Chapter 12 Coordination Chemistry IV Reactions and Mechanisms



![Interchange Mechanism • Reactions typically run under conditions where [Y] >> [ML 5 X] Interchange Mechanism • Reactions typically run under conditions where [Y] >> [ML 5 X]](https://slidetodoc.com/presentation_image_h/78b24a3ddf53b9e7b1515060e7075380/image-11.jpg)



![Example [Co. II(CN)5]3 - + Co. III(NH 3)5 X 2+ Products Those with bridging Example [Co. II(CN)5]3 - + Co. III(NH 3)5 X 2+ Products Those with bridging](https://slidetodoc.com/presentation_image_h/78b24a3ddf53b9e7b1515060e7075380/image-34.jpg)
- Slides: 34

Chapter 12 Coordination Chemistry IV Reactions and Mechanisms

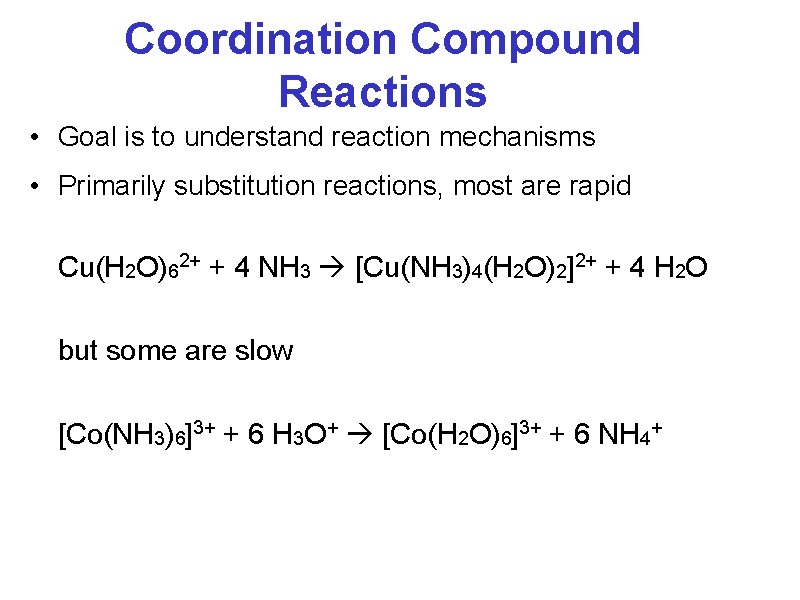
Coordination Compound Reactions • Goal is to understand reaction mechanisms • Primarily substitution reactions, most are rapid Cu(H 2 O)62+ + 4 NH 3 [Cu(NH 3)4(H 2 O)2]2+ + 4 H 2 O but some are slow [Co(NH 3)6]3+ + 6 H 3 O+ [Co(H 2 O)6]3+ + 6 NH 4+

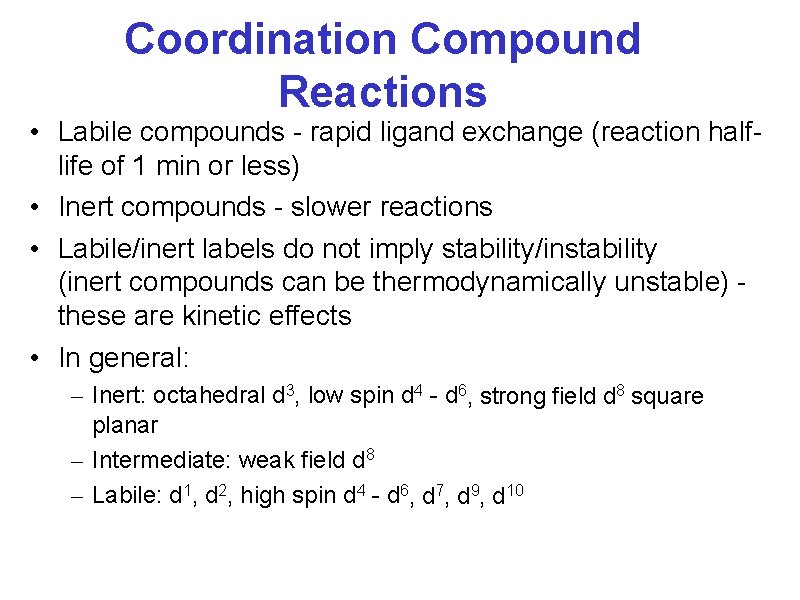
Coordination Compound Reactions • Labile compounds - rapid ligand exchange (reaction halflife of 1 min or less) • Inert compounds - slower reactions • Labile/inert labels do not imply stability/instability (inert compounds can be thermodynamically unstable) these are kinetic effects • In general: – Inert: octahedral d 3, low spin d 4 - d 6, strong field d 8 square planar – Intermediate: weak field d 8 – Labile: d 1, d 2, high spin d 4 - d 6, d 7, d 9, d 10

