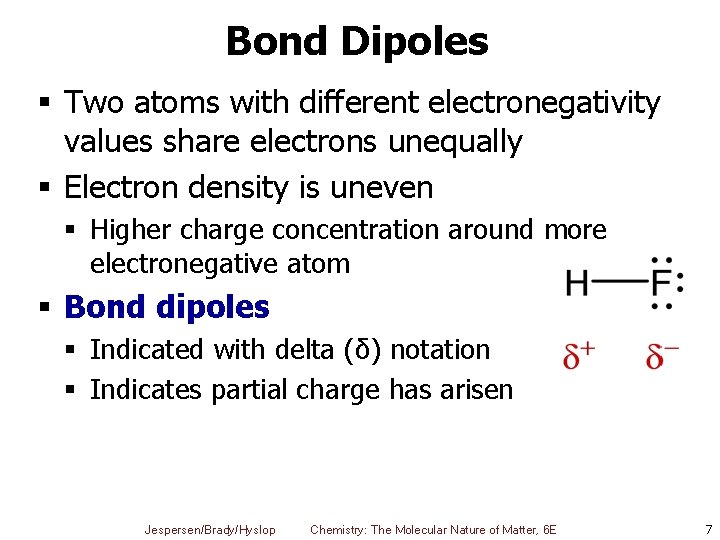
Bond Dipoles § Two atoms with different electronegativity values share electrons unequally § Electron density is uneven § Higher charge concentration around more electronegative atom § Bond dipoles § Indicated with delta (δ) notation § Indicates partial charge has arisen Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 7

Net Dipoles § Symmetrical molecules § Even if they have polar bonds § Are non-polar because bond dipoles cancel § Asymmetrical molecules § Are polar because bond dipoles do not cancel § These molecules have permanent, net dipoles § Molecular dipoles § Cause molecules to interact § Decreased distance between molecules increases amount of interaction Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 8

Intermolecular Forces § When substance melts or boils § § § Intermolecular forces are broken Not covalent bonds Responsible for non-ideal behavior of gases Responsible for existence of condensed states of matter Responsible for bulk properties of matter § § Boiling points and melting points Reflect strength of intermolecular forces Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 9

Three Important Types of Intermolecular Forces 1. London dispersion forces 2. Dipole-dipole forces § Hydrogen bonds 3. Ion-dipole forces § Ion-induced dipole forces Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 10

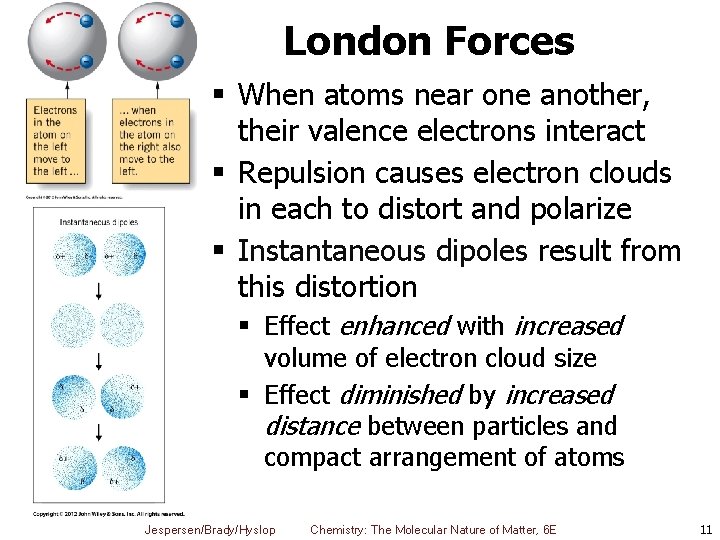
London Forces § When atoms near one another, their valence electrons interact § Repulsion causes electron clouds in each to distort and polarize § Instantaneous dipoles result from this distortion § Effect enhanced with increased volume of electron cloud size § Effect diminished by increased distance between particles and compact arrangement of atoms Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 11

London Forces § Instantaneous dipole-induced dipole attractions § § § London Forces Dispersion forces Operate between all molecules § § Neutral or net charged Nonpolar or polar Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 12

London Dispersion Forces § Ease with which dipole moments can be induced and thus London Forces depend on 1. Polarizability of electron cloud 2. Points of attraction § Number atoms § Molecular shape (compact or elongated) Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 13

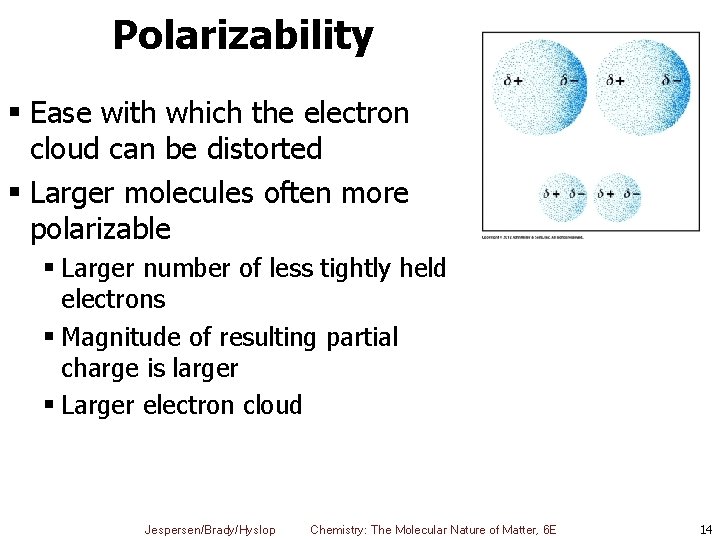
Polarizability § Ease with which the electron cloud can be distorted § Larger molecules often more polarizable § Larger number of less tightly held electrons § Magnitude of resulting partial charge is larger § Larger electron cloud Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 14

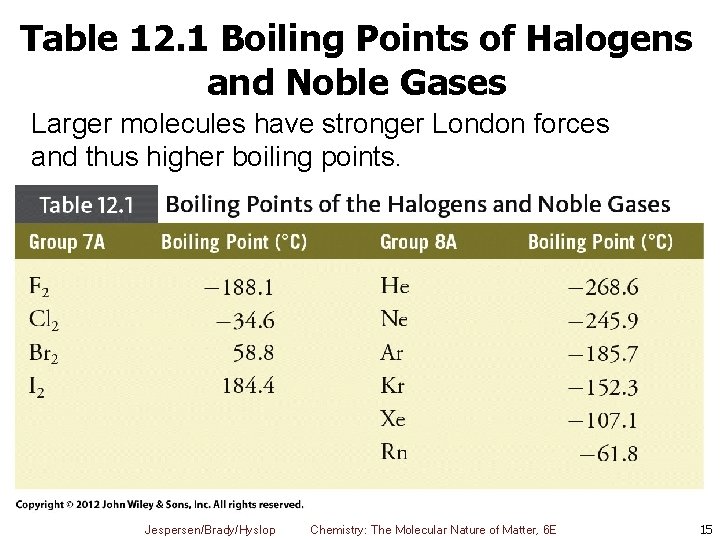
Table 12. 1 Boiling Points of Halogens and Noble Gases Larger molecules have stronger London forces and thus higher boiling points. Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 15

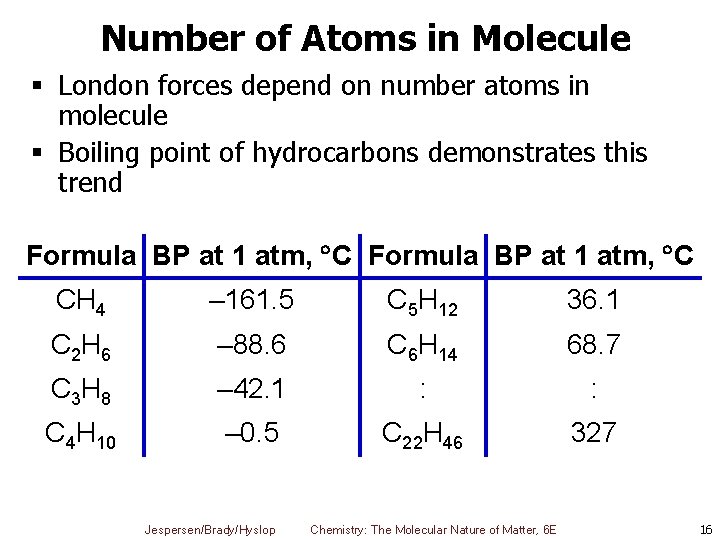
Number of Atoms in Molecule § London forces depend on number atoms in molecule § Boiling point of hydrocarbons demonstrates this trend Formula BP at 1 atm, C CH 4 – 161. 5 C 5 H 12 36. 1 C 2 H 6 – 88. 6 C 6 H 14 68. 7 C 3 H 8 – 42. 1 : : C 4 H 10 – 0. 5 C 22 H 46 327 Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 16

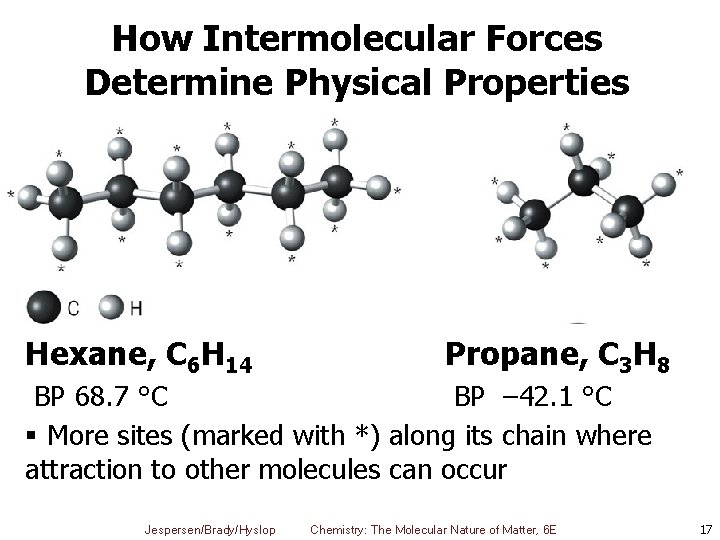
How Intermolecular Forces Determine Physical Properties Hexane, C 6 H 14 Propane, C 3 H 8 BP 68. 7 °C BP – 42. 1 °C § More sites (marked with *) along its chain where attraction to other molecules can occur Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 17

Molecular Shape § Increased surface area available for contact = increased London forces § London dispersion forces between spherical molecules are lower than chain-like molecules § More compact molecules § Hydrogen atoms not as free to interact with hydrogen atoms on other molecules § Less compact molecules § Hydrogen atoms have more chance to interact with hydrogen atoms on other molecules Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 18

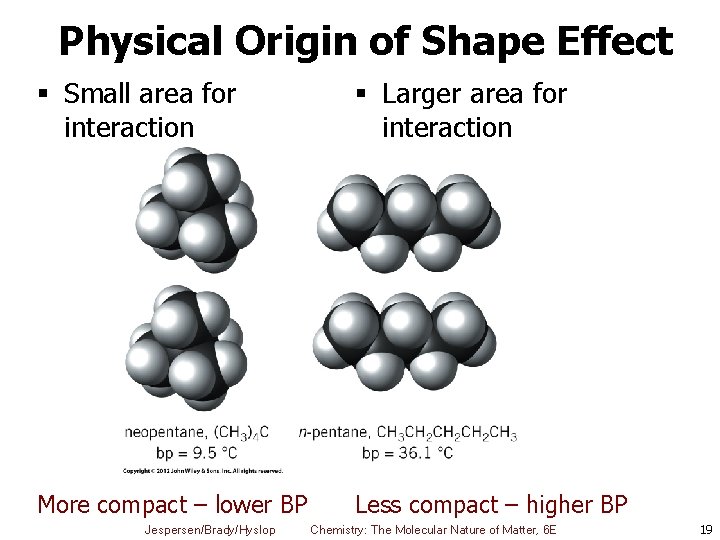
Physical Origin of Shape Effect § Small area for interaction § Larger area for interaction More compact – lower BP Less compact – higher BP Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 19

