Chapter 12 EDTA Titrations Overview 12 1 MetalChelate


![12 -4: Do It with a Spreadsheet Substituting Kf[M][L] from Equation 12 -8 for 12 -4: Do It with a Spreadsheet Substituting Kf[M][L] from Equation 12 -8 for](https://slidetodoc.com/presentation_image/00cd6ac0ccd7853dd0a3f99557b89ec1/image-26.jpg)
![12 -5: Metal-Ligand Equilibria • The mass balance is: Mtot = [M] + [ML 12 -5: Metal-Ligand Equilibria • The mass balance is: Mtot = [M] + [ML](https://slidetodoc.com/presentation_image/00cd6ac0ccd7853dd0a3f99557b89ec1/image-30.jpg)

- Slides: 42

Chapter 12 EDTA Titrations

Overview 12 -1 Metal-Chelate Complexes 12 -2 EDTA 12 -3 EDTA Titration Curves 12 -4 Do It with a Spreadsheet 12 -5 Auxiliary Complexing Agents 12 -6 Metal Ion Indicators 12 -7 EDTA Titration Techniques

12 -2: EDTA • Ethylenediaminetetraacetic acid (EDTA) is a compound that forms strong 1: 1 complexes with most metal ions. • Used in industrial processes and products such as detergents, cleaning agents, and food additives that prevent metal-catalyzed oxidation of food. • Metal-EDTA complexes find their way into the environment because they can pass through wastewater treatment plants unscathed.

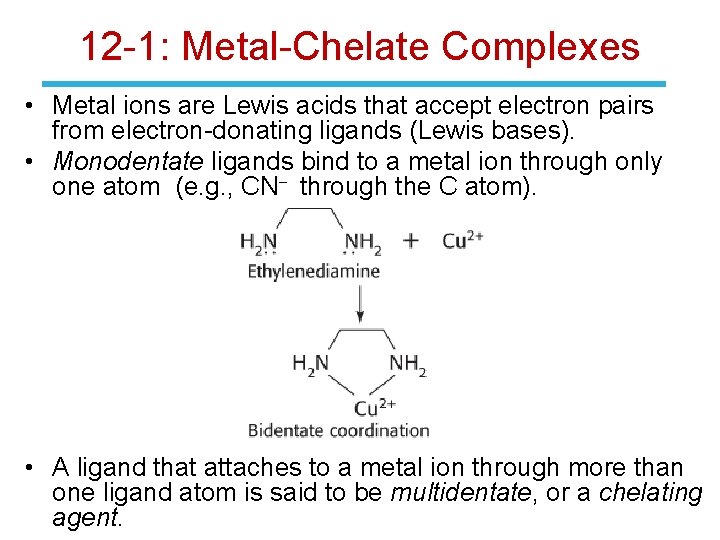
12 -1: Metal-Chelate Complexes • Metal ions are Lewis acids that accept electron pairs from electron-donating ligands (Lewis bases). • Monodentate ligands bind to a metal ion through only one atom (e. g. , CN- through the C atom). • A ligand that attaches to a metal ion through more than one ligand atom is said to be multidentate, or a chelating agent.

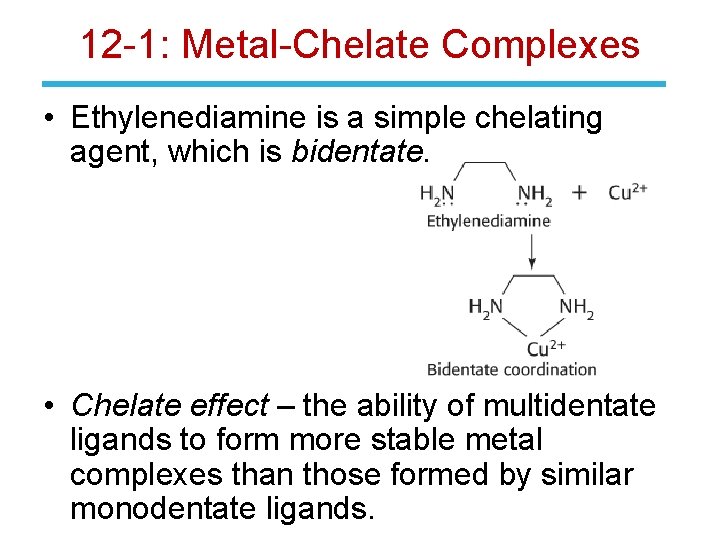
12 -1: Metal-Chelate Complexes • Ethylenediamine is a simple chelating agent, which is bidentate. • Chelate effect – the ability of multidentate ligands to form more stable metal complexes than those formed by similar monodentate ligands.

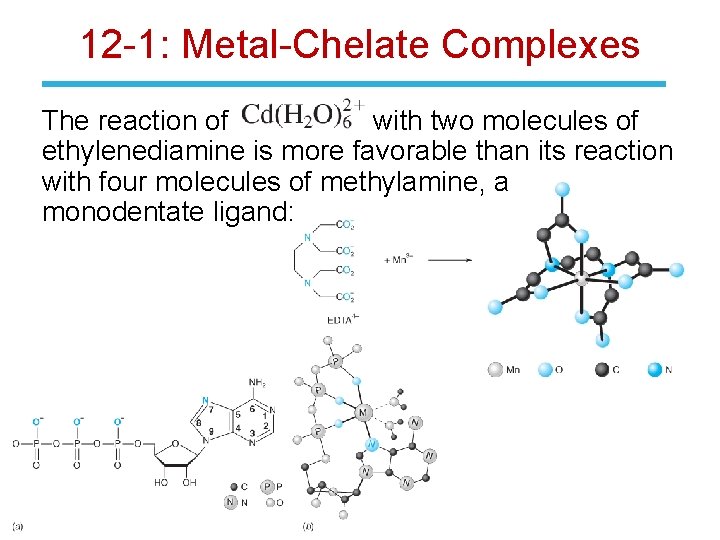
12 -1: Metal-Chelate Complexes The reaction of with two molecules of ethylenediamine is more favorable than its reaction with four molecules of methylamine, a monodentate ligand:

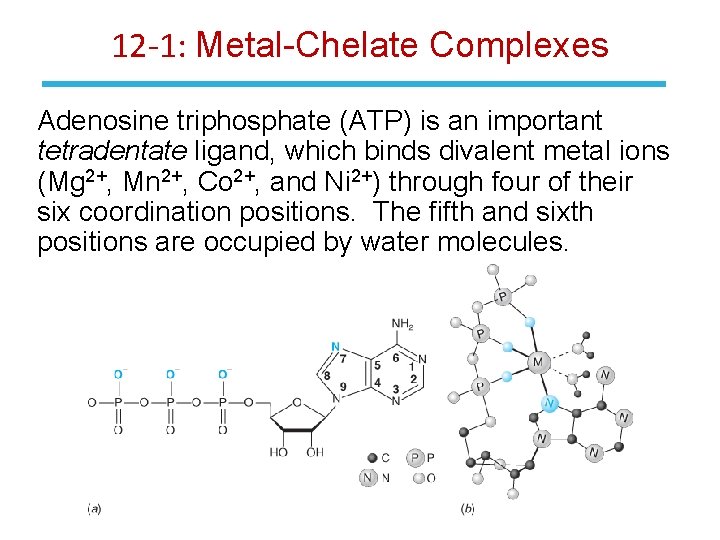
12 -1: Metal-Chelate Complexes Adenosine triphosphate (ATP) is an important tetradentate ligand, which binds divalent metal ions (Mg 2+, Mn 2+, Co 2+, and Ni 2+) through four of their six coordination positions. The fifth and sixth positions are occupied by water molecules.