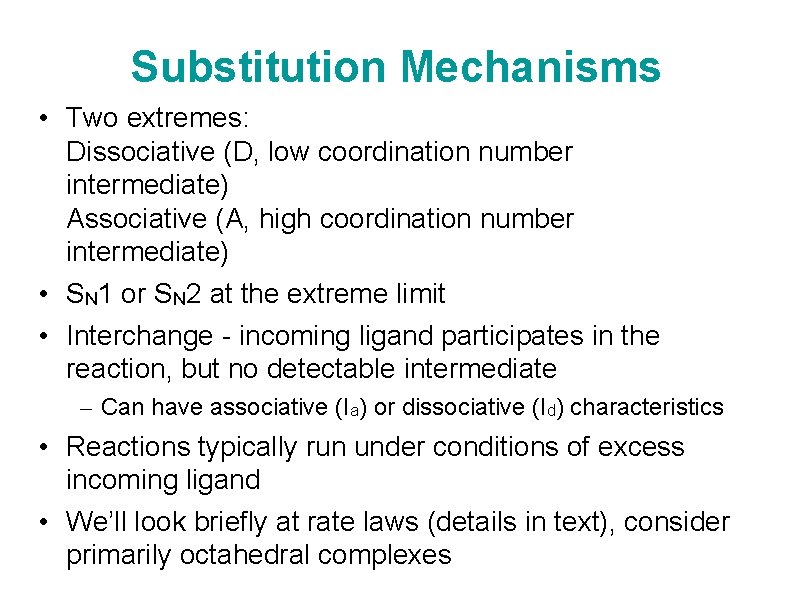
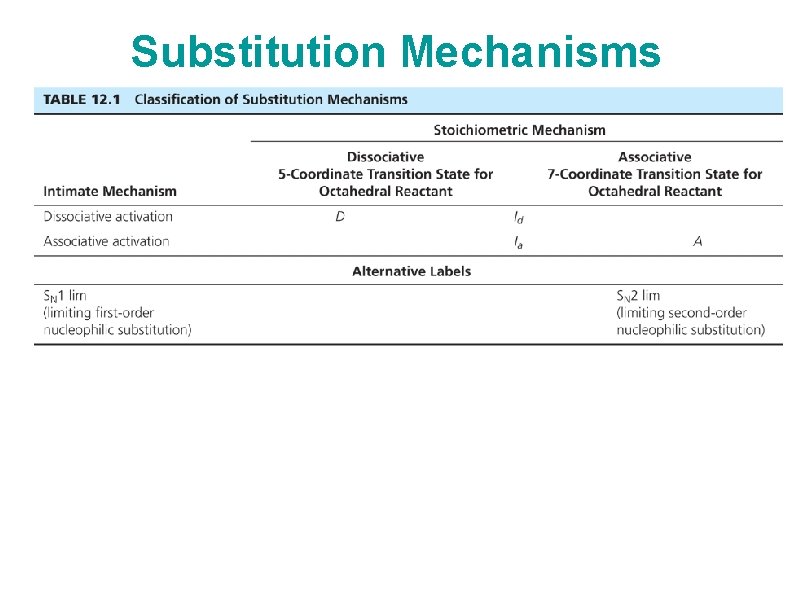
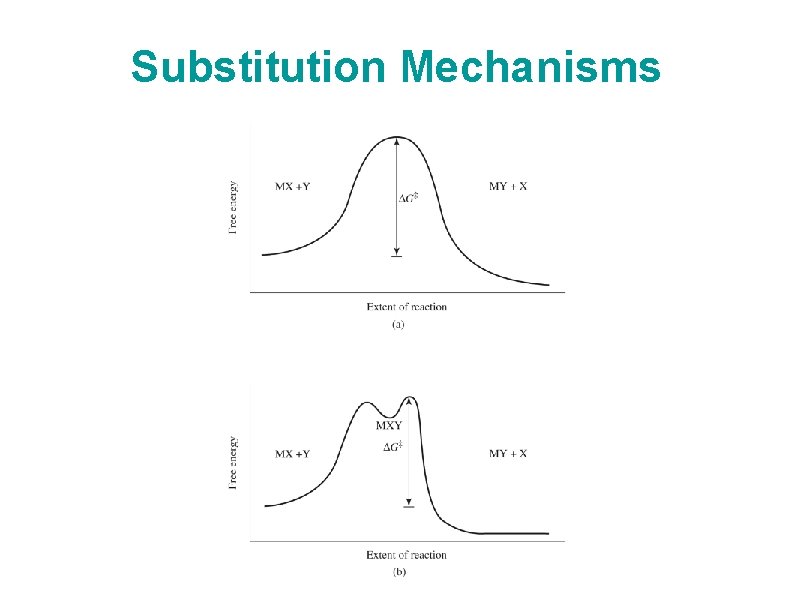
Substitution Mechanisms • Two extremes: Dissociative (D, low coordination number intermediate) Associative (A, high coordination number intermediate) • SN 1 or SN 2 at the extreme limit • Interchange - incoming ligand participates in the reaction, but no detectable intermediate – Can have associative (Ia) or dissociative (Id) characteristics • Reactions typically run under conditions of excess incoming ligand • We’ll look briefly at rate laws (details in text), consider primarily octahedral complexes

