Chapter 12 Forensic Spectroscopy What is it Is




















- Slides: 51

Chapter 12 Forensic Spectroscopy

What is it? • • Is it gasoline in the carpet? Is it Heroin in the syringe? Is it arsenic in the body? Is it polyester in the ski mask?

Spectroscopy • What is the elemental or molecular composition of a substance and how much of it is present? • Based upon measuring how electromagnetic radiation, including light, interacts with matter

Electromagnetic Energy

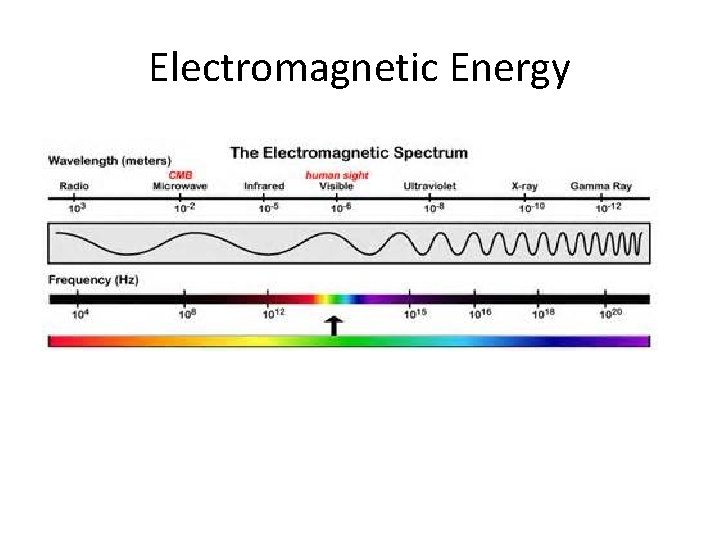
Electromagnetic Radiation Magnetic and Electronic Parts mutually perpendicular

Newton 1666 • Sir Isaac Newton coined term “spectrum” for white light dispersion

Hershel 1800 • Detected heat past the red color zone • Discovered Infrared Radiation • Others made discoveries of other invisible energies at either end of visible spectrum

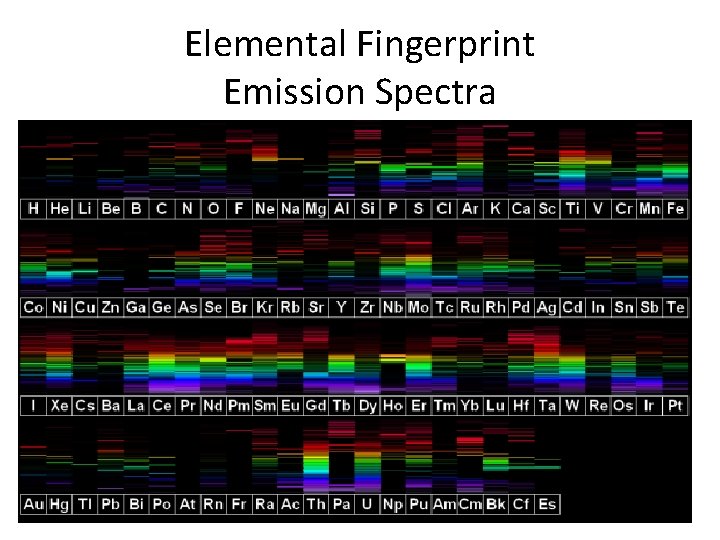
Elements emitting light when heated?

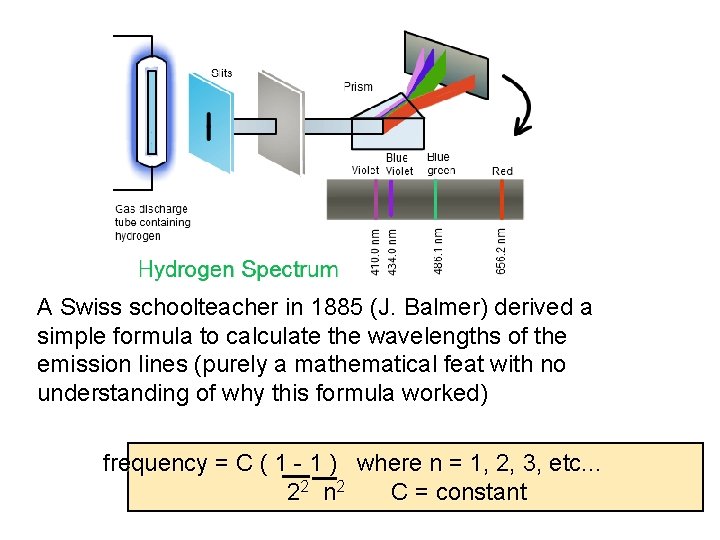
Elemental Fingerprint Emission Spectra

A Swiss schoolteacher in 1885 (J. Balmer) derived a simple formula to calculate the wavelengths of the emission lines (purely a mathematical feat with no understanding of why this formula worked) frequency = C ( 1 - 1 ) where n = 1, 2, 3, etc. . . 22 n 2 C = constant