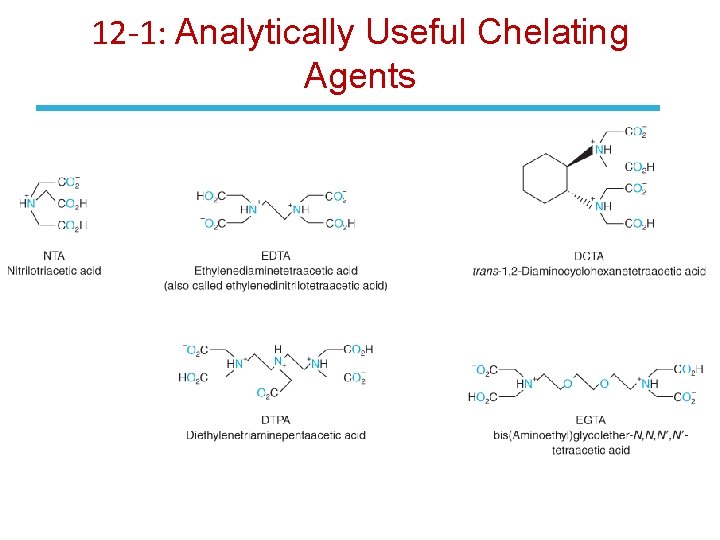
12 -1: Analytically Useful Chelating Agents

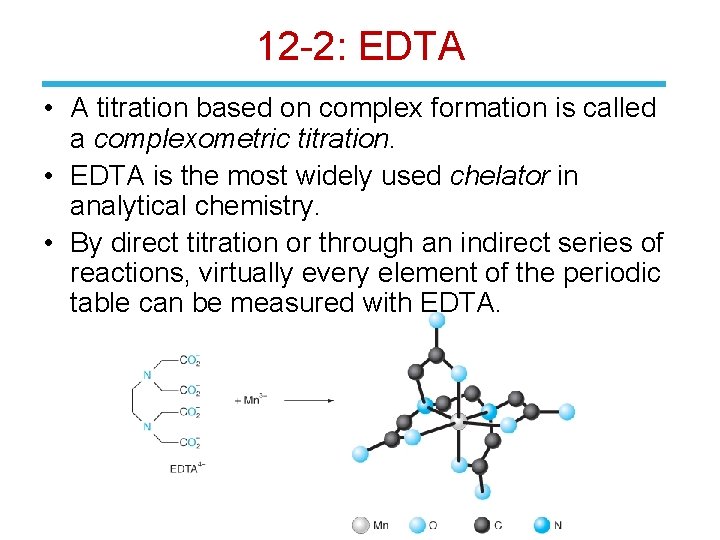
12 -2: EDTA • A titration based on complex formation is called a complexometric titration. • EDTA is the most widely used chelator in analytical chemistry. • By direct titration or through an indirect series of reactions, virtually every element of the periodic table can be measured with EDTA.

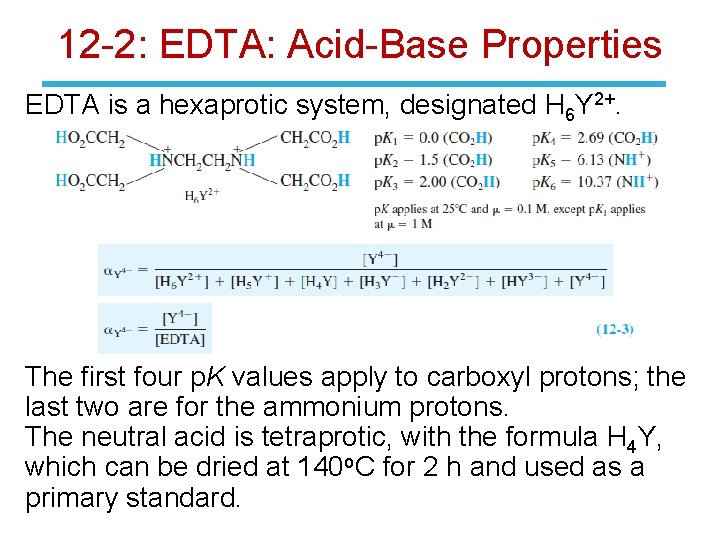
12 -2: EDTA: Acid-Base Properties EDTA is a hexaprotic system, designated H 6 Y 2+. The first four p. K values apply to carboxyl protons; the last two are for the ammonium protons. The neutral acid is tetraprotic, with the formula H 4 Y, which can be dried at 140 o. C for 2 h and used as a primary standard.

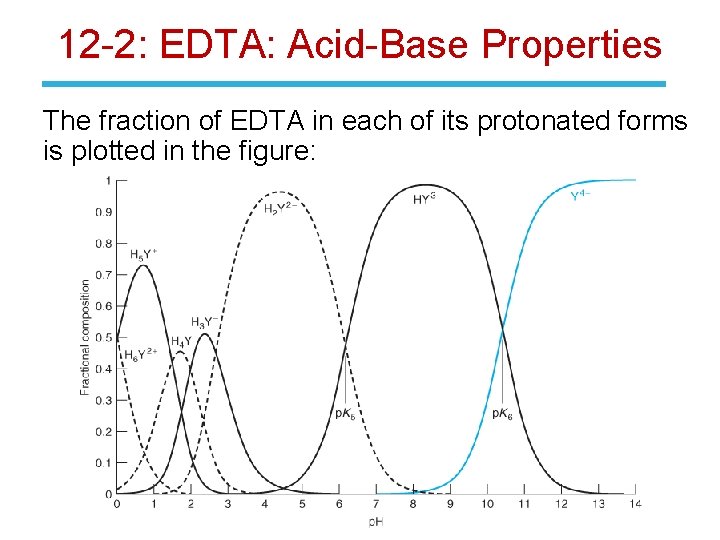
12 -2: EDTA: Acid-Base Properties The fraction of EDTA in each of its protonated forms is plotted in the figure:

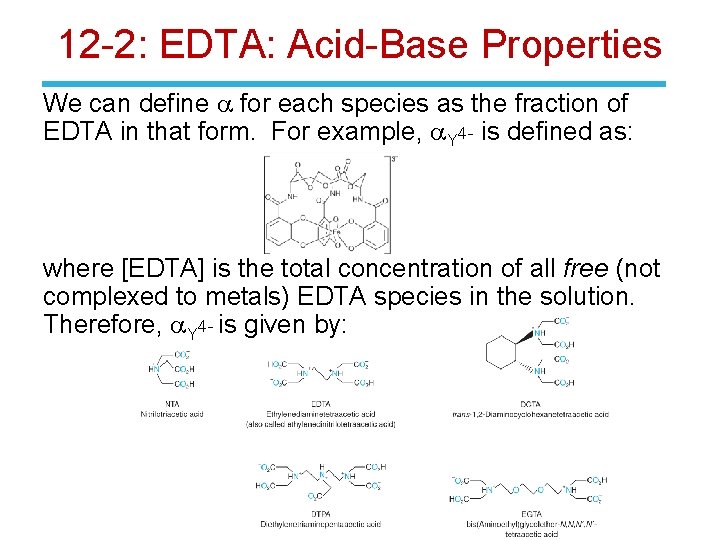
12 -2: EDTA: Acid-Base Properties We can define a for each species as the fraction of EDTA in that form. For example, a. Y 4 - is defined as: where [EDTA] is the total concentration of all free (not complexed to metals) EDTA species in the solution. Therefore, a. Y 4 - is given by:

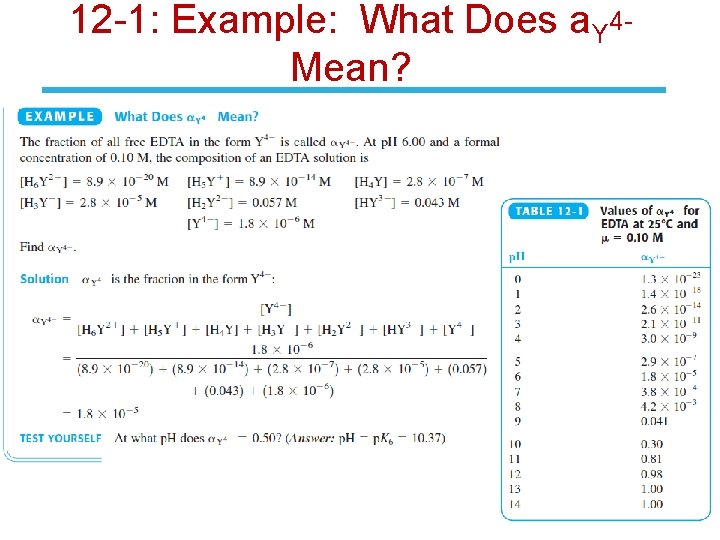
12 -1: Example: What Does a. Y 4 Mean?

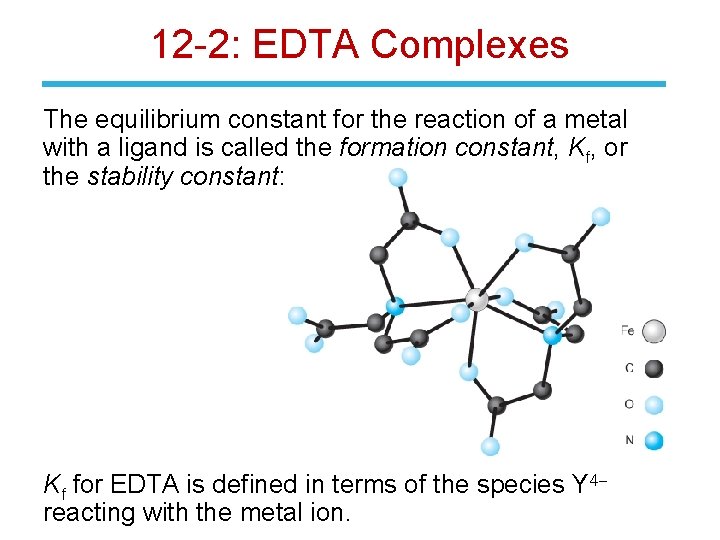
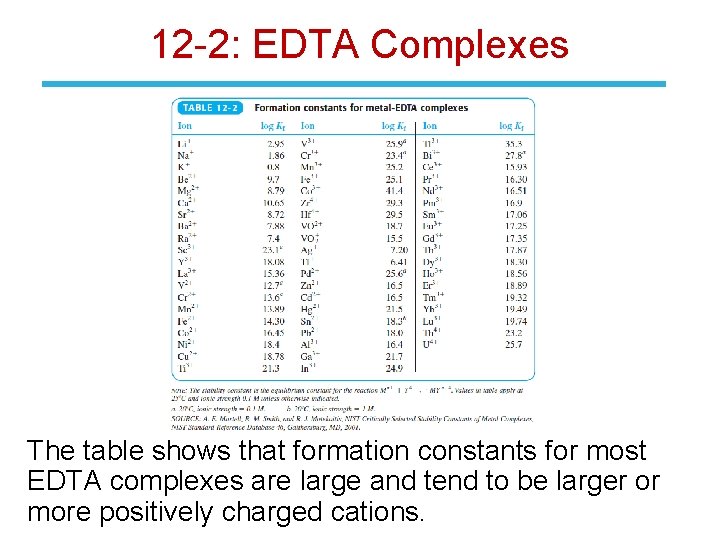
12 -2: EDTA Complexes The equilibrium constant for the reaction of a metal with a ligand is called the formation constant, Kf, or the stability constant: Kf for EDTA is defined in terms of the species Y 4 reacting with the metal ion.

12 -2: EDTA Complexes The table shows that formation constants for most EDTA complexes are large and tend to be larger or more positively charged cations.

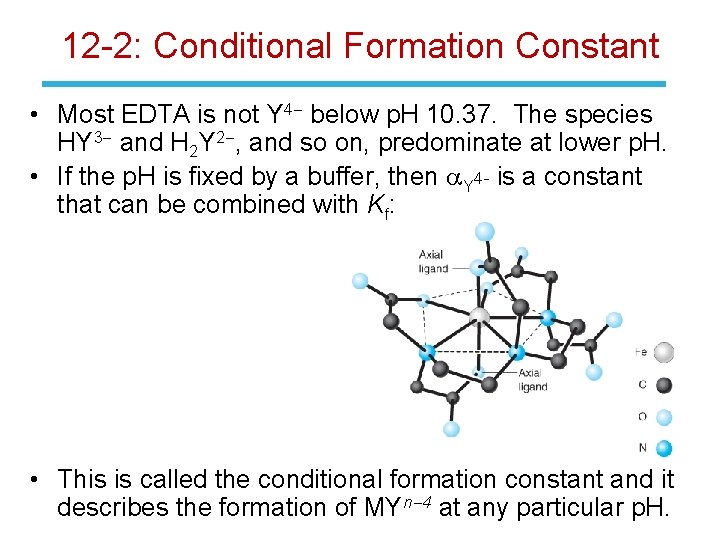
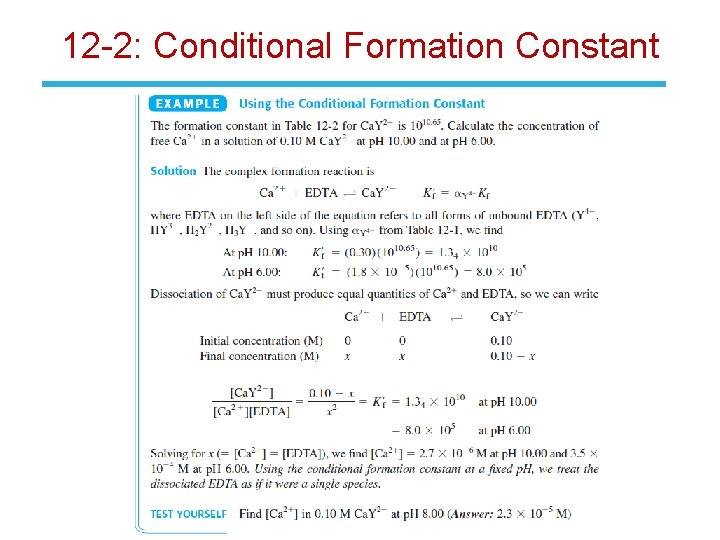
12 -2: Conditional Formation Constant • Most EDTA is not Y 4 - below p. H 10. 37. The species HY 3 - and H 2 Y 2 -, and so on, predominate at lower p. H. • If the p. H is fixed by a buffer, then a. Y 4 - is a constant that can be combined with Kf: • This is called the conditional formation constant and it describes the formation of MYn-4 at any particular p. H.