Substitution Mechanisms

Substitution Mechanisms Pictures:

Substitution Mechanisms

Determining mechanisms What things would you do to determine the mechanism?

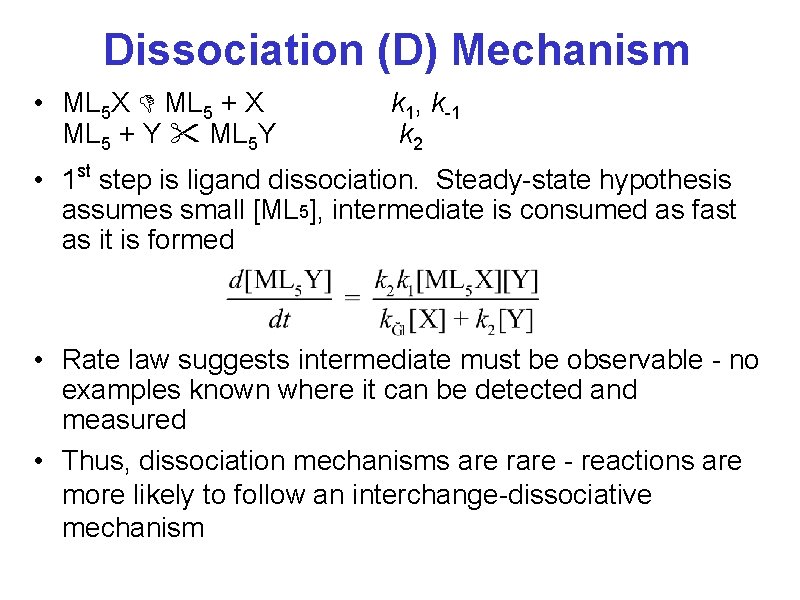
Dissociation (D) Mechanism • ML 5 X ML 5 + X ML 5 + Y ML 5 Y k 1, k-1 k 2 • 1 st step is ligand dissociation. Steady-state hypothesis assumes small [ML 5], intermediate is consumed as fast as it is formed • Rate law suggests intermediate must be observable - no examples known where it can be detected and measured • Thus, dissociation mechanisms are rare - reactions are more likely to follow an interchange-dissociative mechanism