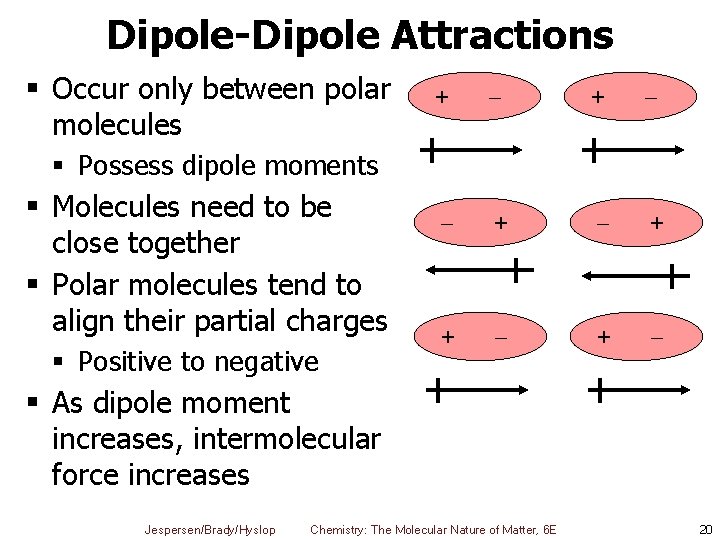
Dipole-Dipole Attractions § Occur only between polar molecules + + + + § Possess dipole moments § Molecules need to be close together § Polar molecules tend to align their partial charges § Positive to negative § As dipole moment increases, intermolecular force increases Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 20

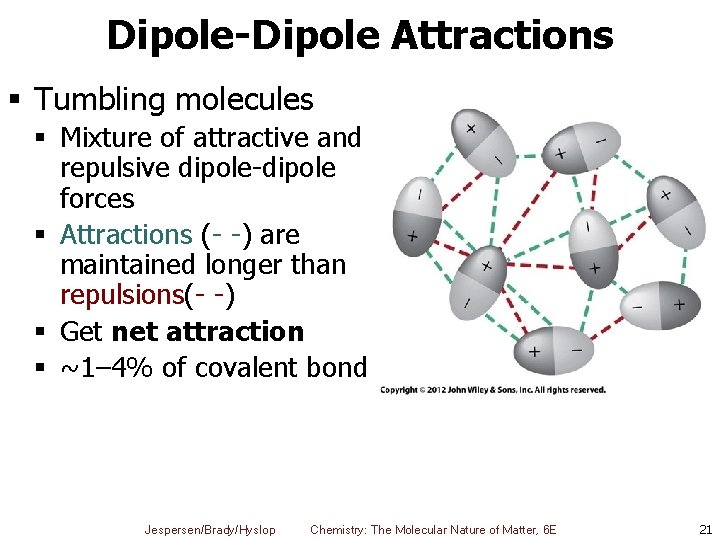
Dipole-Dipole Attractions § Tumbling molecules § Mixture of attractive and repulsive dipole-dipole forces § Attractions (- -) are maintained longer than repulsions(- -) § Get net attraction § ~1– 4% of covalent bond Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 21

Dipole-Dipole Attractions § Interactions between net dipoles in polar molecules § About 1– 4% as strong as a covalent bond § Decrease as molecular distance increases § Dipole-dipole forces increase with increasing polarity Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 22

Hydrogen Bonds § Special type of dipole-dipole Interaction § Very strong dipole-dipole attraction § ~10% of a covalent bond § Occurs between H and highly electronegative atom (O, N, or F) § H—F, H—O, and H—N bonds very polar § Electrons are drawn away from H, so high partial charges § H only has one electron, so +H presents almost bare proton § –X almost full – 1 charge § Element’s small size, means high charge density § Positive end of one can get very close to negative end of another Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 23

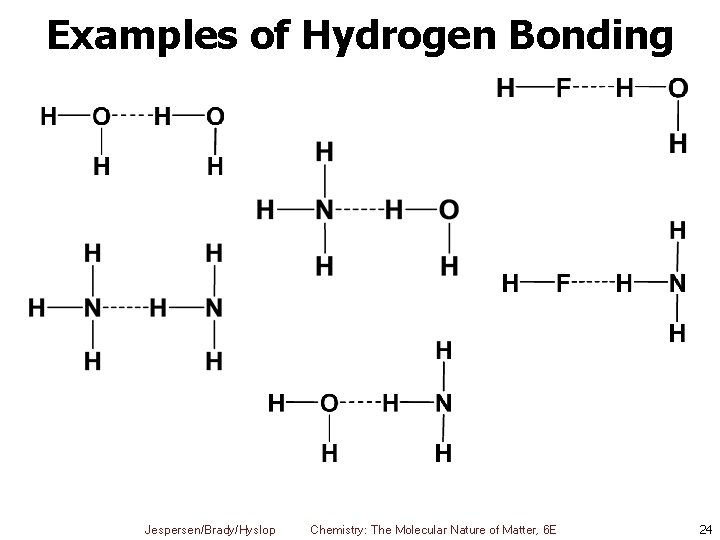
Examples of Hydrogen Bonding Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 24

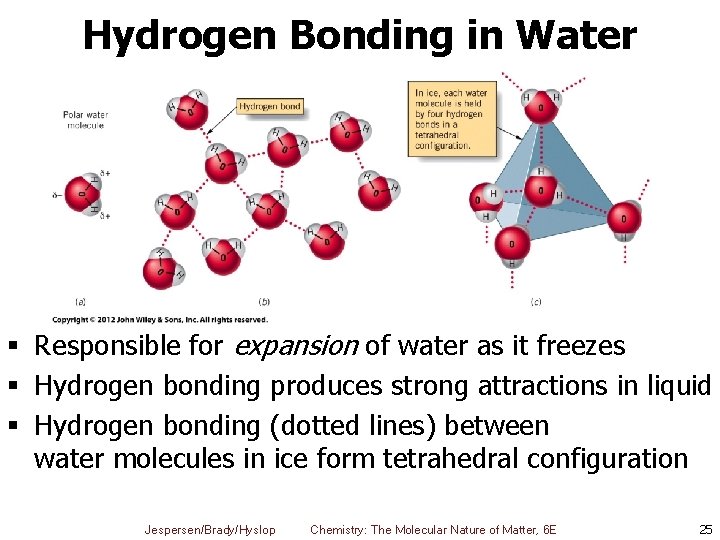
Hydrogen Bonding in Water § Responsible for expansion of water as it freezes § Hydrogen bonding produces strong attractions in liquid § Hydrogen bonding (dotted lines) between water molecules in ice form tetrahedral configuration Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 25

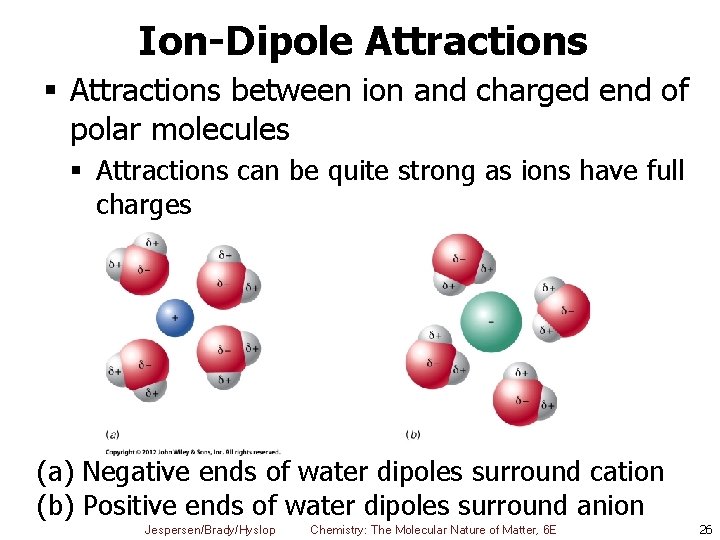
Ion-Dipole Attractions § Attractions between ion and charged end of polar molecules § Attractions can be quite strong as ions have full charges (a) Negative ends of water dipoles surround cation (b) Positive ends of water dipoles surround anion Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 26

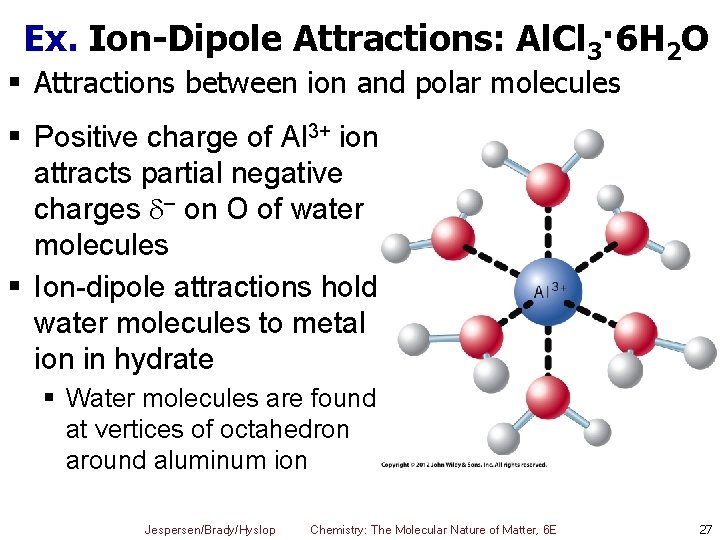
Ex. Ion-Dipole Attractions: Al. Cl 3· 6 H 2 O § Attractions between ion and polar molecules § Positive charge of Al 3+ ion attracts partial negative charges – on O of water molecules § Ion-dipole attractions hold water molecules to metal ion in hydrate § Water molecules are found at vertices of octahedron around aluminum ion Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 27

Ion-Induced Dipole Attractions § Attractions between ion and dipole it induces on neighboring molecules § Depends on § Ion charge and § Polarizability of its neighbor § Attractions can be quite strong as ion charge is constant, unlike instantaneous dipoles of ordinary London forces § E. g. , I– and Benzene Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 28

Summary of Intermolecular Attractions Dipole-dipole § Occur between neutral molecules with permanent dipoles § About 1– 4% of covalent bond § Mid range in terms of intermolecular forces Hydrogen bonding § Special type of dipole-dipole interaction § Occur when molecules contain N—H, H—F and O—H bonds § About 10% of a covalent bond Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 29

Summary of Intermolecular Attractions London dispersion § Present in all substances § Weakest intermolecular force § Weak, but can add up to large net attractions Ion-dipole § Occur when ions interact with polar molecules § Strongest intermolecular attraction Ion-induced dipole § Occur when ion induces dipole on neighboring particle § Depend on ion charge and polarizability of its neighbor Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 30

Using Intermolecular Forces § Often can predict physical properties (like BP, MP and many others) by comparing strengths of intermolecular attractions Strongest § Ion-Dipole § Hydrogen Bonding § Dipole-Dipole Weakest § London Forces § Larger, longer, and therefore heavier molecules often have stronger intermolecular forces § Smaller, more compact, lighter molecules have generally weaker intermolecular forces Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 31

Physical Properties that Depend on How Tightly Molecules Pack § Compressibility § Measure of ability of substance to be forced into smaller volume § Determined by strength of intermolecular forces § Gases highly compressible § Molecules far apart § Weak intermolecular forces § Solids and liquids nearly incompressible § Molecules very close together § Stronger intermolecular forces Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 32

Intermolecular Forces Determine Strength of Many Physical Properties § Retention of volume and shape § Solids retain both volume and shape § Strongest intermolecular attractions § Molecules closest § Liquids retain volume, but not shape § Attractions intermediate § Gases, expand to fill their containers § Weakest intermolecular attractions § Molecules farthest apart Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 33

Intermolecular Forces and Temperature § Decrease with increasing temperature § Increasing kinetic energy overcomes attractive forces § If allowed to expand, increasing temperature increases distance between gas particles and decreases attractive forces Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 34

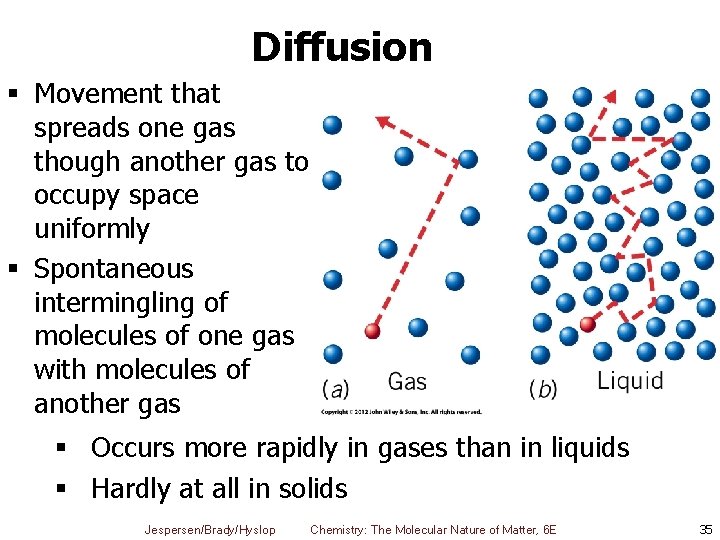
Diffusion § Movement that spreads one gas though another gas to occupy space uniformly § Spontaneous intermingling of molecules of one gas with molecules of another gas § Occurs more rapidly in gases than in liquids § Hardly at all in solids Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 35