12 -2: Conditional Formation Constant

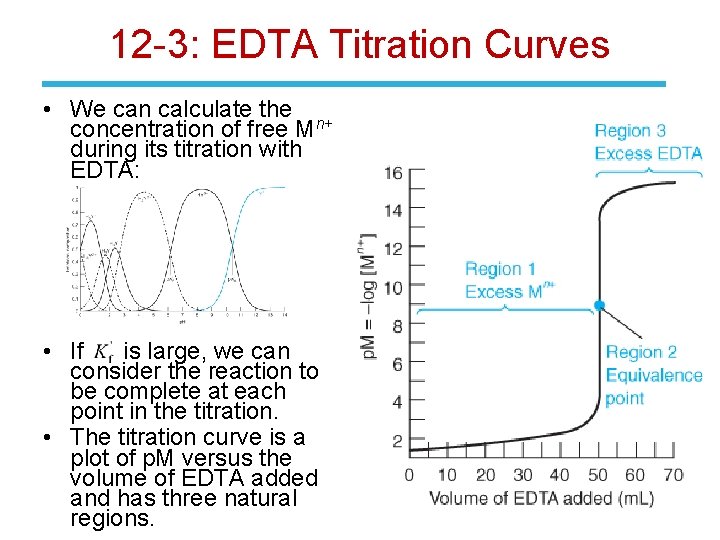
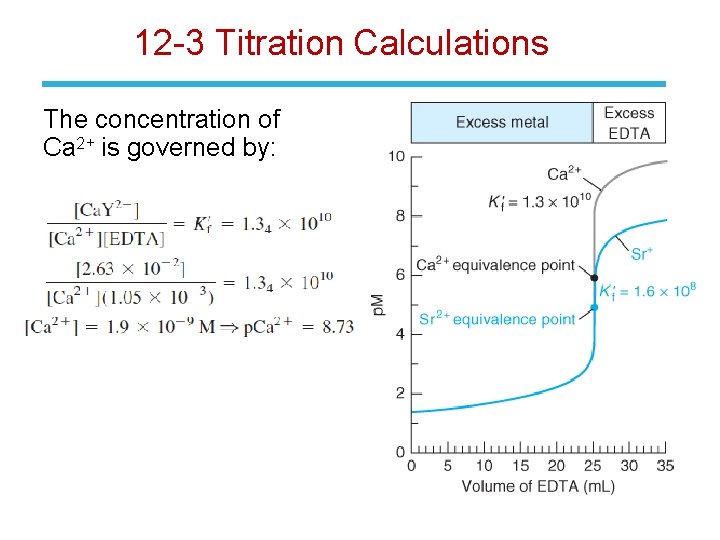
12 -3: EDTA Titration Curves • We can calculate the n+ concentration of free M during its titration with EDTA: • If is large, we can consider the reaction to be complete at each point in the titration. • The titration curve is a plot of p. M versus the volume of EDTA added and has three natural regions.

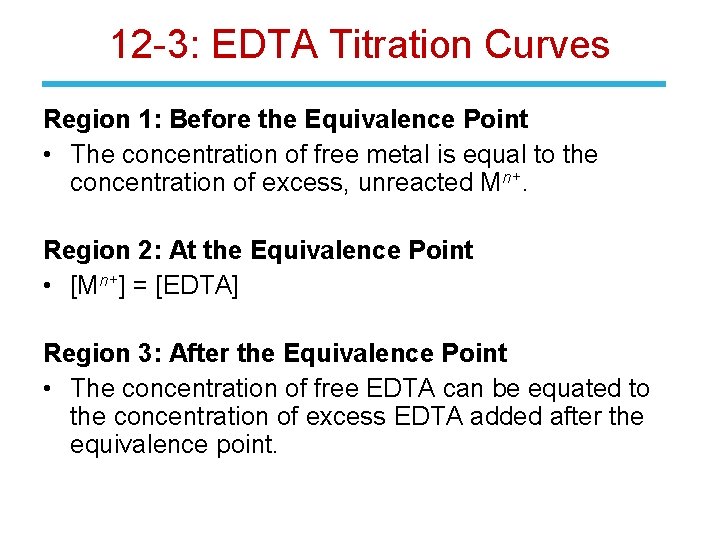
12 -3: EDTA Titration Curves Region 1: Before the Equivalence Point • The concentration of free metal is equal to the concentration of excess, unreacted Mn+. Region 2: At the Equivalence Point • [Mn+] = [EDTA] Region 3: After the Equivalence Point • The concentration of free EDTA can be equated to the concentration of excess EDTA added after the equivalence point.

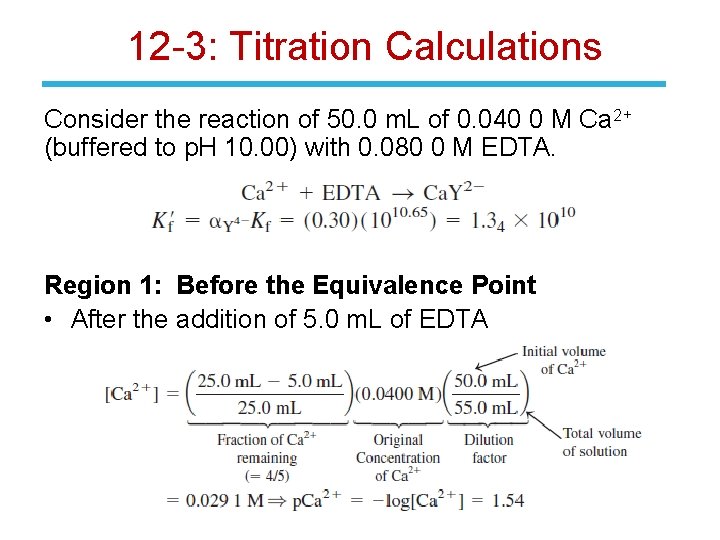
12 -3: Titration Calculations Consider the reaction of 50. 0 m. L of 0. 040 0 M Ca 2+ (buffered to p. H 10. 00) with 0. 080 0 M EDTA. Region 1: Before the Equivalence Point • After the addition of 5. 0 m. L of EDTA