Birth of Quantum Mechanics 1900 -1930 • Quantized properties-atoms can change energy states • Light travels in particle form? -not waves? • Matter can behave like a wave? -even if made of particles? Quantm Mech. Video

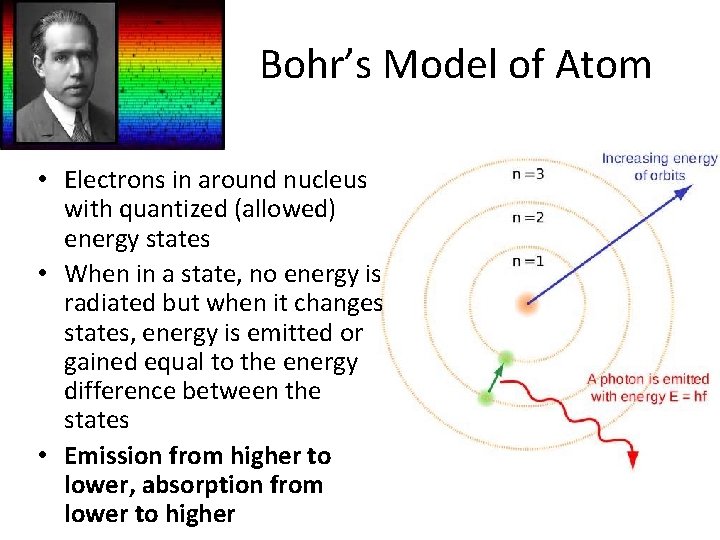
Bohr’s Model of Atom • Electrons in around nucleus with quantized (allowed) energy states • When in a state, no energy is radiated but when it changes states, energy is emitted or gained equal to the energy difference between the states • Emission from higher to lower, absorption from lower to higher

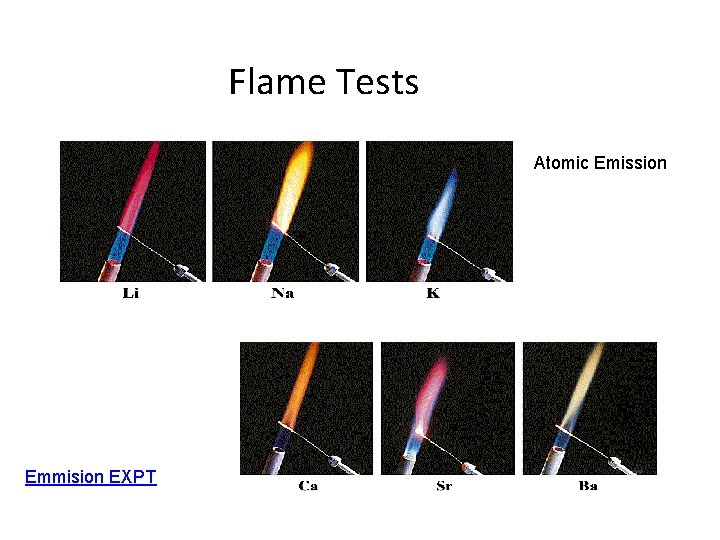
Flame Tests Atomic Emission Emmision EXPT

Atomic Emission Fireworks Science

Atomic Emission Spectroscopy AES • Measures the optical emission from excited atoms to determine analyte concentration. • Analyte atomized by a flame, discharge, or plasma to promote the atoms into high energy levels. • The atoms decay back to lower levels by emitting light.

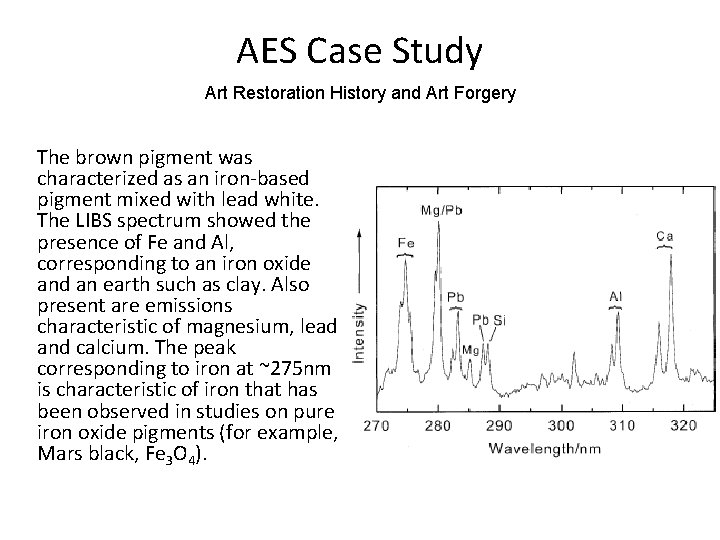
AES Case Study Art Restoration History and Art Forgery • Russian Icon of St. Nicholas - The pigments present on this mid-19 th Century painting were characterized by AES • The identification of pigments on the original work along with those applied in restoration of cracks in the varnish and painting surface were analyzed.