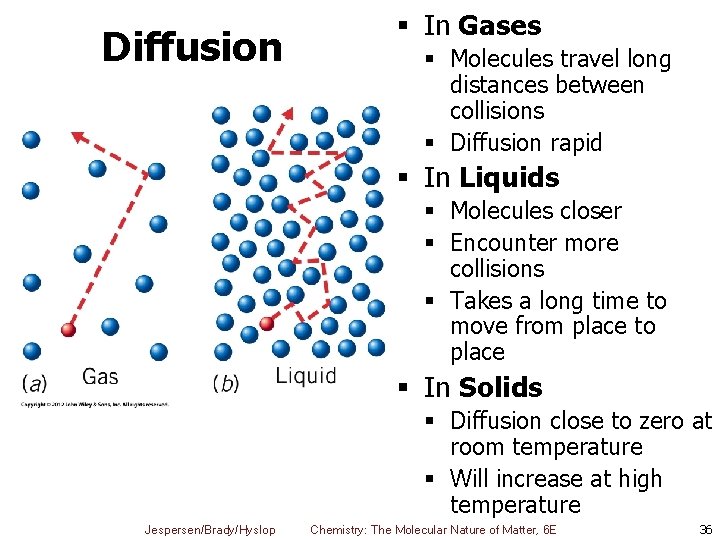
Diffusion § In Gases § Molecules travel long distances between collisions § Diffusion rapid § In Liquids § Molecules closer § Encounter more collisions § Takes a long time to move from place to place § In Solids § Diffusion close to zero at room temperature § Will increase at high temperature Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 36

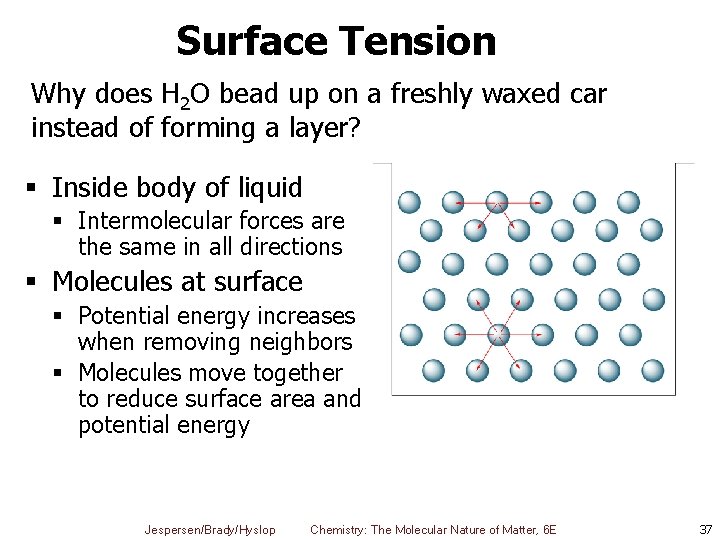
Surface Tension Why does H 2 O bead up on a freshly waxed car instead of forming a layer? § Inside body of liquid § Intermolecular forces are the same in all directions § Molecules at surface § Potential energy increases when removing neighbors § Molecules move together to reduce surface area and potential energy Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 37

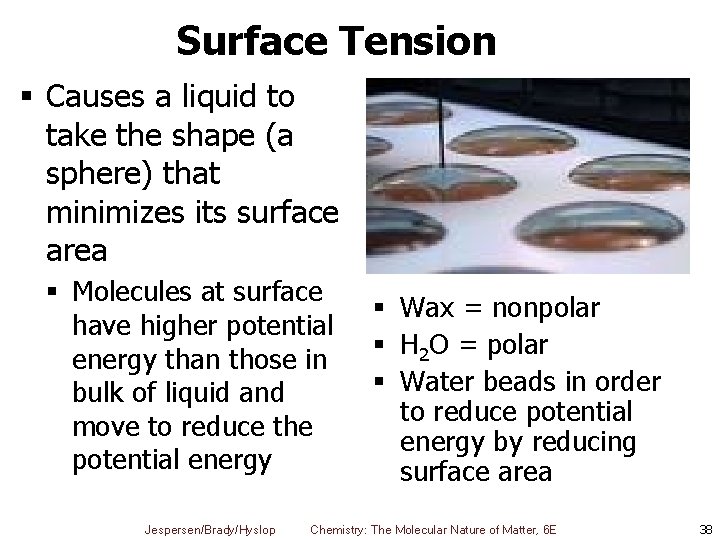
Surface Tension § Causes a liquid to take the shape (a sphere) that minimizes its surface area § Molecules at surface have higher potential energy than those in bulk of liquid and move to reduce the potential energy Jespersen/Brady/Hyslop § Wax = nonpolar § H 2 O = polar § Water beads in order to reduce potential energy by reducing surface area Chemistry: The Molecular Nature of Matter, 6 E 38

Surface Tension § Liquids containing molecules with strong intermolecular forces have high surface tension § Allows us to fill glass above rim § Gives surface rounded appearance § Surface acts as “skin” that lets water pile up § Surface resists expansion and pushes back Jespersen/Brady/Hyslop § Surface tension increases as intermolecular forces increase § Surface tension decreases as temperature increases Chemistry: The Molecular Nature of Matter, 6 E 39

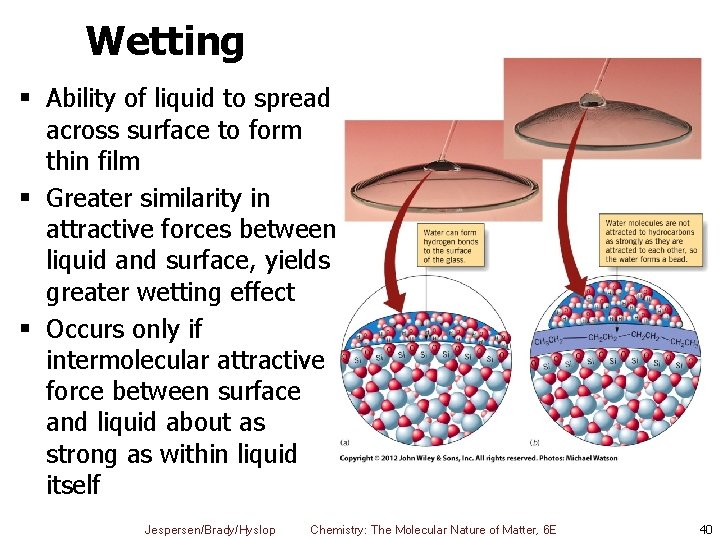
Wetting § Ability of liquid to spread across surface to form thin film § Greater similarity in attractive forces between liquid and surface, yields greater wetting effect § Occurs only if intermolecular attractive force between surface and liquid about as strong as within liquid itself Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 40

Wetting Ex. H 2 O wets clean glass surface as it forms H–bonds to Si. O 2 surface § Does not wet greasy glass, because grease is nonpolar and water is very polar § Only London forces § Forms beads instead Surfactants § Added to detergents to lower surface tension of H 2 O § Now water can spread out on greasy glass Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 41

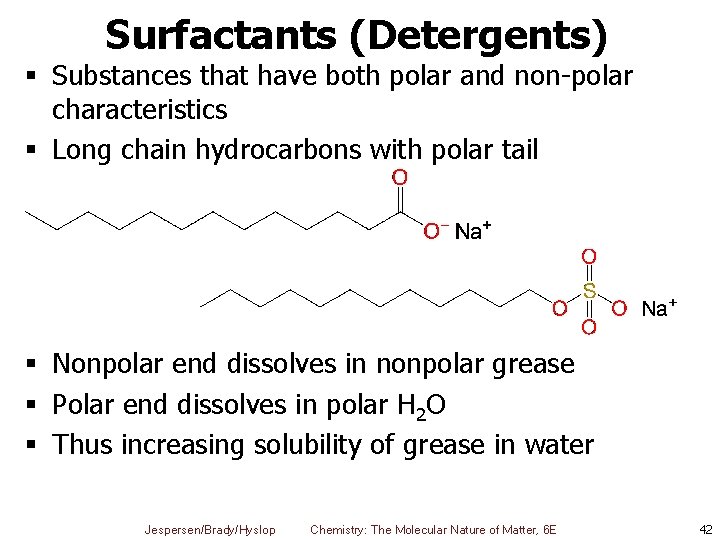
Surfactants (Detergents) § Substances that have both polar and non-polar characteristics § Long chain hydrocarbons with polar tail § Nonpolar end dissolves in nonpolar grease § Polar end dissolves in polar H 2 O § Thus increasing solubility of grease in water Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 42

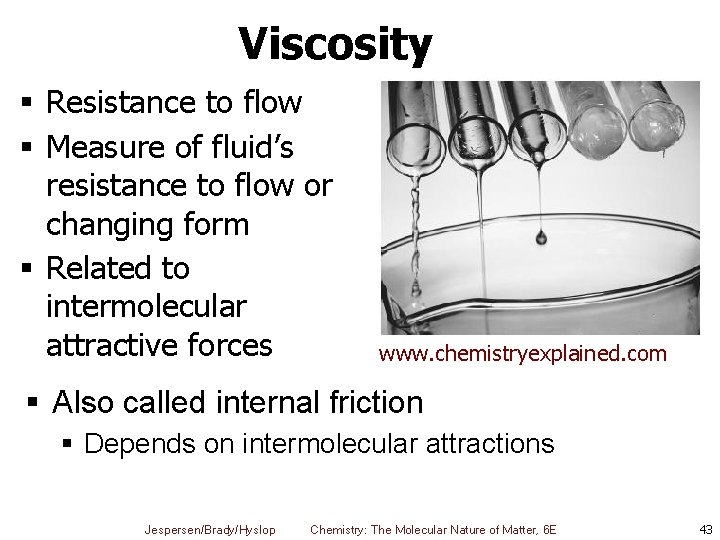
Viscosity § Resistance to flow § Measure of fluid’s resistance to flow or changing form § Related to intermolecular attractive forces www. chemistryexplained. com § Also called internal friction § Depends on intermolecular attractions Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 43

Viscosity § Viscosity decreases when temperature increases § Most people associate liquids with viscosity § Syrup more viscous than water § Gases have viscosity § Respond almost instantly to form-changing forces § Solids, such as rocks and glass have viscosity § Normally respond very slowly to forces acting to change their shape Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 44

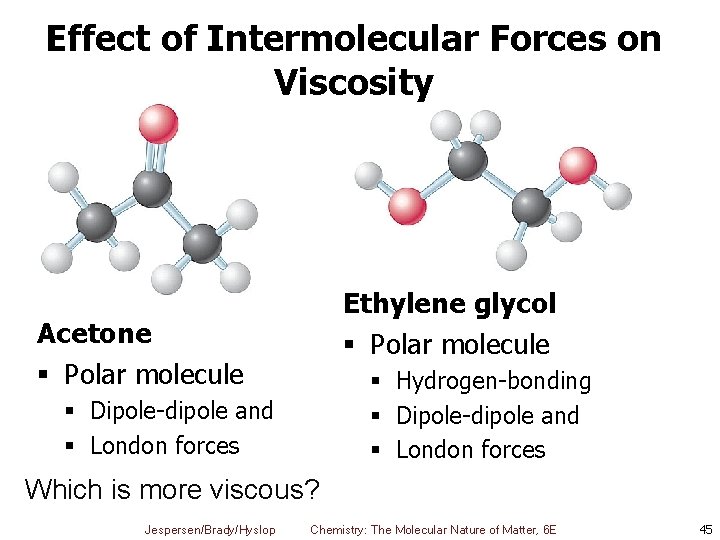
Effect of Intermolecular Forces on Viscosity Ethylene glycol § Polar molecule Acetone § Polar molecule § Hydrogen-bonding § Dipole-dipole and § London forces Which is more viscous? Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 45