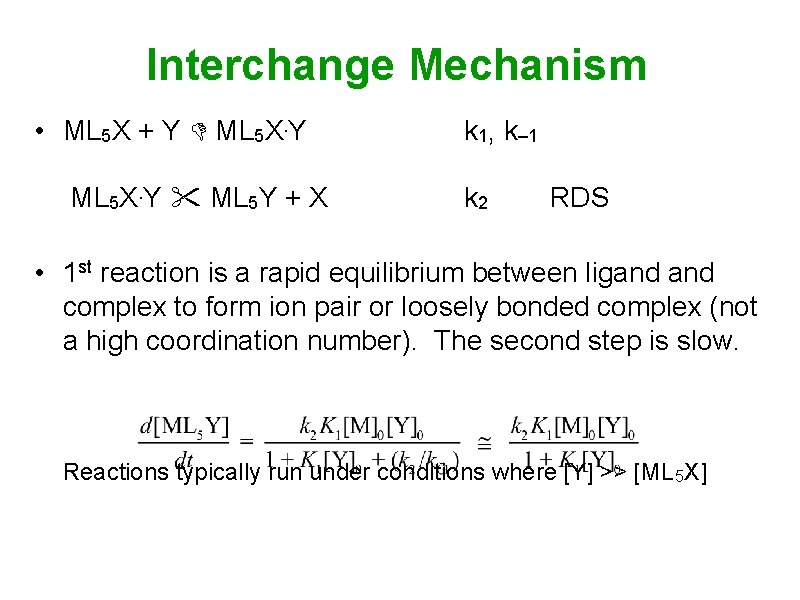
Interchange Mechanism • ML 5 X + Y ML 5 X. Y ML 5 Y + X k 1, k– 1 k 2 RDS • 1 st reaction is a rapid equilibrium between ligand complex to form ion pair or loosely bonded complex (not a high coordination number). The second step is slow. Reactions typically run under conditions where [Y] >> [ML 5 X]
![Interchange Mechanism Reactions typically run under conditions where Y ML 5 X Interchange Mechanism • Reactions typically run under conditions where [Y] >> [ML 5 X]](https://slidetodoc.com/presentation_image_h/78b24a3ddf53b9e7b1515060e7075380/image-11.jpg)
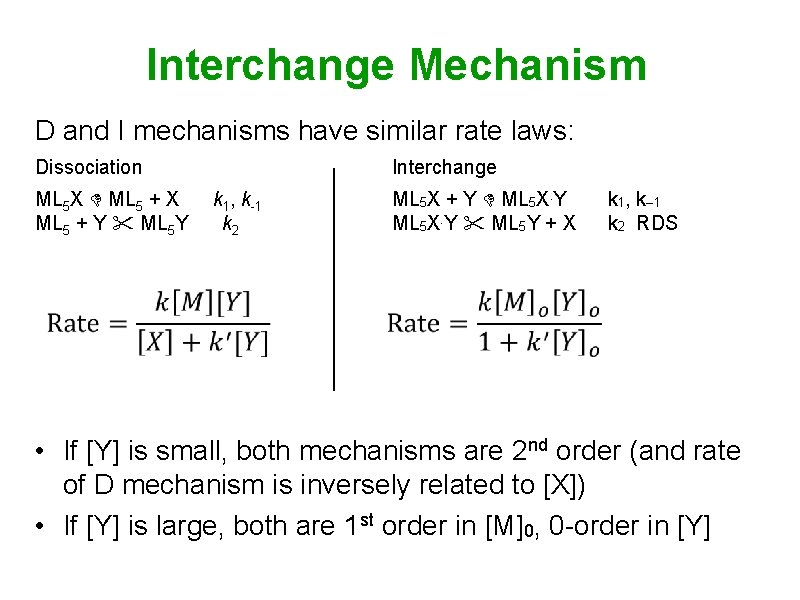
Interchange Mechanism • Reactions typically run under conditions where [Y] >> [ML 5 X] [M]0 = [ML 5 X] + [ML 5 X. Y] [Y]0 [Y] • Both D and I have similar rate laws: • If [Y] is small, both mechanisms are 2 nd order (rate of D is inversely related to [X]) If [Y] is large, both are 1 st order in [M]0, 0 -order in [Y]