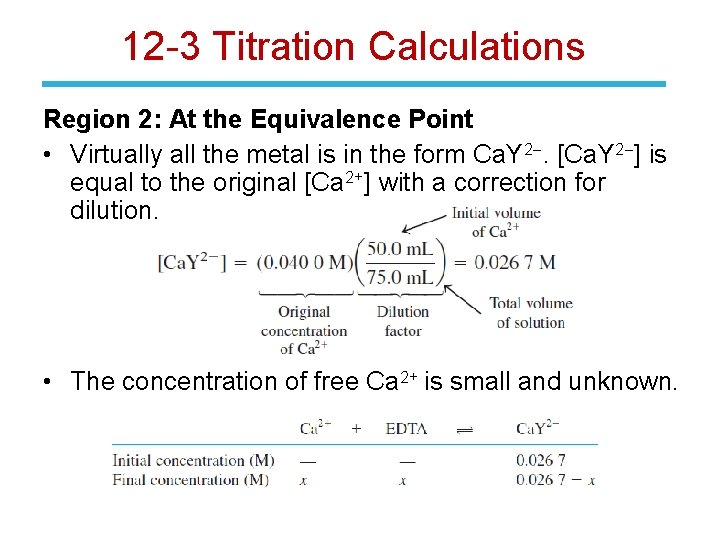
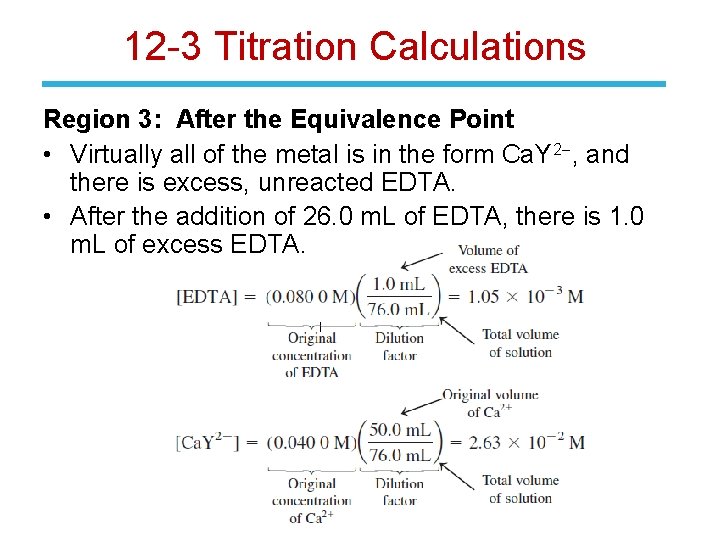
12 -3 Titration Calculations Region 2: At the Equivalence Point • Virtually all the metal is in the form Ca. Y 2 -. [Ca. Y 2 -] is equal to the original [Ca 2+] with a correction for dilution. • The concentration of free Ca 2+ is small and unknown.

12 -3 Titration Calculations

12 -3 Titration Calculations Region 3: After the Equivalence Point • Virtually all of the metal is in the form Ca. Y 2 -, and there is excess, unreacted EDTA. • After the addition of 26. 0 m. L of EDTA, there is 1. 0 m. L of excess EDTA.

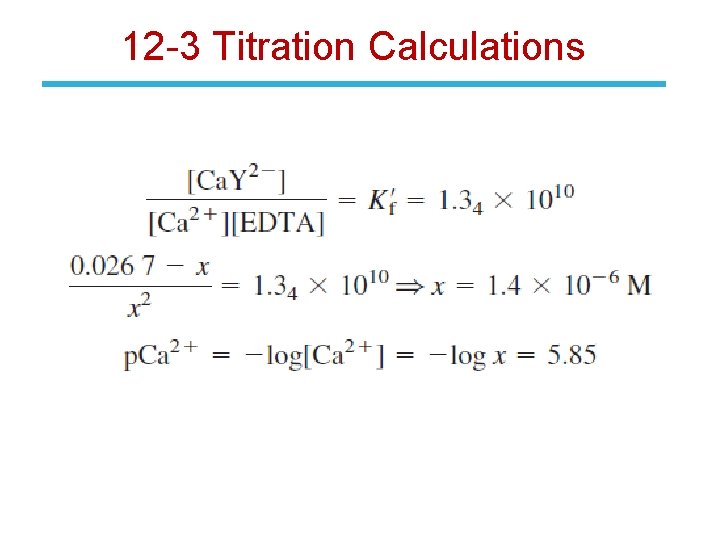
12 -3 Titration Calculations The concentration of Ca 2+ is governed by:

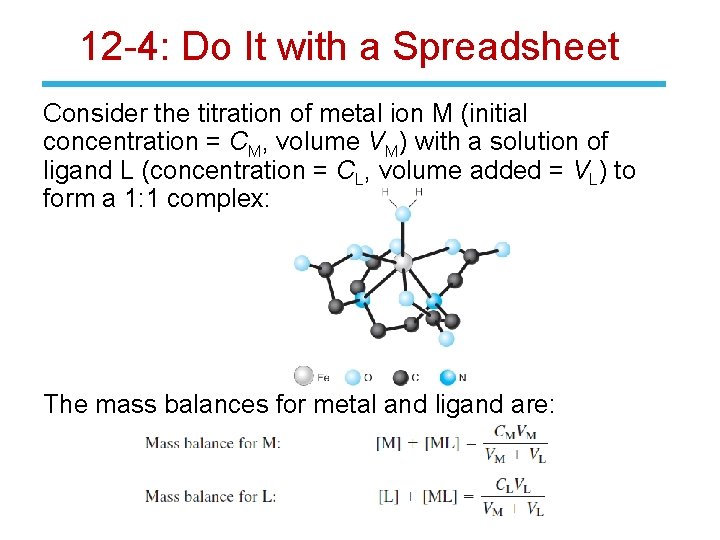
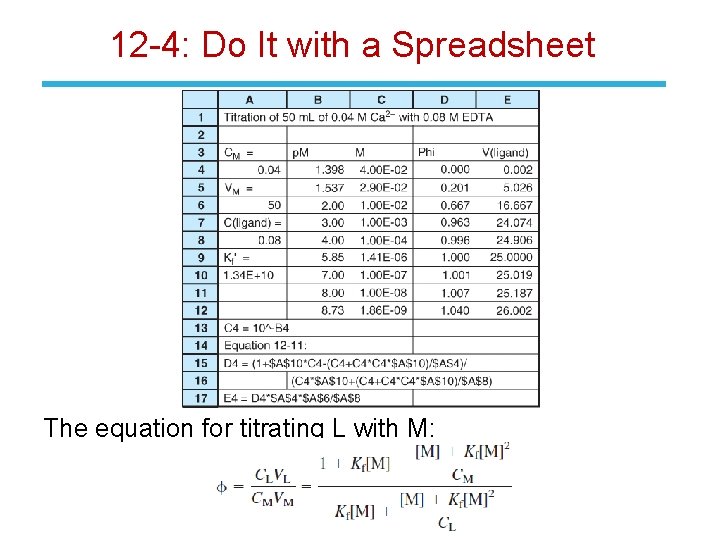
12 -4: Do It with a Spreadsheet Consider the titration of metal ion M (initial concentration = CM, volume VM) with a solution of ligand L (concentration = CL, volume added = VL) to form a 1: 1 complex: The mass balances for metal and ligand are:
![12 4 Do It with a Spreadsheet Substituting KfML from Equation 12 8 for 12 -4: Do It with a Spreadsheet Substituting Kf[M][L] from Equation 12 -8 for](https://slidetodoc.com/presentation_image/00cd6ac0ccd7853dd0a3f99557b89ec1/image-26.jpg)
12 -4: Do It with a Spreadsheet Substituting Kf[M][L] from Equation 12 -8 for [ML] in the mass balance: Now substitute the expression for [L] from Equation 12 -10 back into Equation 12 -9, then solve for the fraction of titration, .