Solubility § “Like dissolves like” § To dissolve polar substance, use polar solvent § To dissolve nonpolar substance, use nonpolar solvent § Compare relative polarity § Similar polarity means greater ability to dissolve in each other § Differing polarity means that they don’t dissolve, they are insoluble § Surfactants § Both polar and non-polar characteristics § Used to increase solubility Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 46

Phase Changes § Changes of physical state § Deal with motion of molecules § As temperature changes § Matter will undergo phase changes § Liquid Gas § Evaporation, vaporization § As heat is added, H 2 O, forms steam or water vapor § Requires energy or source of heat Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 47

Phase Changes § Solid Gas § Sublimation § Ice cubes in freezer, leave in long enough disappear § Endothermic § Gas Liquid § § Condensation Dew is H 2 O vapor condensing onto cooler ground Exothermic Often limits lower night time temperature Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 48

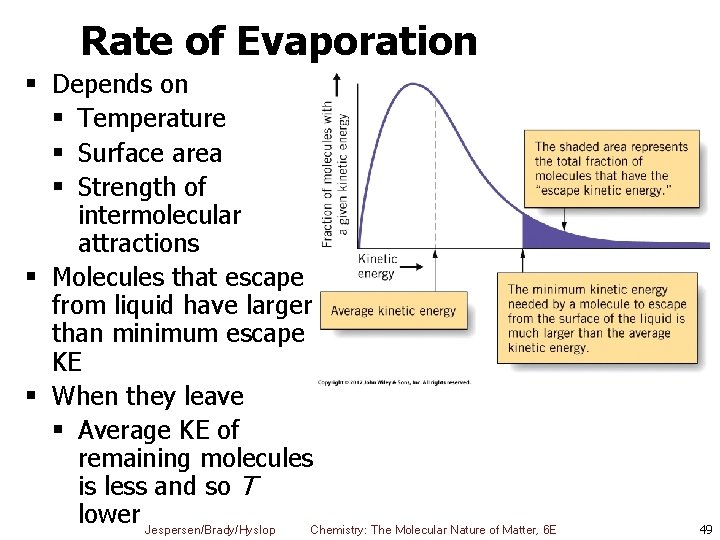
Rate of Evaporation § Depends on § Temperature § Surface area § Strength of intermolecular attractions § Molecules that escape from liquid have larger than minimum escape KE § When they leave § Average KE of remaining molecules is less and so T lower Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 49

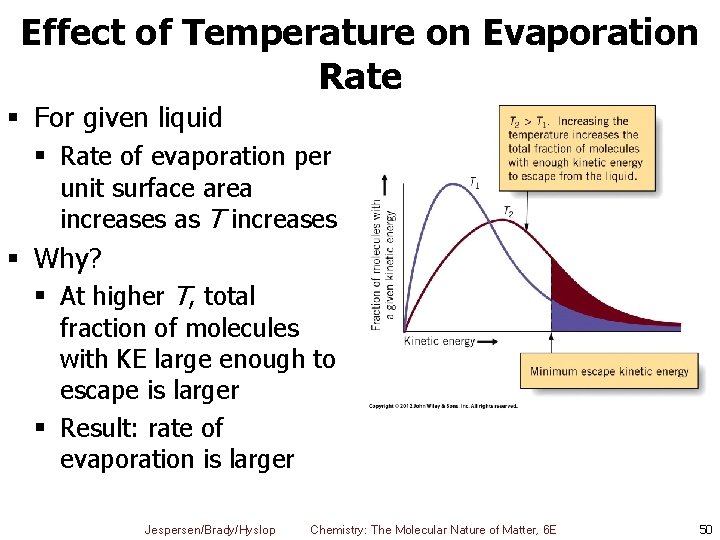
Effect of Temperature on Evaporation Rate § For given liquid § Rate of evaporation per unit surface area increases as T increases § Why? § At higher T, total fraction of molecules with KE large enough to escape is larger § Result: rate of evaporation is larger Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 50

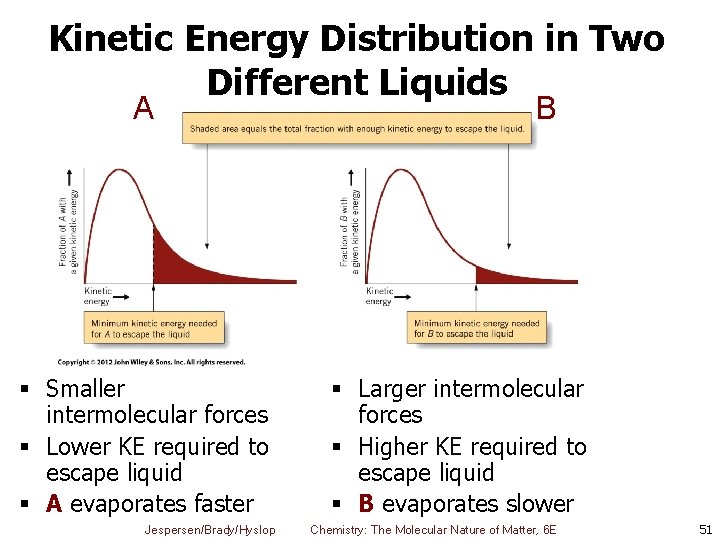
Kinetic Energy Distribution in Two Different Liquids A § Smaller intermolecular forces § Lower KE required to escape liquid § A evaporates faster Jespersen/Brady/Hyslop B § Larger intermolecular forces § Higher KE required to escape liquid § B evaporates slower Chemistry: The Molecular Nature of Matter, 6 E 51

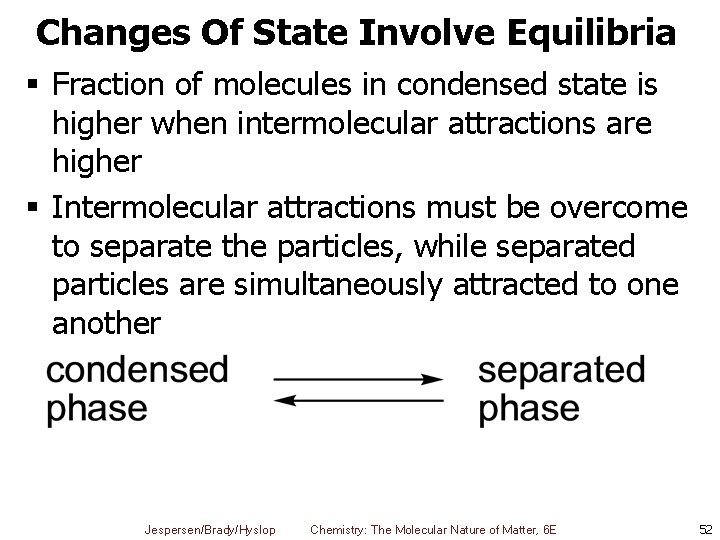
Changes Of State Involve Equilibria § Fraction of molecules in condensed state is higher when intermolecular attractions are higher § Intermolecular attractions must be overcome to separate the particles, while separated particles are simultaneously attracted to one another Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 52

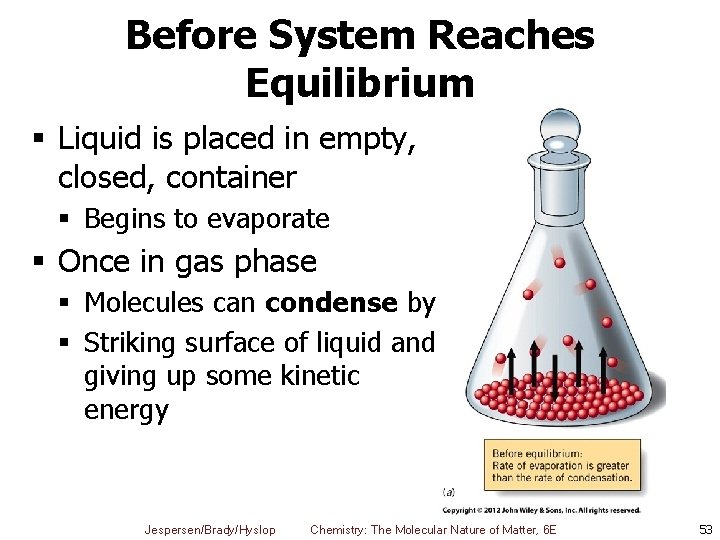
Before System Reaches Equilibrium § Liquid is placed in empty, closed, container § Begins to evaporate § Once in gas phase § Molecules can condense by § Striking surface of liquid and giving up some kinetic energy Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 53

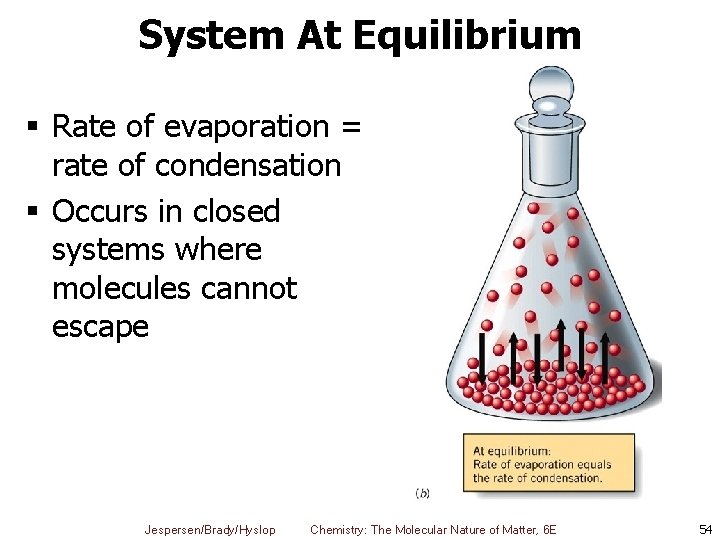
System At Equilibrium § Rate of evaporation = rate of condensation § Occurs in closed systems where molecules cannot escape Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 54

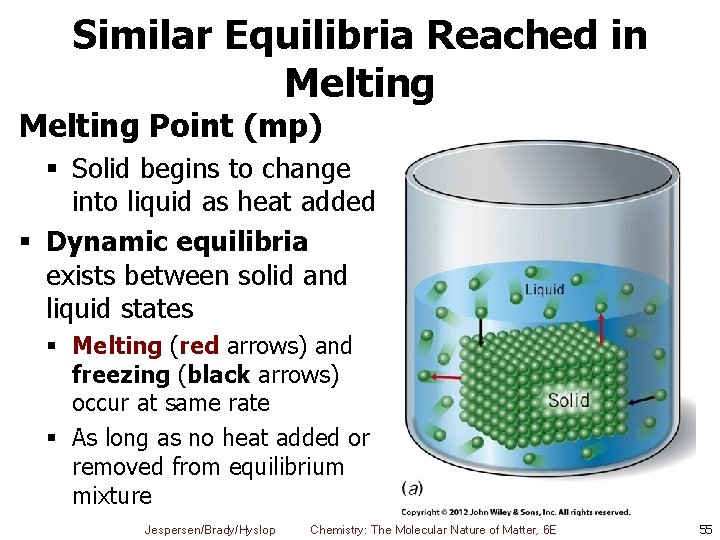
Similar Equilibria Reached in Melting Point (mp) § Solid begins to change into liquid as heat added § Dynamic equilibria exists between solid and liquid states § Melting (red arrows) and freezing (black arrows) occur at same rate § As long as no heat added or removed from equilibrium mixture Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 55

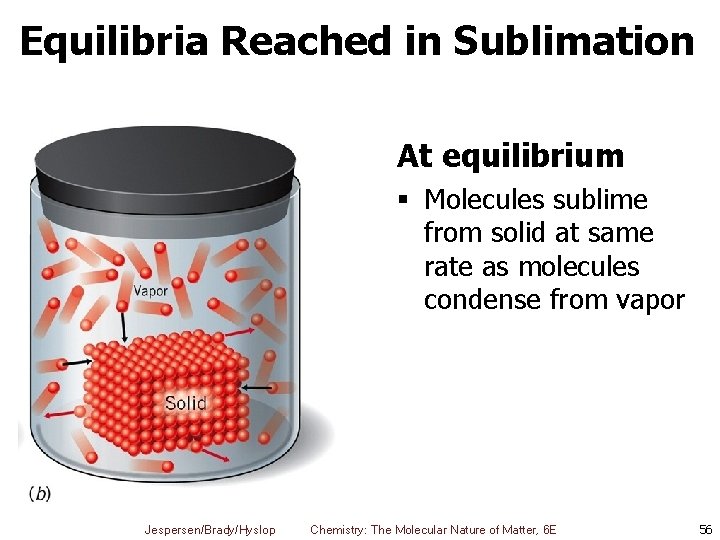
Equilibria Reached in Sublimation At equilibrium § Molecules sublime from solid at same rate as molecules condense from vapor Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 56