Interchange Mechanism D and I mechanisms have similar rate laws: Dissociation ML 5 X ML 5 + X ML 5 + Y ML 5 Y Interchange k 1, k-1 k 2 ML 5 X + Y ML 5 X. Y ML 5 Y + X k 1, k– 1 k 2 RDS • If [Y] is small, both mechanisms are 2 nd order (and rate of D mechanism is inversely related to [X]) • If [Y] is large, both are 1 st order in [M]0, 0 -order in [Y]

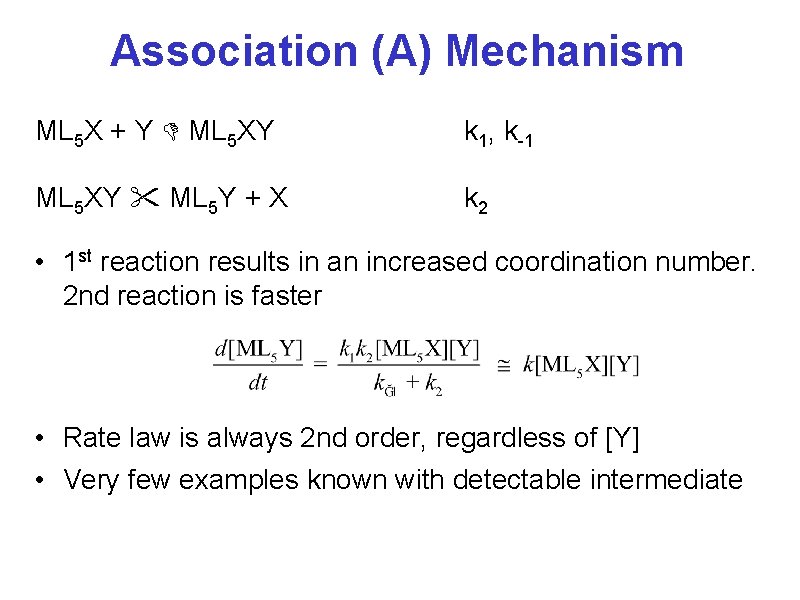
Association (A) Mechanism ML 5 X + Y ML 5 XY k 1, k-1 ML 5 XY ML 5 Y + X k 2 • 1 st reaction results in an increased coordination number. 2 nd reaction is faster • Rate law is always 2 nd order, regardless of [Y] • Very few examples known with detectable intermediate

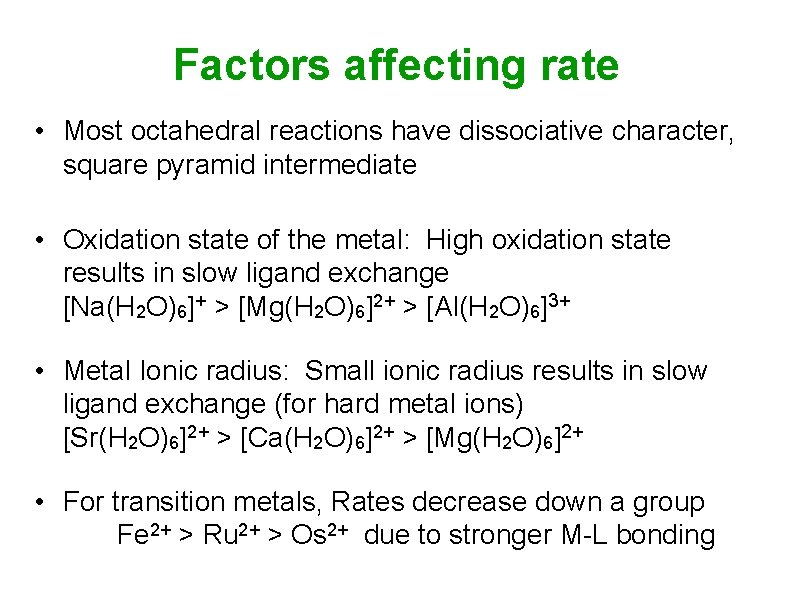
Factors affecting rate • Most octahedral reactions have dissociative character, square pyramid intermediate • Oxidation state of the metal: High oxidation state results in slow ligand exchange [Na(H 2 O)6]+ > [Mg(H 2 O)6]2+ > [Al(H 2 O)6]3+ • Metal Ionic radius: Small ionic radius results in slow ligand exchange (for hard metal ions) [Sr(H 2 O)6]2+ > [Ca(H 2 O)6]2+ > [Mg(H 2 O)6]2+ • For transition metals, Rates decrease down a group Fe 2+ > Ru 2+ > Os 2+ due to stronger M-L bonding