AES Case Study Art Restoration History and Art Forgery The brown pigment was characterized as an iron-based pigment mixed with lead white. The LIBS spectrum showed the presence of Fe and Al, corresponding to an iron oxide and an earth such as clay. Also present are emissions characteristic of magnesium, lead and calcium. The peak corresponding to iron at ~275 nm is characteristic of iron that has been observed in studies on pure iron oxide pigments (for example, Mars black, Fe 3 O 4).

AES Uses • • Trace elements in glass analysis Trace elements in metals analysis Trace elements in soil Trace elements in biological samples for Arsenic and Mercury

AES • Advantages: – Reliable – Rapid – Simple to operate • Disadvantages: – Spectral interferences (many emission lines), – cost and operating expenses

Atomic Absorption Spectroscopy AAS • Uses the absorption of light to measure the concentration of gas-phase atoms. • Since samples are usually liquids or solids, the analyte atoms or ions must be vaporized in a flame or graphite furnace. • The atoms absorb ultraviolet or visible light and make transitions to higher electronic energy levels. The analyte concentration is determined from the amount of absorption (black bands). AAS Machine

AAS Uses • Detect trace elements in hair, nails and fluids • Analyze elements in soil on suspect’s shoe and compare to other soil samples • Detect elemental gun shot residue on suspects • Lead in the paint of toys, cribs etc.

AAS • Advantages: – 60 elements of Periodic Table detectable – More sensitive than AES – Rapid – Simple to operate • Disadvantages: – Quantification requires calibration curves – Need to quantify one element at a time with special light sources – Tech doesn’t work well to measure nonmetals

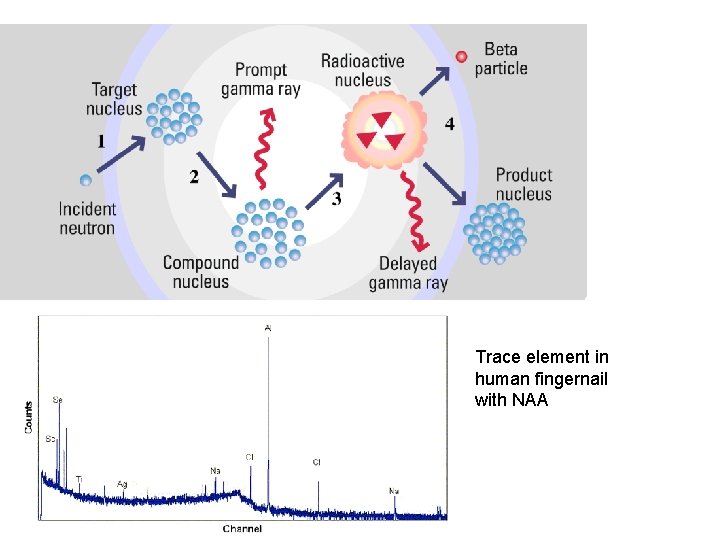
Neutron Activation Analysis 1. 2. 3. 4. Shoot neutrons from a reactor at sample Atoms of sample absorb this extra neutron Atoms emit gamma rays (-a form of EME) Atom becomes radioactive and spits out a beta particle (ie. Electron) 5. Another gamma ray emitted, atom stabilizes 6. From this gamma radiation can tell type and amount of elements

Trace element in human fingernail with NAA

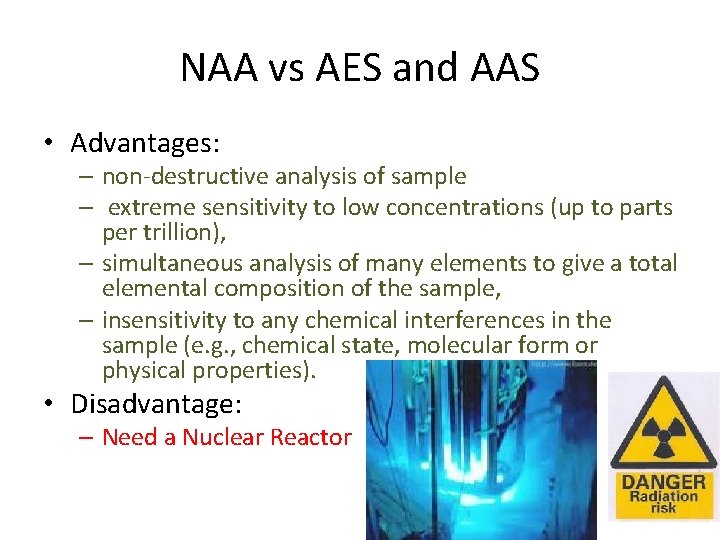
NAA vs AES and AAS • Advantages: – non-destructive analysis of sample – extreme sensitivity to low concentrations (up to parts per trillion), – simultaneous analysis of many elements to give a total elemental composition of the sample, – insensitivity to any chemical interferences in the sample (e. g. , chemical state, molecular form or physical properties). • Disadvantage: – Need a Nuclear Reactor