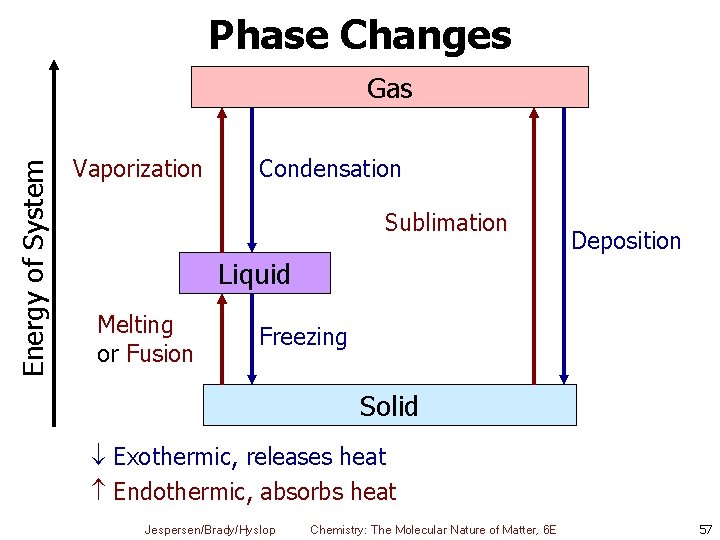
Phase Changes Energy of System Gas Vaporization Condensation Sublimation Deposition Liquid Melting or Fusion Freezing Solid Exothermic, releases heat Endothermic, absorbs heat Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 57

Energy Changes Accompanying Phase Changes § All phase changes are possible under the right conditions § Following sequence is endothermic heat solid melt heat liquid boil heat gas § Following sequence is exothermic cool gas condense cool liquid freeze cool solid Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 58

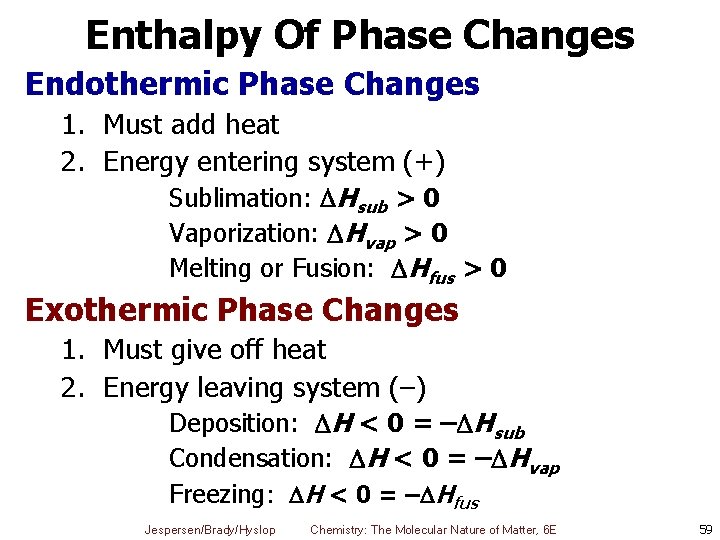
Enthalpy Of Phase Changes Endothermic Phase Changes 1. Must add heat 2. Energy entering system (+) Sublimation: Hsub > 0 Vaporization: Hvap > 0 Melting or Fusion: Hfus > 0 Exothermic Phase Changes 1. Must give off heat 2. Energy leaving system (–) Deposition: H < 0 = – Hsub Condensation: H < 0 = – Hvap Freezing: H < 0 = – Hfus Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 59

Phase Changes § As T changes, matter undergoes phase changes § Phase Change § Transformation from one phase to another § Liquid-Vapor Equilibrium § Molecules in liquid § Not in rigid lattice § In constant motion § Denser than gas, so more collisions § Some have enough kinetic energy to escape, some don’t Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 60

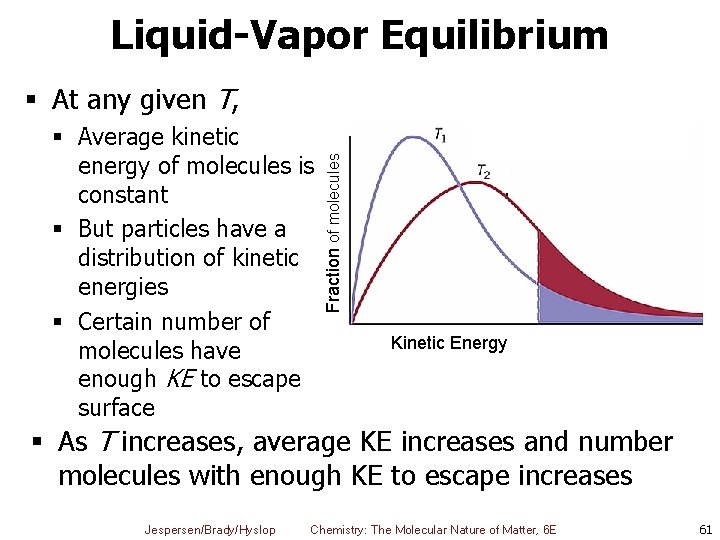
Liquid-Vapor Equilibrium § Average kinetic energy of molecules is constant § But particles have a distribution of kinetic energies § Certain number of molecules have enough KE to escape surface Fraction of molecules § At any given T, Kinetic Energy § As T increases, average KE increases and number molecules with enough KE to escape increases Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 61

Vapor Pressure § Pressure molecules exert when they evaporate or escape into gas (vapor) phase § Pressure of gas when liquid or solid is at equilibrium with its gas phase § Increasing temperature increases vapor pressure because vaporization is endothermic § liquid + heat of vaporization ↔ gas Equilibrium Vapor Pressure § VP once dynamic equilibrium reached § Usually referred to as simply vapor pressure Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 62

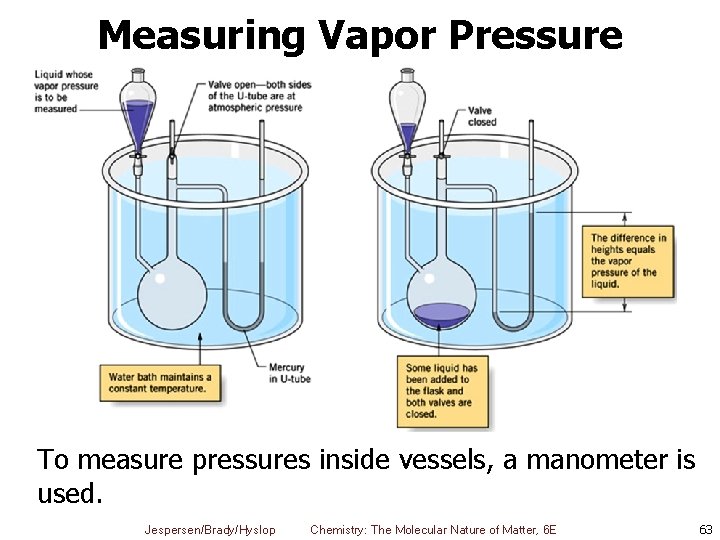
Measuring Vapor Pressure To measure pressures inside vessels, a manometer is used. Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 63

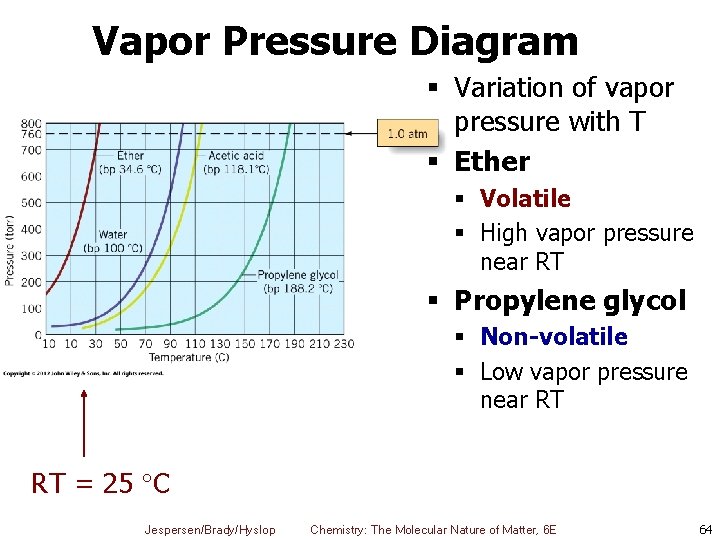
Vapor Pressure Diagram § Variation of vapor pressure with T § Ether § Volatile § High vapor pressure near RT § Propylene glycol § Non-volatile § Low vapor pressure near RT RT = 25 C Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 64

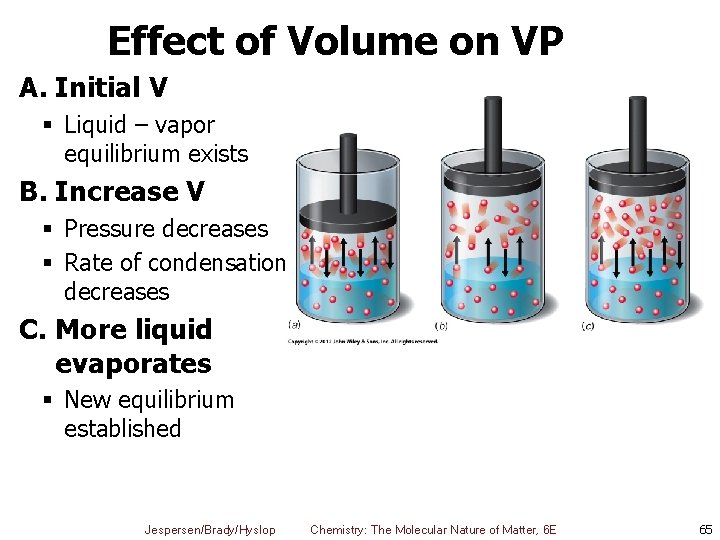
Effect of Volume on VP A. Initial V § Liquid – vapor equilibrium exists B. Increase V § Pressure decreases § Rate of condensation decreases C. More liquid evaporates § New equilibrium established Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 65

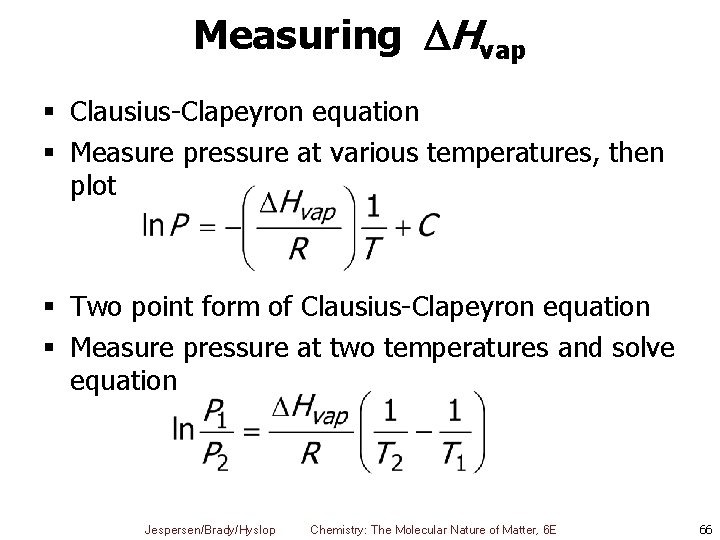
Measuring Hvap § Clausius-Clapeyron equation § Measure pressure at various temperatures, then plot § Two point form of Clausius-Clapeyron equation § Measure pressure at two temperatures and solve equation Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 66

Do Solids Have Vapor Pressures? § Yes § At given temperature § Some solid particles have enough KE to escape into vapor phase § When vapor particles collide with surface § They can be captured § Equilibrium vapor pressure of solid § Pressure of vapor in equilibrium with solid Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 67

Boiling Point (bp) § T at which vapor pressure of liquid = atmospheric pressure. § Bp increases as strength of intermolecular forces increase Normal Boiling Point § T at which vapor pressure of liquid = 1 atm Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 68