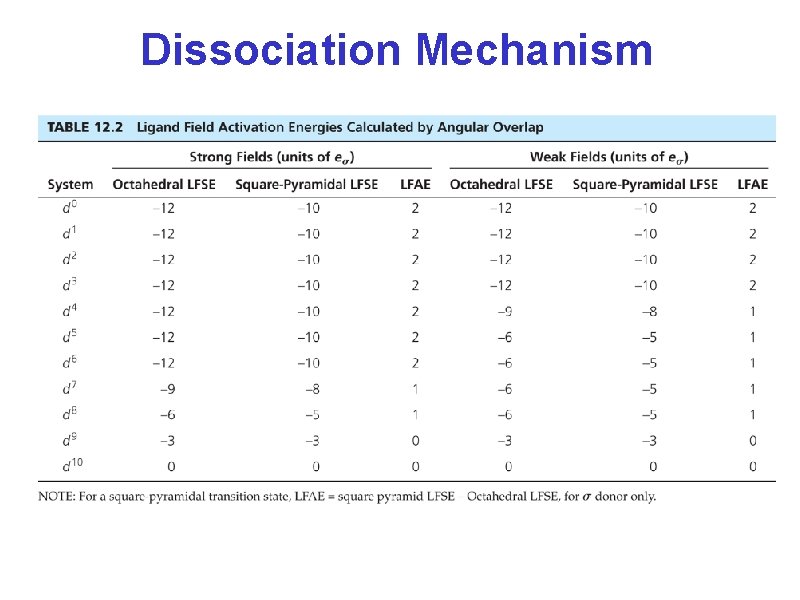
Dissociation Mechanism

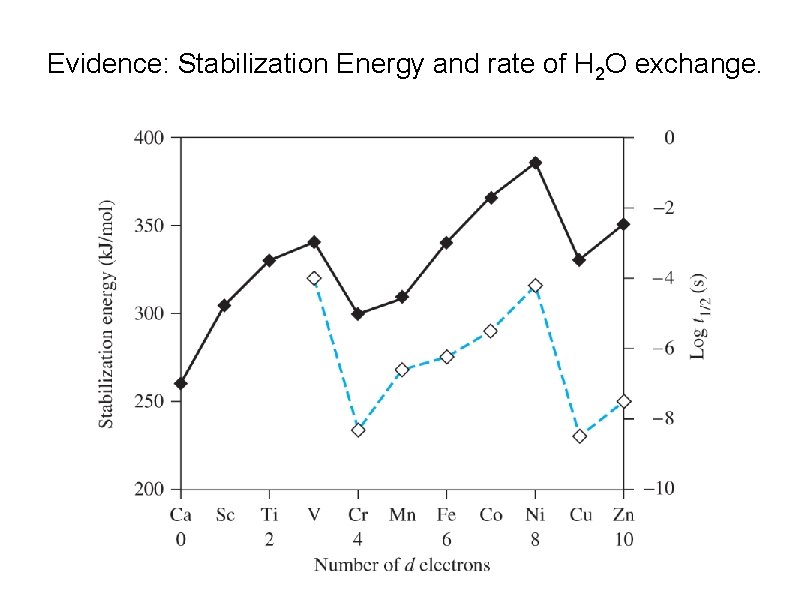
Evidence: Stabilization Energy and rate of H 2 O exchange.