NAA Uses • When you want a nondestructive mechanism. . • When you have a very small sample. . • When you are looking for elements at lowest levels. .

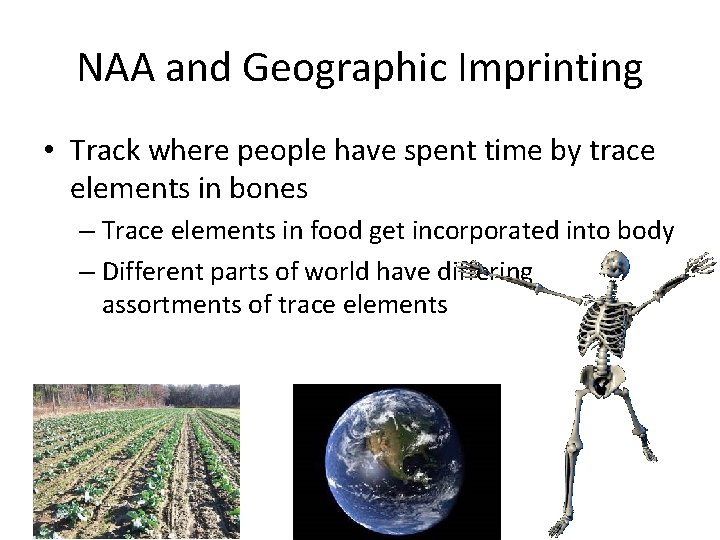
NAA and Geographic Imprinting • Track where people have spent time by trace elements in bones – Trace elements in food get incorporated into body – Different parts of world have differing assortments of trace elements

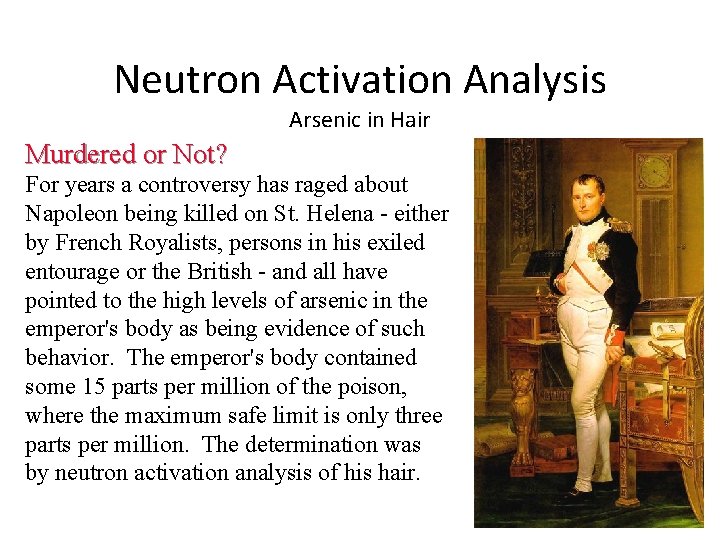
Case Study Neutron Activation Analysis Arsenic in Hair Napoleon Bonaparte One of the most brilliant individuals in history, Napoleon Bonaparte was a masterful soldier, grand tactician, sublime statesman and exceedingly capable administrator. After an extraordinary career, he was finally defeated and exiled to Elba. He returned from Elba to be ultimately defeated at Waterloo. He was finally exiled to the remote tiny volcanic island of St. Helena, south of the Equator. The nearest land is Ascension Island, 700 miles to the north.

Neutron Activation Analysis Arsenic in Hair Murdered or Not? For years a controversy has raged about Napoleon being killed on St. Helena - either by French Royalists, persons in his exiled entourage or the British - and all have pointed to the high levels of arsenic in the emperor's body as being evidence of such behavior. The emperor's body contained some 15 parts per million of the poison, where the maximum safe limit is only three parts per million. The determination was by neutron activation analysis of his hair.