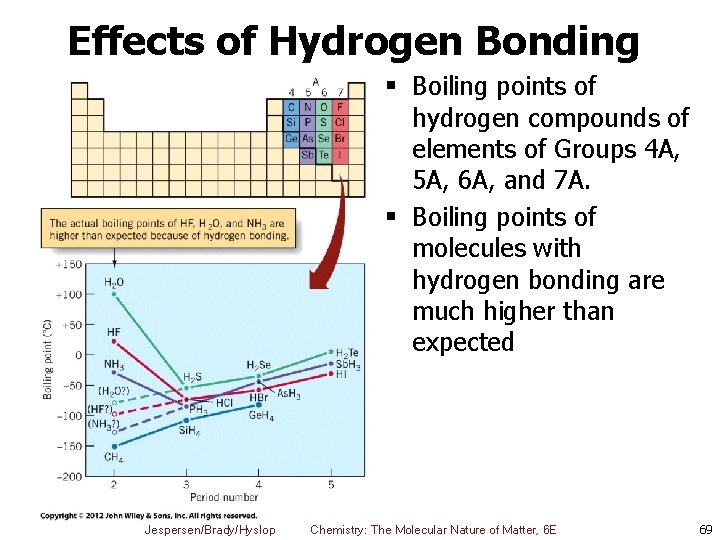
Effects of Hydrogen Bonding § Boiling points of hydrogen compounds of elements of Groups 4 A, 5 A, 6 A, and 7 A. § Boiling points of molecules with hydrogen bonding are much higher than expected Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 69

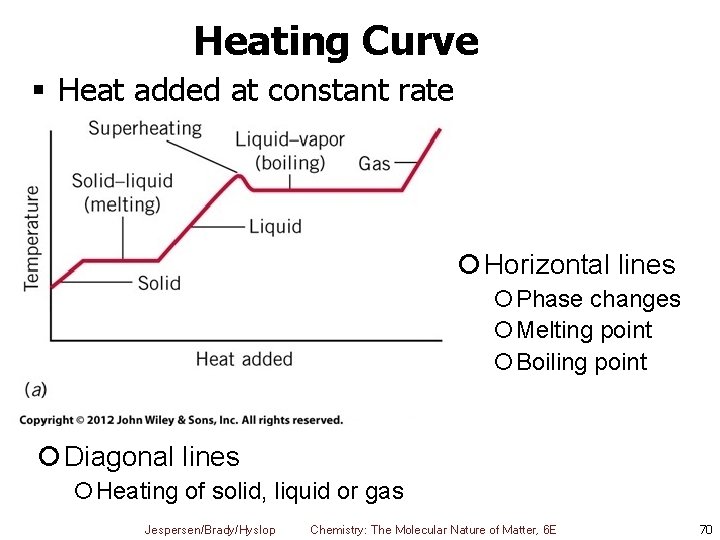
Heating Curve § Heat added at constant rate ¡ Horizontal lines ¡Phase changes ¡Melting point ¡Boiling point ¡ Diagonal lines ¡Heating of solid, liquid or gas Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 70

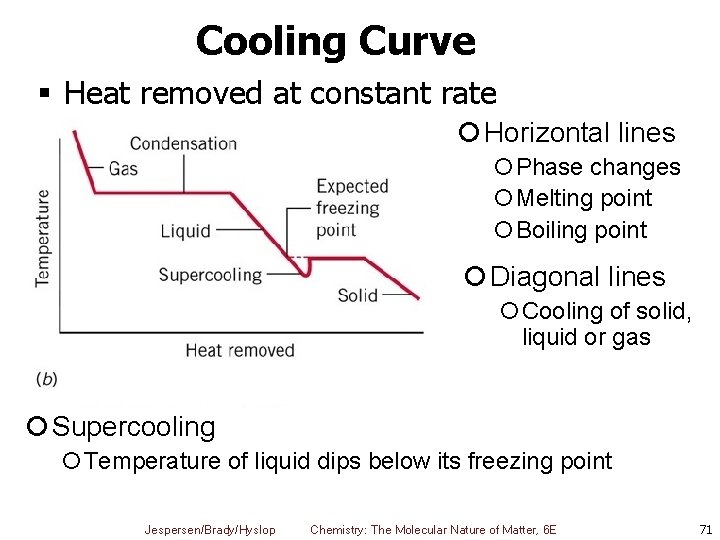
Cooling Curve § Heat removed at constant rate ¡ Horizontal lines ¡Phase changes ¡Melting point ¡Boiling point ¡ Diagonal lines ¡Cooling of solid, liquid or gas ¡ Supercooling ¡Temperature of liquid dips below its freezing point Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 71

Energies of Phase Changes § Expressed per mole § Molar heat of fusion ( Hfus) § Heat absorbed by one mole of solid when it melts to give liquid at constant. T and P § Molar heat of vaporization ( Hvap ) § Heat absorbed when one mole of liquid is changed to one mole of vapor at constant T and P § Molar heat of sublimation ( Hsub ) § Heat absorbed by one mole of solid when it sublimes to give one mole of vapor at constant T and P § All of these quantities tend to increase with increasing intermolecular forces Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 72

Le Chatelier’s Principle § Equilibria are often disturbed or upset § When dynamic equilibrium of system is upset by a disturbance § System responds in direction that tends to counteract disturbance and, if possible, restore equilibrium § Position of equilibrium § Used to refer to relative amounts of substance on each side of double (equilibrium) arrows Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 73

Liquid Vapor Equilibrium Liquid + Heat Vapor § Increasing T § Increases amount of vapor § Decreases amount of liquid § Equilibrium has shifted § Shifted to the right § More vapor is produced at expense of liquid § Temperature-pressure relationships can be represented using a phase diagram Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 74

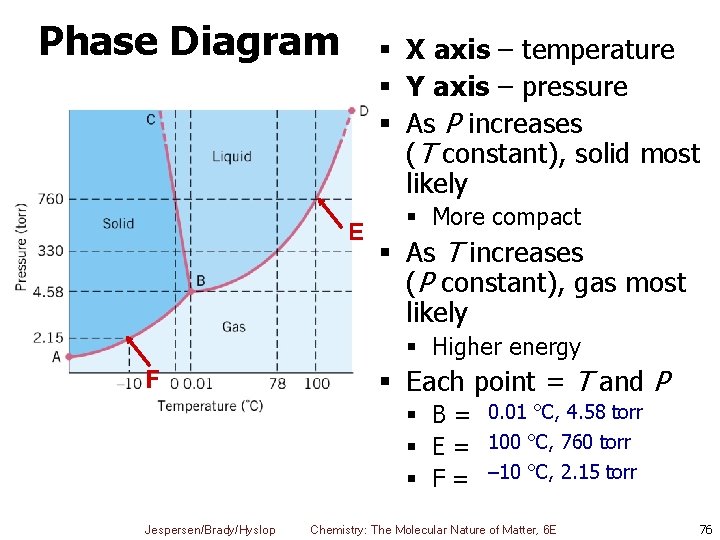
Phase Diagrams § Show the effects of both pressure and temperature on phase changes § Boundaries between phases indicate equilibrium § Triple point: § The temperature and pressure at which s, l, and g are all at equilibrium § Critical point: § The temperature and pressure at which a gas can no longer be condensed § TC = temperature at critical point § PC = pressure at critical point Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 75

Phase Diagram § X axis – temperature § Y axis – pressure § As P increases (T constant), solid most likely E § More compact § As T increases (P constant), gas most likely § Higher energy F § Each point = T and P § B = 0. 01 °C, 4. 58 torr § E = 100 °C, 760 torr § F = – 10 °C, 2. 15 torr Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 76

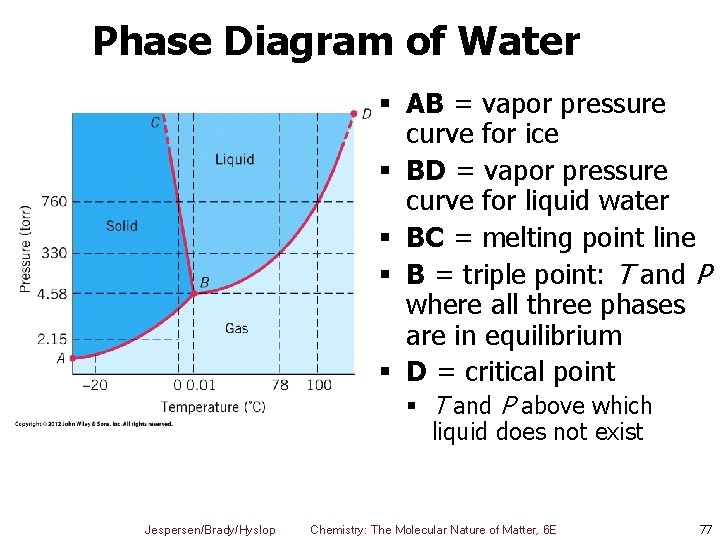
Phase Diagram of Water § AB = vapor pressure curve for ice § BD = vapor pressure curve for liquid water § BC = melting point line § B = triple point: T and P where all three phases are in equilibrium § D = critical point § T and P above which liquid does not exist Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 77

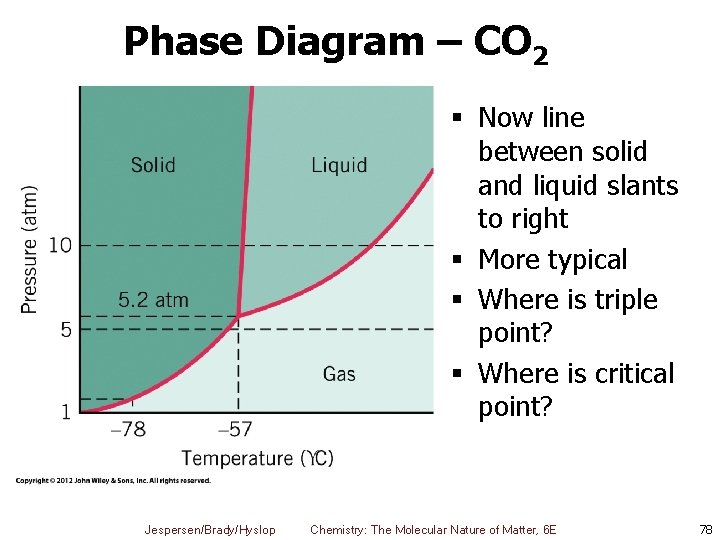
Phase Diagram – CO 2 § Now line between solid and liquid slants to right § More typical § Where is triple point? § Where is critical point? Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 78

Supercritical Fluid § Substance with temperature above its critical temperature (TC) and density near its liquid density § Have unique properties that make them excellent solvents § Values of TC tend to increase with increased intermolecular attractions between particles Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 79

Types of Solids § Crystalline Solids § Solids with highly regular arrangements of components § Amorphous Solids § Solids with considerable disorder in their structures Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 80

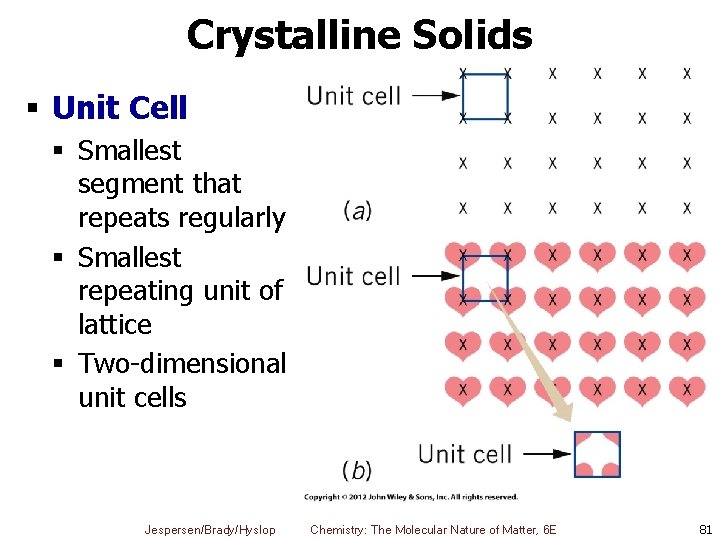
Crystalline Solids § Unit Cell § Smallest segment that repeats regularly § Smallest repeating unit of lattice § Two-dimensional unit cells Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 81