12 -4: Do It with a Spreadsheet The equation for titrating L with M:

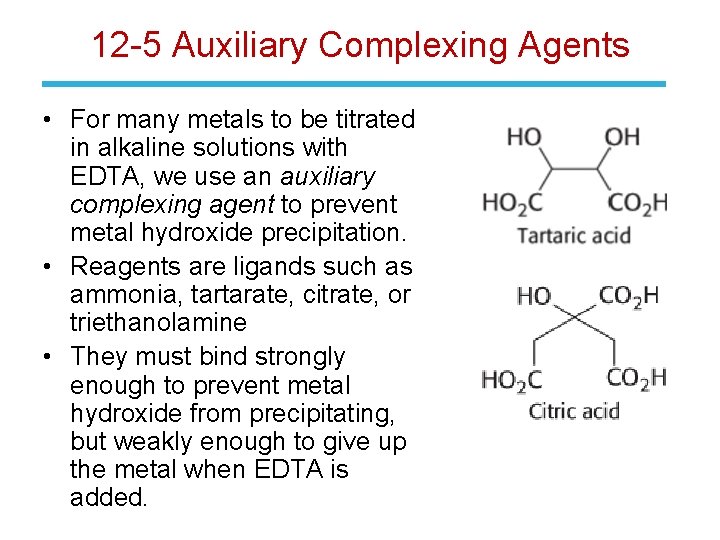
12 -5 Auxiliary Complexing Agents • For many metals to be titrated in alkaline solutions with EDTA, we use an auxiliary complexing agent to prevent metal hydroxide precipitation. • Reagents are ligands such as ammonia, tartarate, citrate, or triethanolamine • They must bind strongly enough to prevent metal hydroxide from precipitating, but weakly enough to give up the metal when EDTA is added.

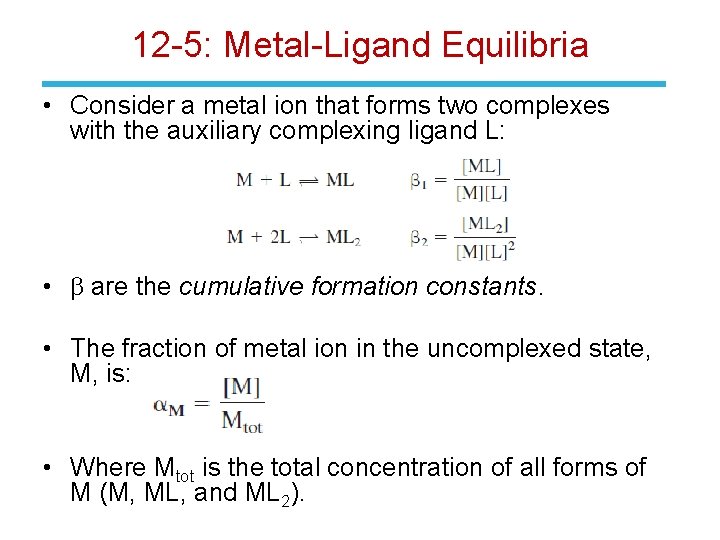
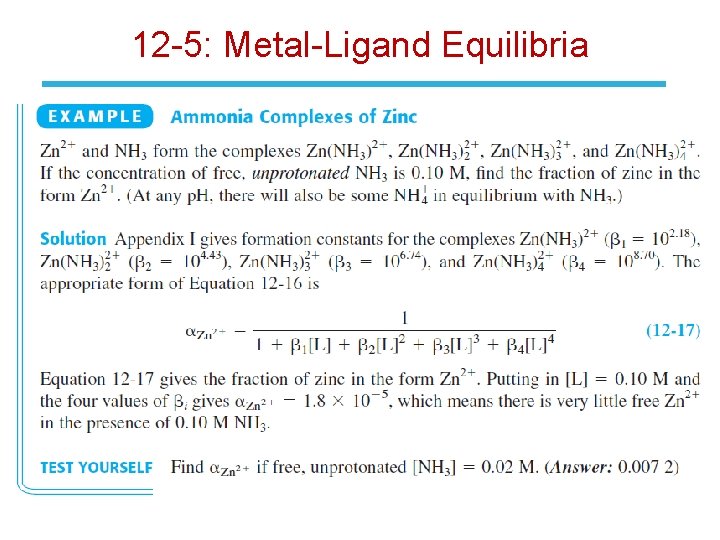
12 -5: Metal-Ligand Equilibria • Consider a metal ion that forms two complexes with the auxiliary complexing ligand L: • b are the cumulative formation constants. • The fraction of metal ion in the uncomplexed state, M, is: • Where Mtot is the total concentration of all forms of M (M, ML, and ML 2).
![12 5 MetalLigand Equilibria The mass balance is Mtot M ML 12 -5: Metal-Ligand Equilibria • The mass balance is: Mtot = [M] + [ML](https://slidetodoc.com/presentation_image/00cd6ac0ccd7853dd0a3f99557b89ec1/image-30.jpg)
12 -5: Metal-Ligand Equilibria • The mass balance is: Mtot = [M] + [ML 2] • Equations 12 -13 and 12 -14 allow us to say [ML] = b 1[M][L] and [ML 2] = b 2[M][L]2 • Therefore, Mtot = [M] + b 1[M][L] + b 2[M][L]2 = [M]{1 + b 1[L] + b 2[L]2} • Substituting into Equation 12 -15:

12 -5: Metal-Ligand Equilibria

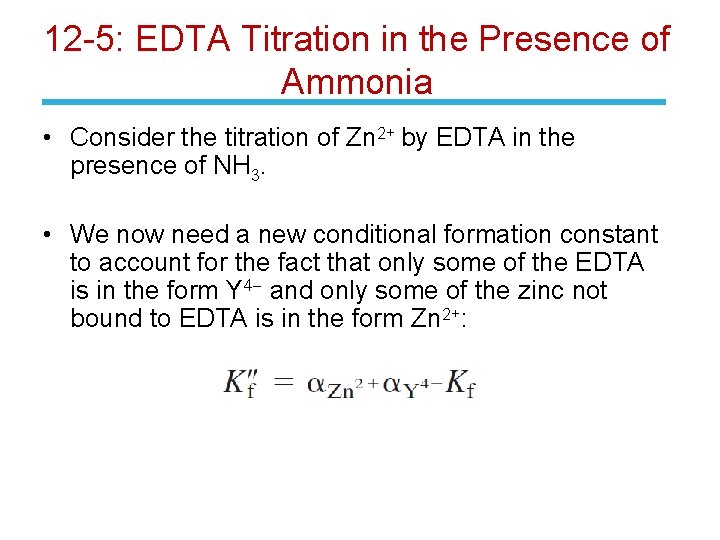
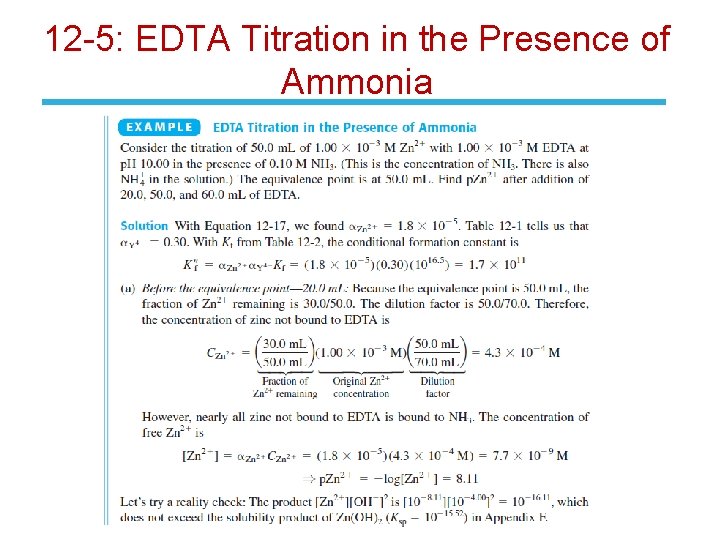
12 -5: EDTA Titration in the Presence of Ammonia • Consider the titration of Zn 2+ by EDTA in the presence of NH 3. • We now need a new conditional formation constant to account for the fact that only some of the EDTA is in the form Y 4 - and only some of the zinc not bound to EDTA is in the form Zn 2+:

12 -5: EDTA Titration in the Presence of Ammonia

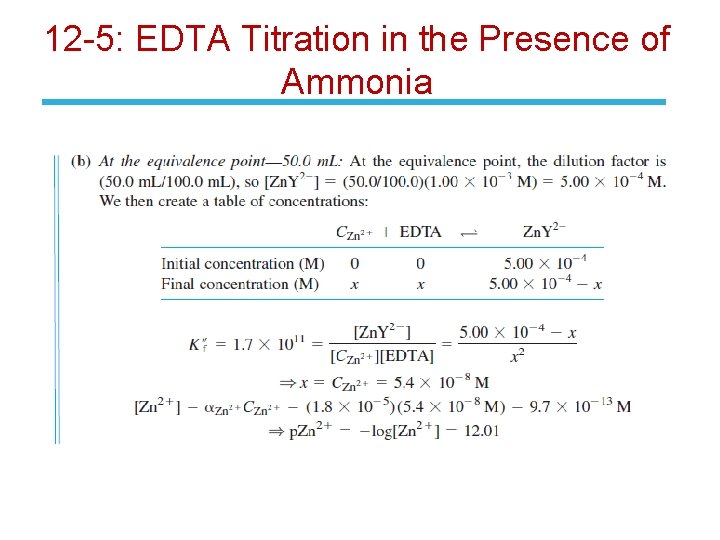
12 -5: EDTA Titration in the Presence of Ammonia

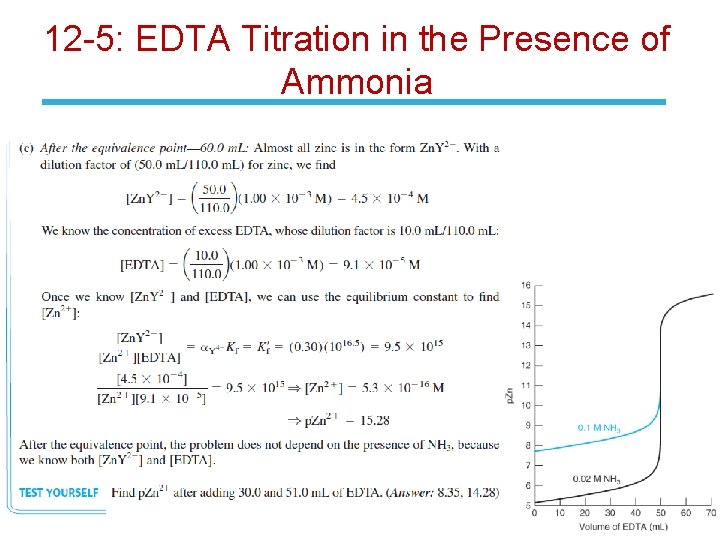
12 -5: EDTA Titration in the Presence of Ammonia

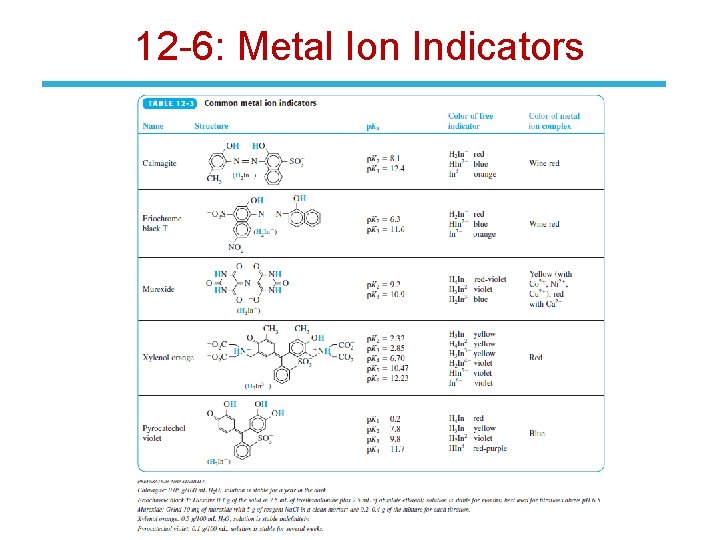
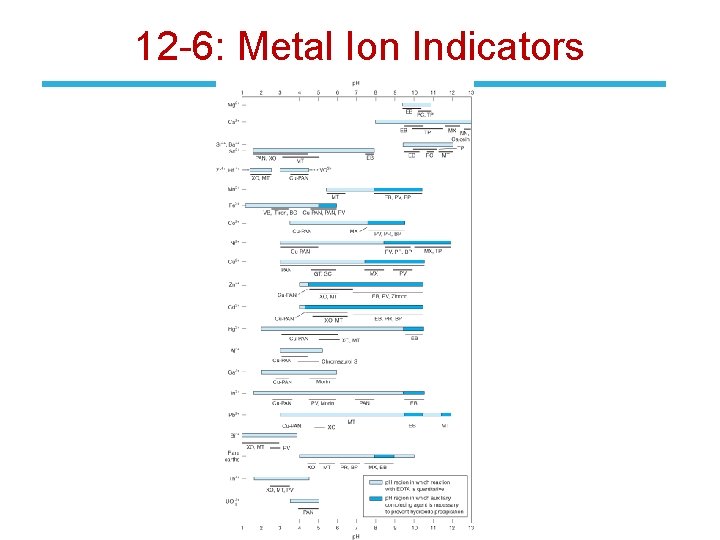
12 -6: Metal Ion Indicators End-point detection methods: 1. Metal ion indicators (most common) 2. Mercury electrode 3. Ion-selective electrode 4. Glass (p. H) electrode • Metal ion indicators are compounds that change color when they bind to a metal ion – must bind metal less strongly than does EDTA. • Example: the reaction of Mg 2+ with EDTA at p. H 10 with Calmagite indicator.