Neutron Activation Analysis Arsenic in Hair “So Who Done It? ” (if it was done at all) British Authorities - The Allied heads of state had no greater wish than to ensure that Napoleon was permanently “out of the way”. Strong hatred by British local commander. Royalists - Revenge and insurance against Napoleon for declaring himself Emperor and dismantling the aristocracy. Exiled Entourage - Jealousy (romantic triangles), intrigue, revenge.

Neutron Activation Analysis Arsenic in Hair The wallpaper in his room was dyed with Scheele's Green (Paris Green), a coloring pigment that had been used in fabrics and wallpapers from around 1770. Named after the Swedish chemist who invented it, the dye contained copper arsenite. It was discovered that if wallpaper containing Scheele’s Green became damp, the mold converted the copper arsenite to a poisonous vapor form of arsenic. Breathing the arsenic on its own might not have been enough to kill Napoleon, but he was ill already with a stomach ulcer/cancer. On the 5 May 1821, the arsenic tipped the scale against "the little corporal. "

Atomic and Molecular Spectroscopy • Combustion Analysis • Mass Spectrometry (MS) • Atomic Spectroscopy – Atomic Absorption (AAS) and Emission Analysis (AES) – Neutron Activation Analysis (NAA) • Molecular Spectroscopy – Electronic Spectroscopy – Vibrational Spectroscopy – Nuclear Magnetic Resonance (NMR or MRI) • X-ray Methods – X-ray Diffraction (XRD and CAT) – Energy Dispersive X-ray Fluorescence (EDXRF)

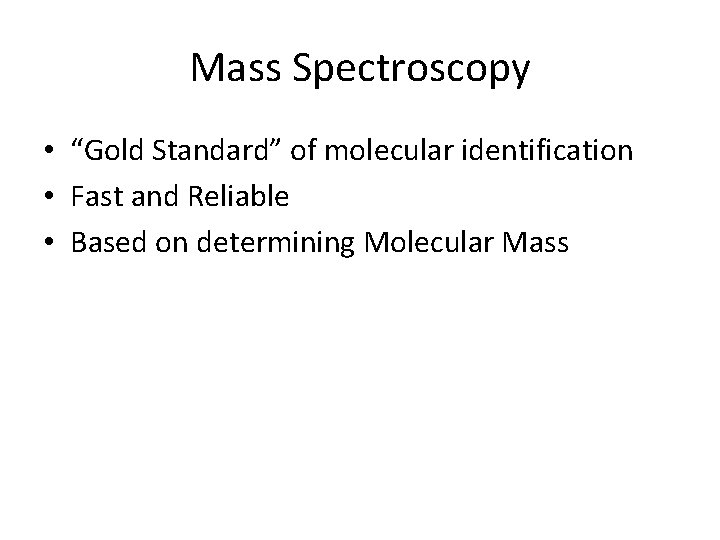
Mass Spectroscopy • “Gold Standard” of molecular identification • Fast and Reliable • Based on determining Molecular Mass

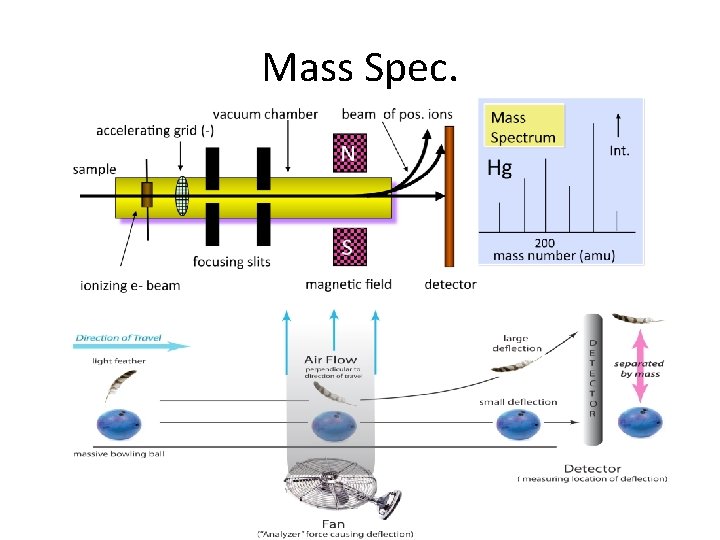
Mass Spectroscopy Steps 1. 2. 3. 4. 5. Put sample in gas form in vacuum Shoot electrons at target molecule Knock a negative electron out of molecule Molecule now an positive ion Propel molecular ions down a negatively charged tube 6. Use magnets to bend the ions’ path 7. Degree of path distortion related to mass 8. Detector records the placements of hits

Mass Spec.