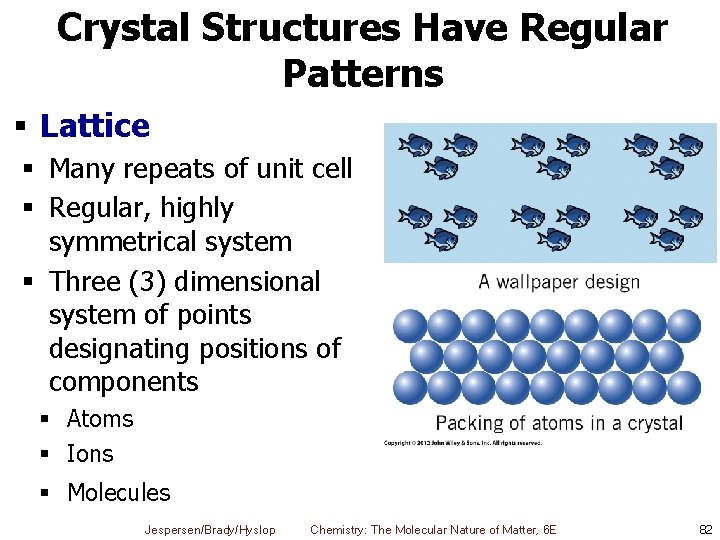
Crystal Structures Have Regular Patterns § Lattice § Many repeats of unit cell § Regular, highly symmetrical system § Three (3) dimensional system of points designating positions of components § Atoms § Ions § Molecules Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 82

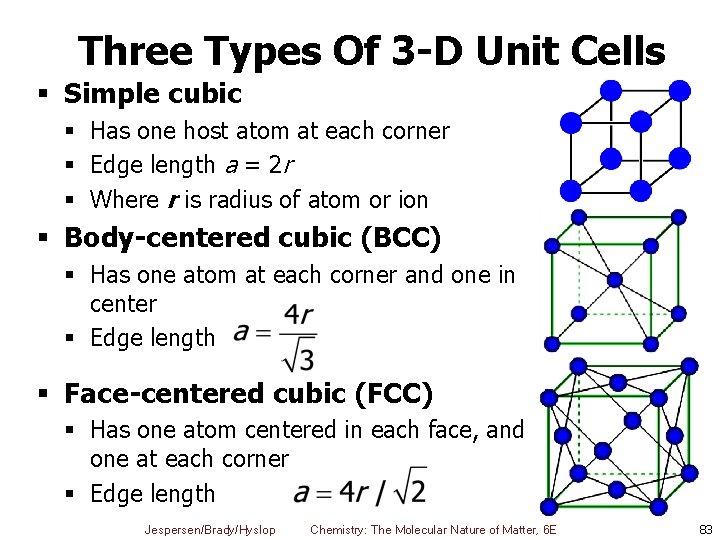
Three Types Of 3 -D Unit Cells § Simple cubic § Has one host atom at each corner § Edge length a = 2 r § Where r is radius of atom or ion § Body-centered cubic (BCC) § Has one atom at each corner and one in center § Edge length § Face-centered cubic (FCC) § Has one atom centered in each face, and one at each corner § Edge length Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 83

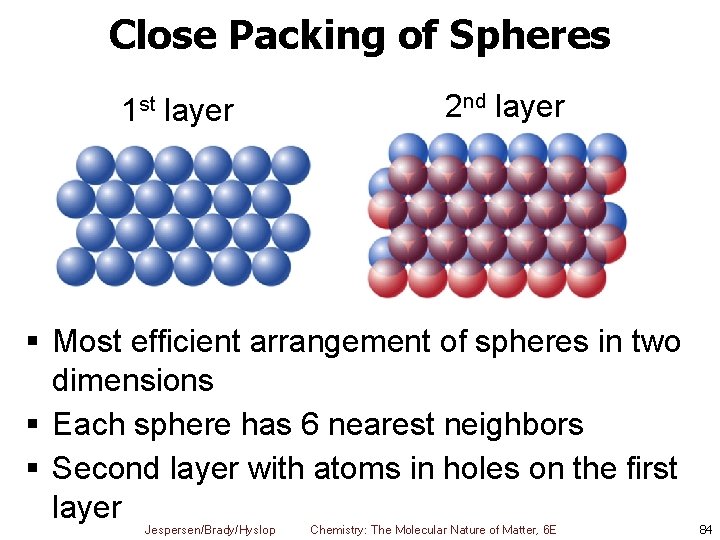
Close Packing of Spheres 1 st layer 2 nd layer § Most efficient arrangement of spheres in two dimensions § Each sphere has 6 nearest neighbors § Second layer with atoms in holes on the first layer Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 84

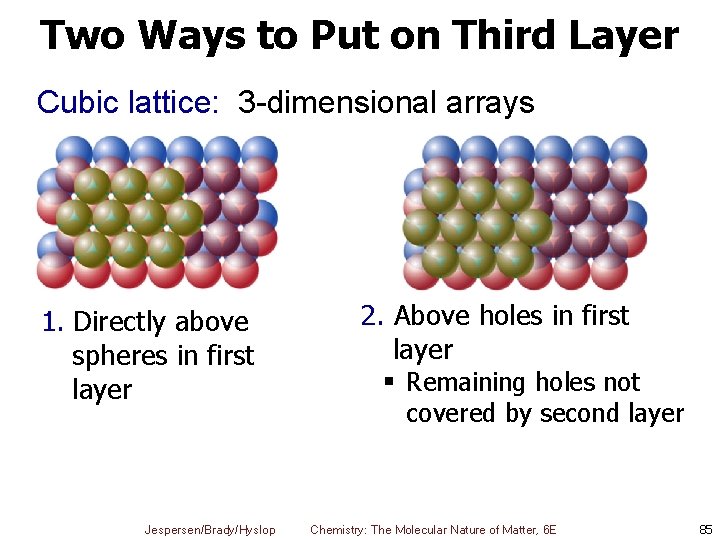
Two Ways to Put on Third Layer Cubic lattice: 3 -dimensional arrays 1. Directly above spheres in first layer Jespersen/Brady/Hyslop 2. Above holes in first layer § Remaining holes not covered by second layer Chemistry: The Molecular Nature of Matter, 6 E 85

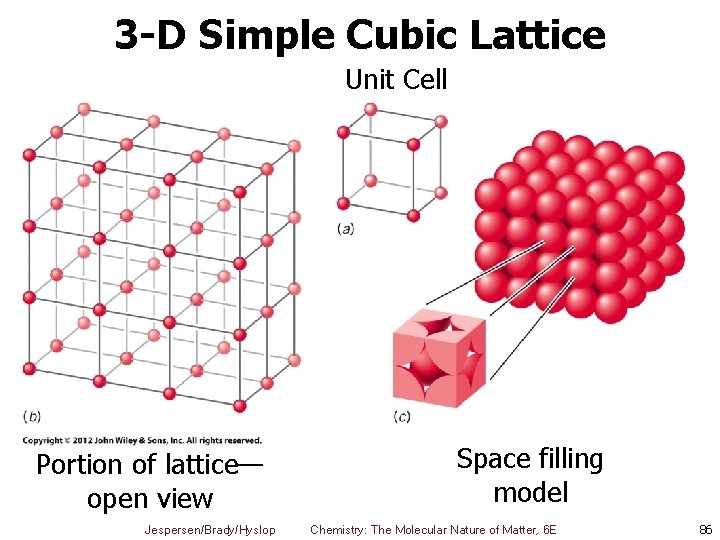
3 -D Simple Cubic Lattice Unit Cell Portion of lattice— open view Jespersen/Brady/Hyslop Space filling model Chemistry: The Molecular Nature of Matter, 6 E 86

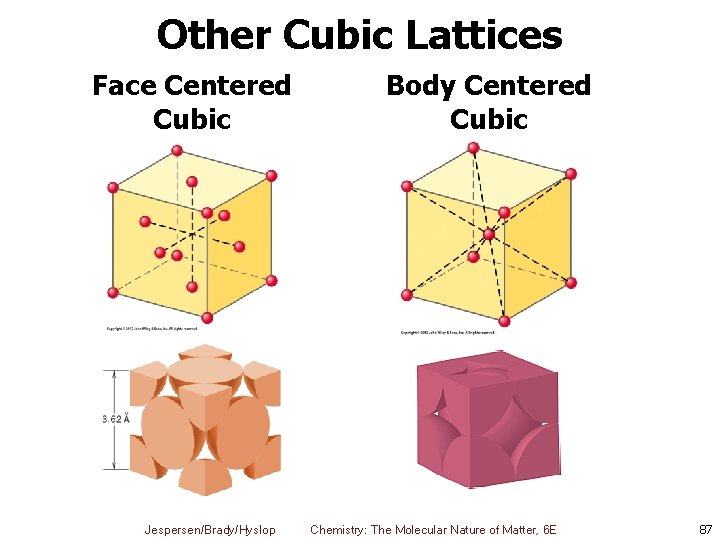
Other Cubic Lattices Face Centered Cubic Jespersen/Brady/Hyslop Body Centered Cubic Chemistry: The Molecular Nature of Matter, 6 E 87

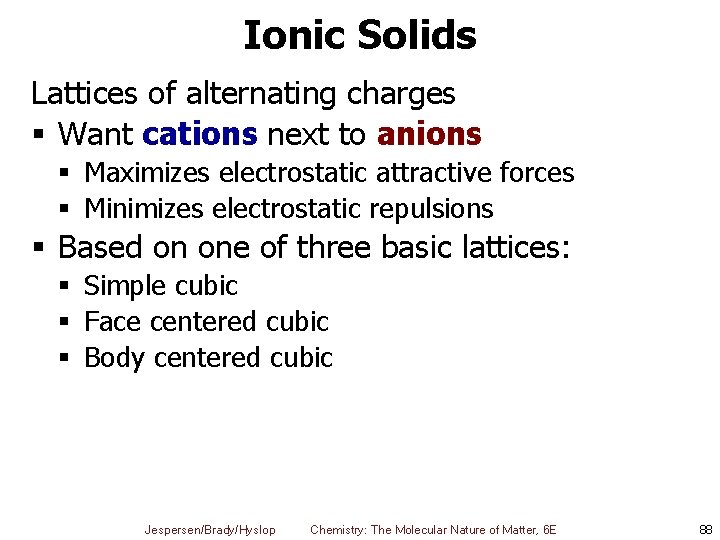
Ionic Solids Lattices of alternating charges § Want cations next to anions § Maximizes electrostatic attractive forces § Minimizes electrostatic repulsions § Based on one of three basic lattices: § Simple cubic § Face centered cubic § Body centered cubic Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 88

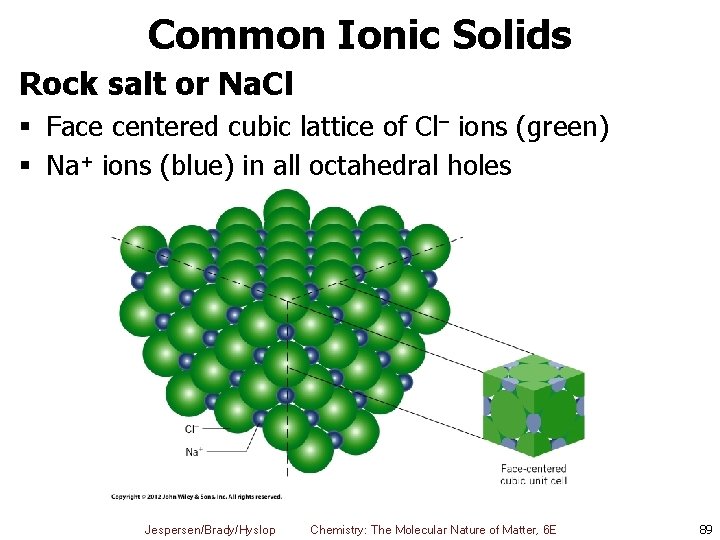
Common Ionic Solids Rock salt or Na. Cl § Face centered cubic lattice of Cl– ions (green) § Na+ ions (blue) in all octahedral holes Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 89