12 -6: Metal Ion Indicators

12 -6: Metal Ion Indicators

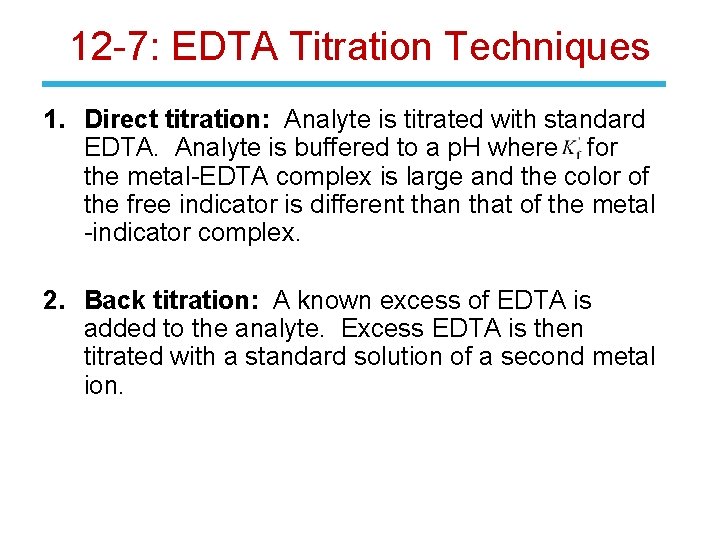
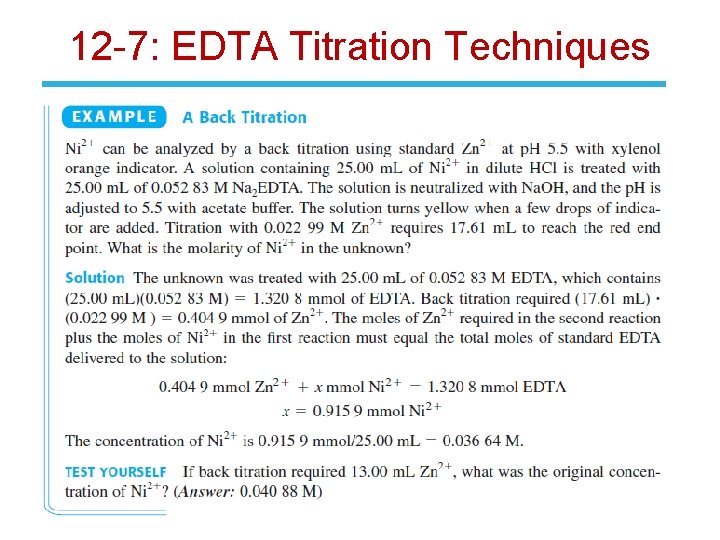
12 -7: EDTA Titration Techniques 1. Direct titration: Analyte is titrated with standard EDTA. Analyte is buffered to a p. H where for the metal-EDTA complex is large and the color of the free indicator is different than that of the metal -indicator complex. 2. Back titration: A known excess of EDTA is added to the analyte. Excess EDTA is then titrated with a standard solution of a second metal ion.

12 -7: EDTA Titration Techniques

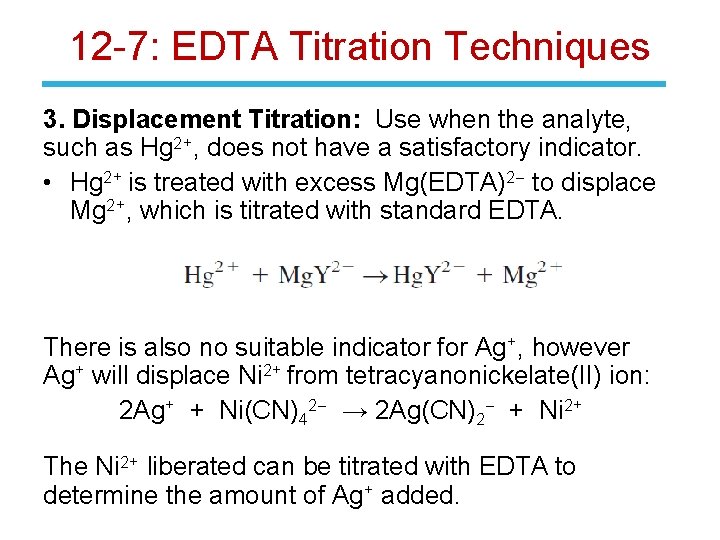
12 -7: EDTA Titration Techniques 3. Displacement Titration: Use when the analyte, such as Hg 2+, does not have a satisfactory indicator. • Hg 2+ is treated with excess Mg(EDTA)2 - to displace Mg 2+, which is titrated with standard EDTA. There is also no suitable indicator for Ag+, however Ag+ will displace Ni 2+ from tetracyanonickelate(II) ion: 2 Ag+ + Ni(CN)42 - → 2 Ag(CN)2 - + Ni 2+ The Ni 2+ liberated can be titrated with EDTA to determine the amount of Ag+ added.

12 -7: EDTA Titration Techniques 4. Indirect Titration: Anions that precipitate with certain metal ions can be analyzed with EDTA using an indirect titration. Example: Sulfate can be analyzed by precipitation with excess Ba 2+ at p. H 1. • Ba. SO 4 (s) is washed then boiled with excess standard EDTA at p. H 10 to bring Ba 2+ back into solution as Ba(EDTA)2 -. • Excess EDTA is back-titrated with Mg 2+. 5. Masking: A masking agent is a reagent that protects a component of the analyte from reaction with EDTA. Example: Al 3+ in a mixture of Mg 2+ and Al 3+ can be measured by masking the Ag+ with F-, leaving only the Mg 2+ to react with EDTA.