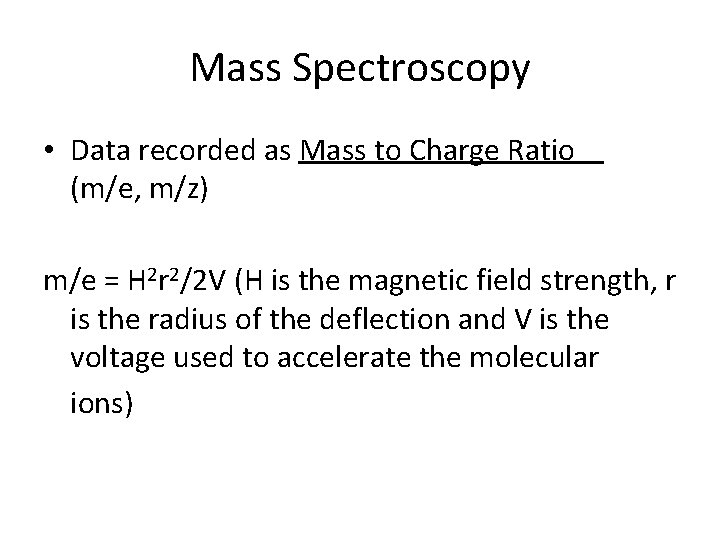
Mass Spectroscopy • Data recorded as Mass to Charge Ratio (m/e, m/z) m/e = H 2 r 2/2 V (H is the magnetic field strength, r is the radius of the deflection and V is the voltage used to accelerate the molecular ions)
Mass Spec. • So far straight forward?

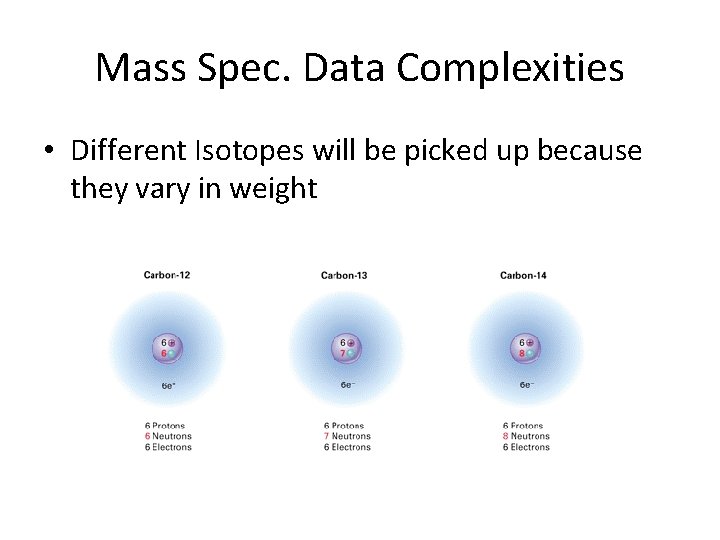
Mass Spec. Data Complexities • Different Isotopes will be picked up because they vary in weight

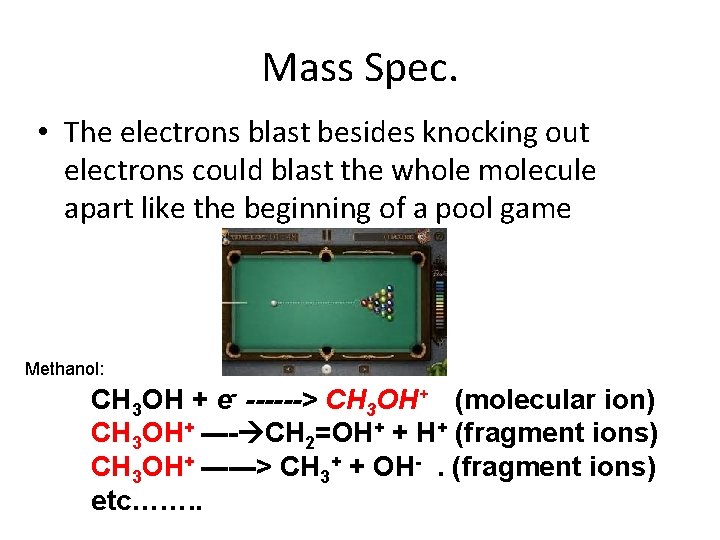
Mass Spec. • The electrons blast besides knocking out electrons could blast the whole molecule apart like the beginning of a pool game Methanol: CH 3 OH + e- ------> CH 3 OH+ (molecular ion) CH 3 OH+ ---- CH 2=OH+ + H+ (fragment ions) CH 3 OH+ ------> CH 3+ + OH-. (fragment ions) etc……. .