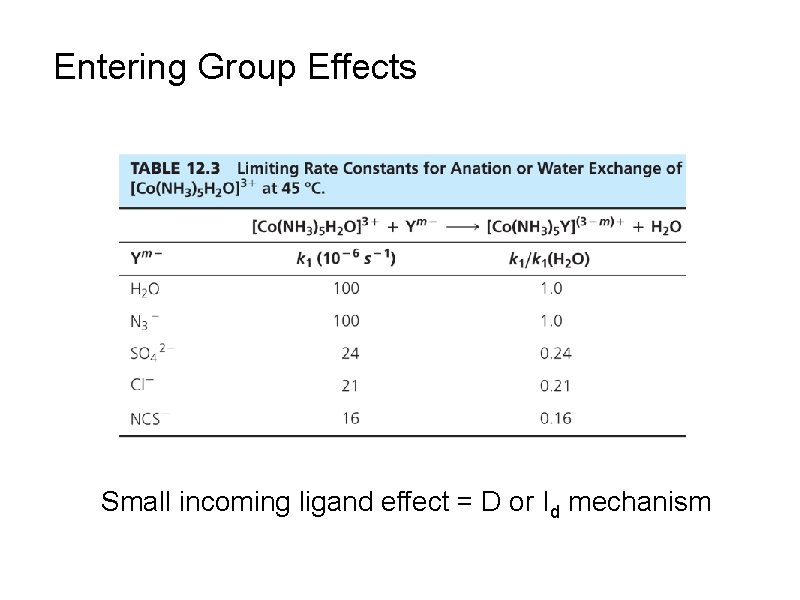
Entering Group Effects Small incoming ligand effect = D or Id mechanism

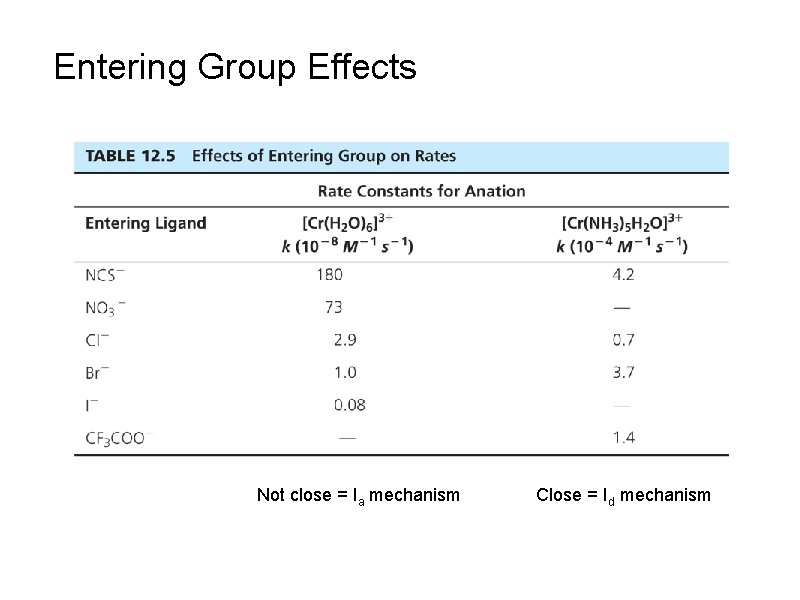
Entering Group Effects Not close = Ia mechanism Close = Id mechanism