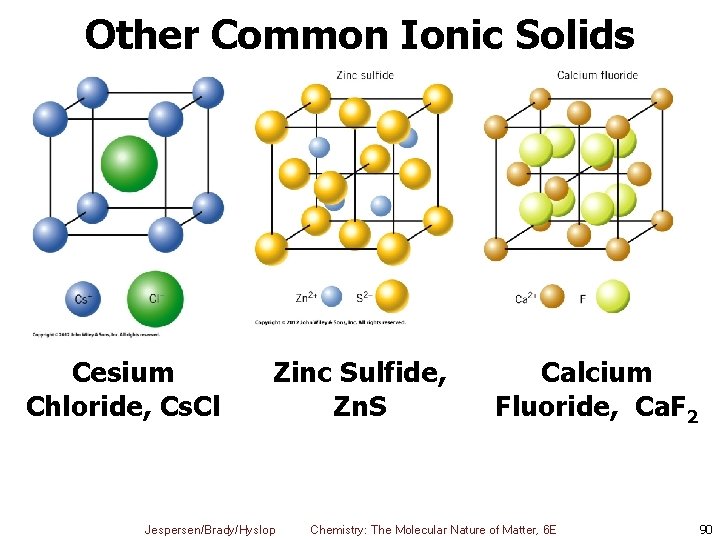
Other Common Ionic Solids Cesium Chloride, Cs. Cl Zinc Sulfide, Zn. S Jespersen/Brady/Hyslop Calcium Fluoride, Ca. F 2 Chemistry: The Molecular Nature of Matter, 6 E 90

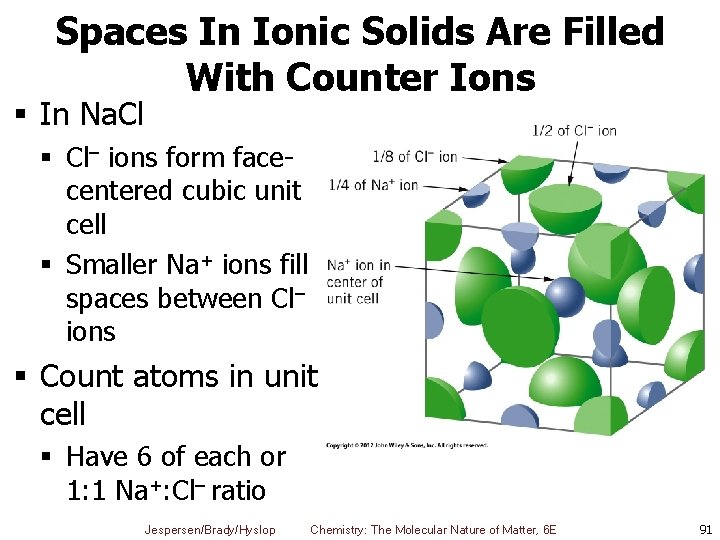
Spaces In Ionic Solids Are Filled With Counter Ions § In Na. Cl § Cl– ions form facecentered cubic unit cell § Smaller Na+ ions fill spaces between Cl– ions § Count atoms in unit cell § Have 6 of each or 1: 1 Na+: Cl– ratio Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 91

Some Factors Affecting Crystalline Structure § Size of atoms or ions involved § Stoichiometry of salt § Materials involved § Some substances do not form crystalline solids Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 92

Amorphous Solids (Glass) § Have little order, thus referred to as “super cooled liquids” § Edges are not clean, but ragged due to the lack of order Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 93

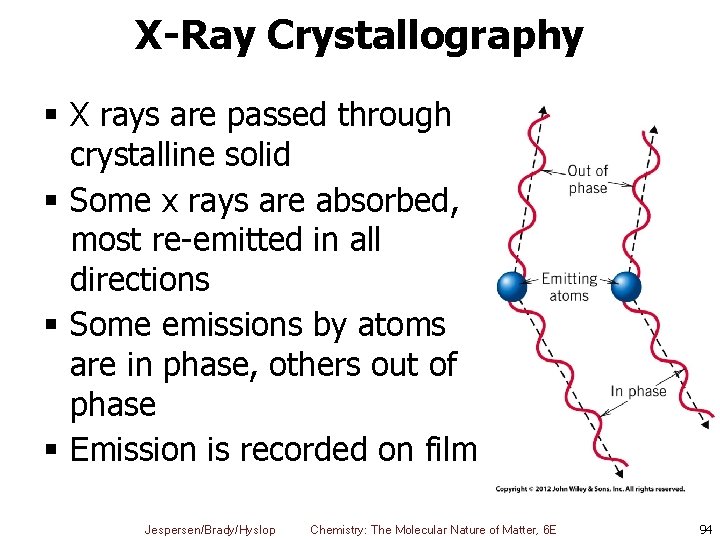
X-Ray Crystallography § X rays are passed through crystalline solid § Some x rays are absorbed, most re-emitted in all directions § Some emissions by atoms are in phase, others out of phase § Emission is recorded on film Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 94

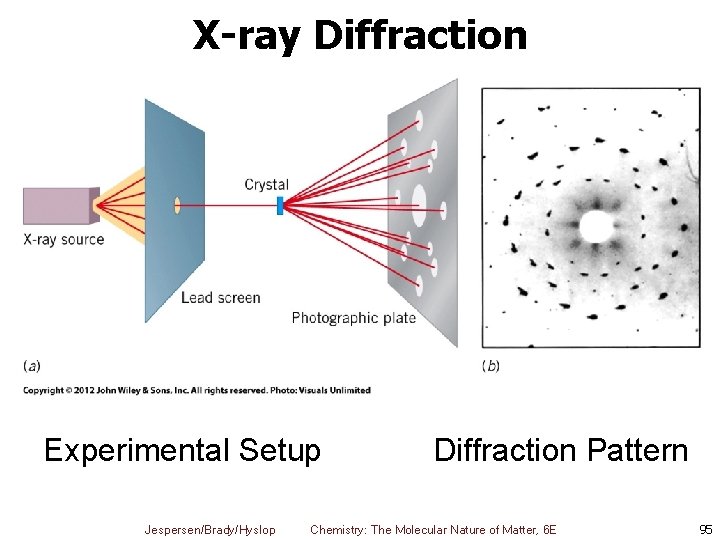
X-ray Diffraction Experimental Setup Jespersen/Brady/Hyslop Diffraction Pattern Chemistry: The Molecular Nature of Matter, 6 E 95

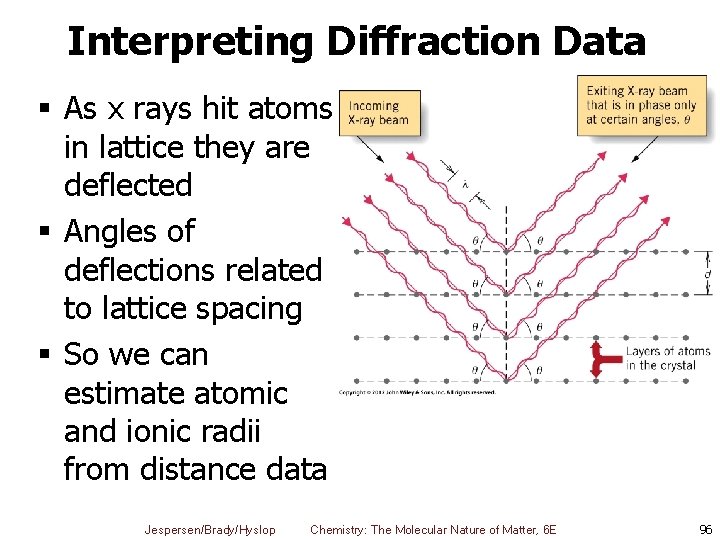
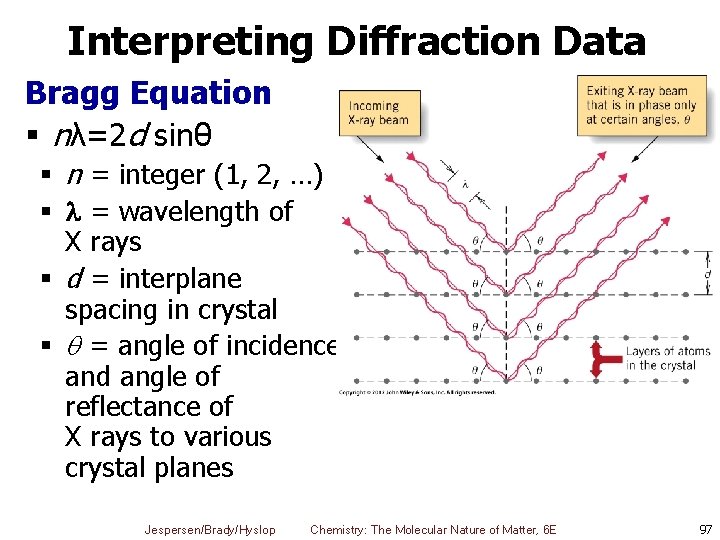
Interpreting Diffraction Data § As x rays hit atoms in lattice they are deflected § Angles of deflections related to lattice spacing § So we can estimate atomic and ionic radii from distance data Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 96

Interpreting Diffraction Data Bragg Equation § nλ=2 d sinθ § n = integer (1, 2, …) § = wavelength of X rays § d = interplane spacing in crystal § = angle of incidence and angle of reflectance of X rays to various crystal planes Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 97

Ionic Crystals (e. g. Na. Cl, Na. NO 3) Have cations and anions at lattice sites Are relatively hard Have high melting points Are brittle Have strong attractive forces between ions Do not conduct electricity in their solid states § Conduct electricity well when molten § § § Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 98

Covalent Crystals § Lattice positions occupied by atoms that are covalently bonded to other atoms at neighboring lattice sites § Also called network solids § Interlocking network of covalent bonds extending all directions § Covalent crystals tend to § Be very hard § Have very high melting points § Have strong attractions between covalently bonded atoms Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 99

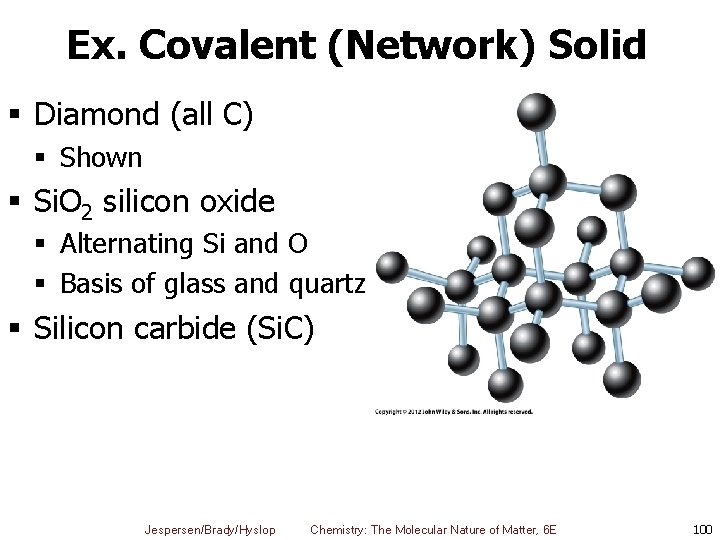
Ex. Covalent (Network) Solid § Diamond (all C) § Shown § Si. O 2 silicon oxide § Alternating Si and O § Basis of glass and quartz § Silicon carbide (Si. C) Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 100

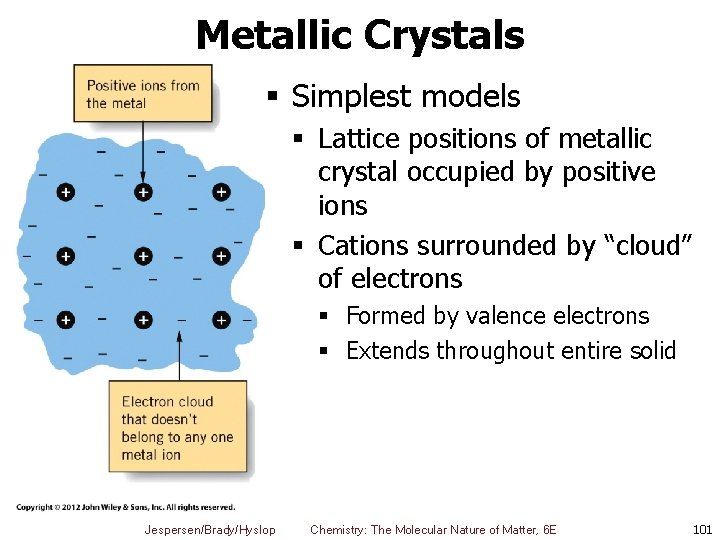
Metallic Crystals § Simplest models § Lattice positions of metallic crystal occupied by positive ions § Cations surrounded by “cloud” of electrons § Formed by valence electrons § Extends throughout entire solid Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 101

Metallic Crystals § Conduct heat and electricity § By their movement, electrons transmit kinetic energy rapidly through solid § Have the luster characteristically associated with metals § When light shines on metal § Loosely held electrons vibrate easily § Re-emit light with essentially same frequency and intensity Jespersen/Brady/Hyslop Chemistry: The Molecular Nature of Matter, 6 E 102