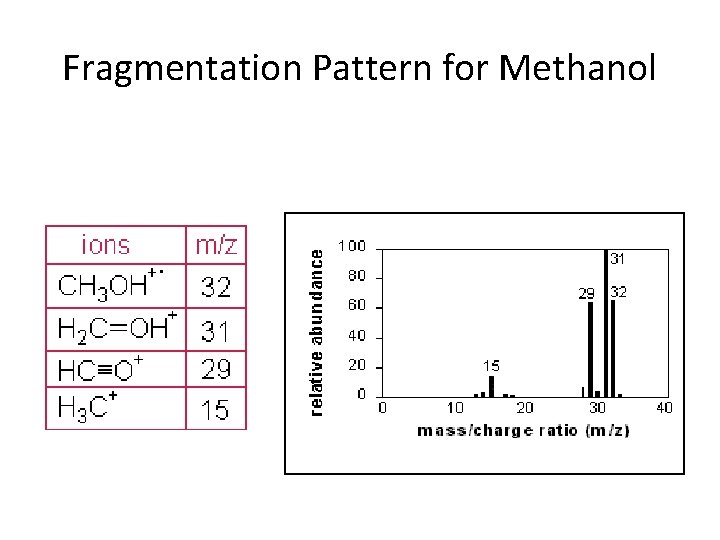
Fragmentation Pattern for Methanol

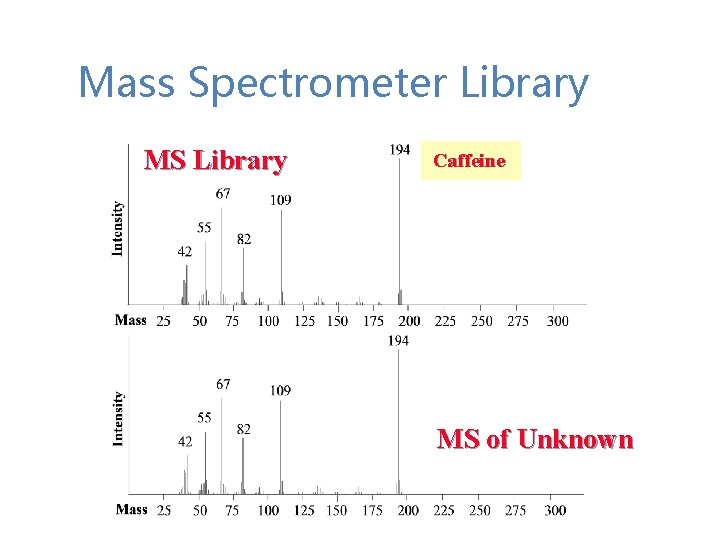
Mass Spectrometer Library MS Library Caffeine MS of Unknown

https: //www. youtube. com/watch? v=Jwao 0 O 0_q. M

Molecular Spectroscopy • Electronic Spectroscopy (or UV visible) • Vibrational Spectroscopy – Infrared – Raman • Nuclear Magnetic Resonance Spectroscopy (NMR or MRI)

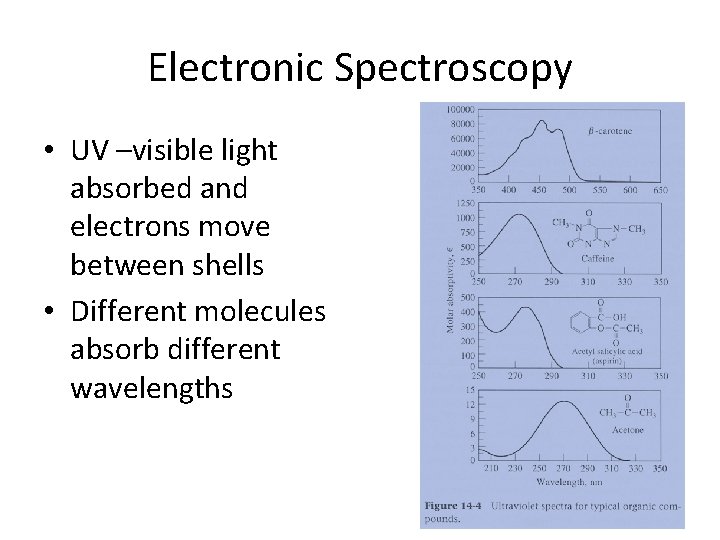
Electronic Spectroscopy • UV –visible light absorbed and electrons move between shells • Different molecules absorb different wavelengths

Electronic Spectroscopy Uses in forensics • Important in quantifying the color of an object, such as: – comparing paint chips, dyes in fabrics, tint glass and any other colored evidence – Clinical Chemistry

• https: //www. youtube. com/watch? v=x. HQM 4 B b. R 040&feature=results_video&playnext=1&lis t=PL 967 C 03 F 996296203

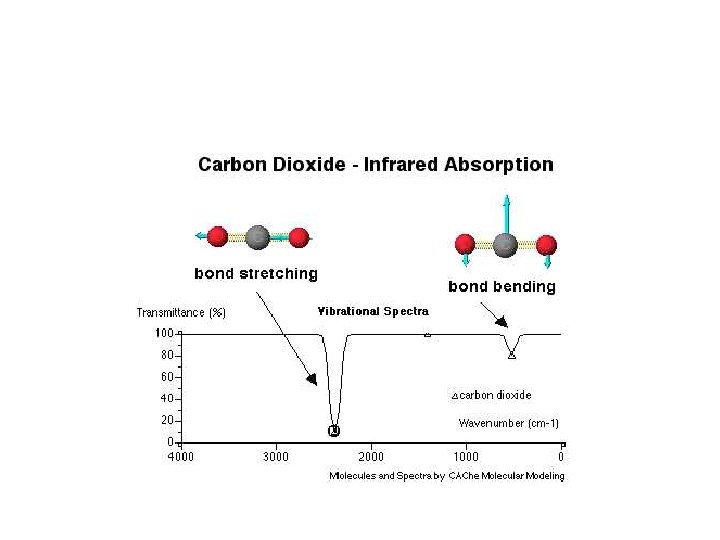
Infrared Spectroscopy • Energy absorbed by molecule to vibrate its bonds that hold it together • No electrons moving between shells • The more energy to vibrate the bond, the stronger the bond


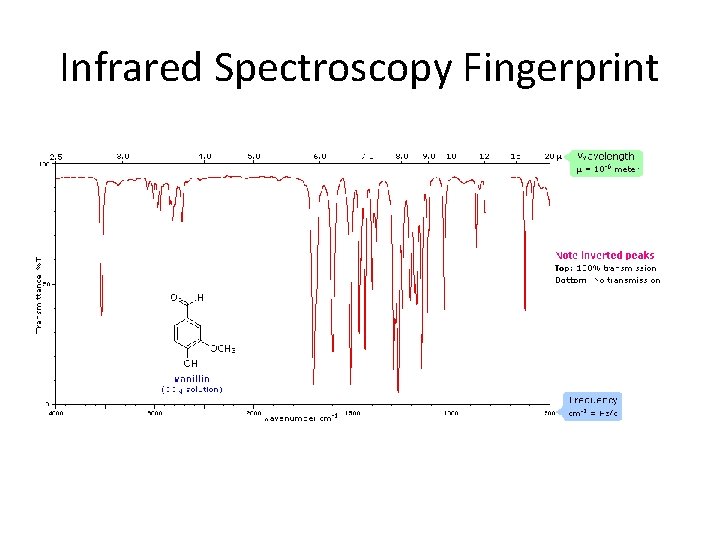
Infrared Spectroscopy Fingerprint

Infrared Spectroscopy Uses • • • Breathalyzers ID of unknown drugs, poisons, pollutants Fiber Analysis Paint Analysis Ink Analysis

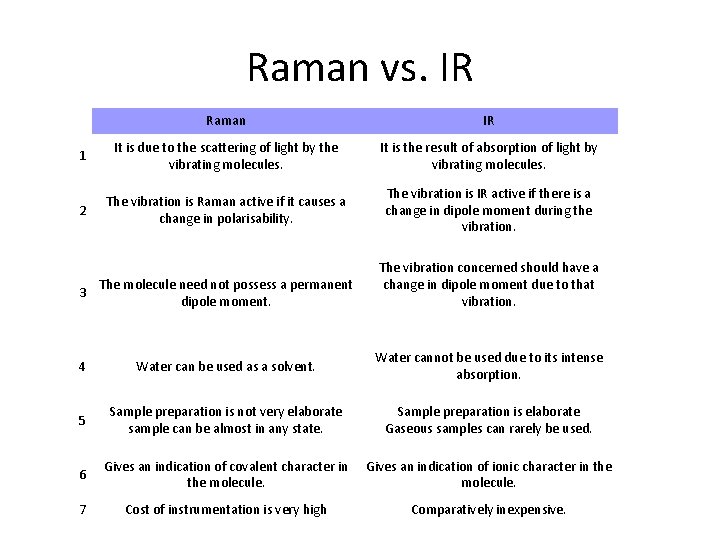
Raman vs. IR Raman IR 1 It is due to the scattering of light by the vibrating molecules. It is the result of absorption of light by vibrating molecules. 2 The vibration is Raman active if it causes a change in polarisability. The vibration is IR active if there is a change in dipole moment during the vibration. The vibration concerned should have a change in dipole moment due to that vibration. 3 The molecule need not possess a permanent dipole moment. 4 Water can be used as a solvent. Water cannot be used due to its intense absorption. 5 Sample preparation is not very elaborate sample can be almost in any state. Sample preparation is elaborate Gaseous samples can rarely be used. 6 Gives an indication of covalent character in the molecule. Gives an indication of ionic character in the molecule. 7 Cost of instrumentation is very high Comparatively inexpensive.