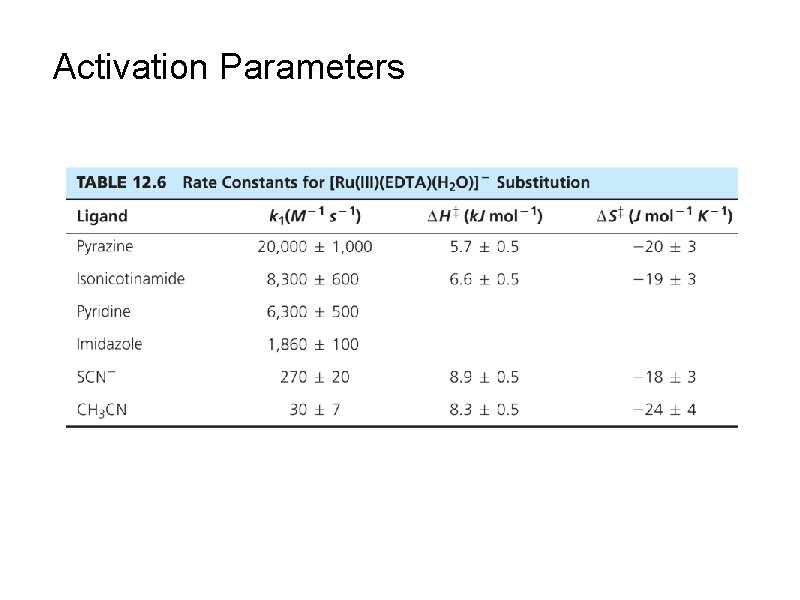
Activation Parameters

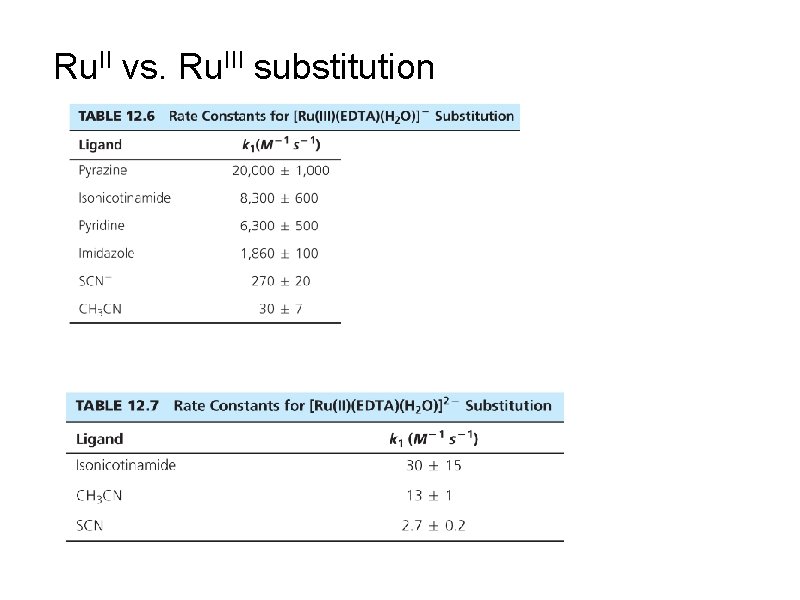
Ru. II vs. Ru. III substitution

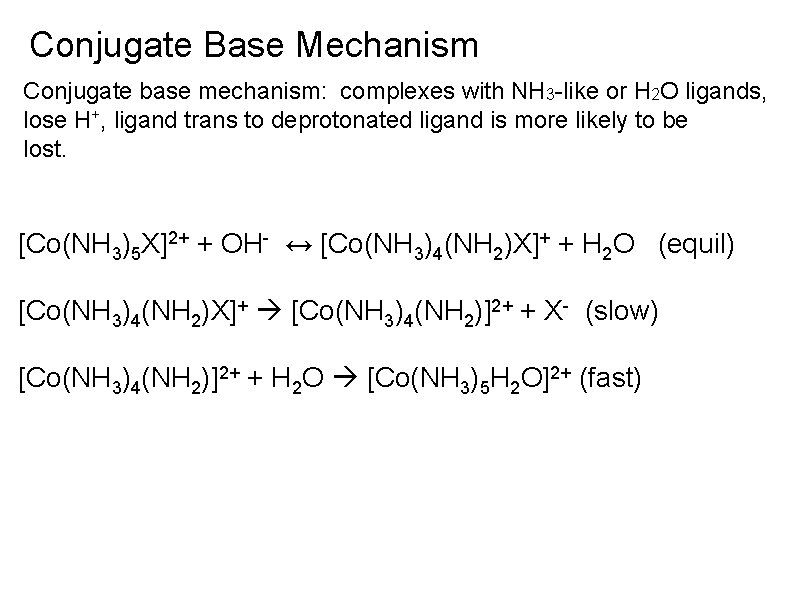
Conjugate Base Mechanism Conjugate base mechanism: complexes with NH 3 -like or H 2 O ligands, lose H+, ligand trans to deprotonated ligand is more likely to be lost. [Co(NH 3)5 X]2+ + OH- ↔ [Co(NH 3)4(NH 2)X]+ + H 2 O (equil) [Co(NH 3)4(NH 2)X]+ [Co(NH 3)4(NH 2)]2+ + X- (slow) [Co(NH 3)4(NH 2)]2+ + H 2 O [Co(NH 3)5 H 2 O]2+ (fast)

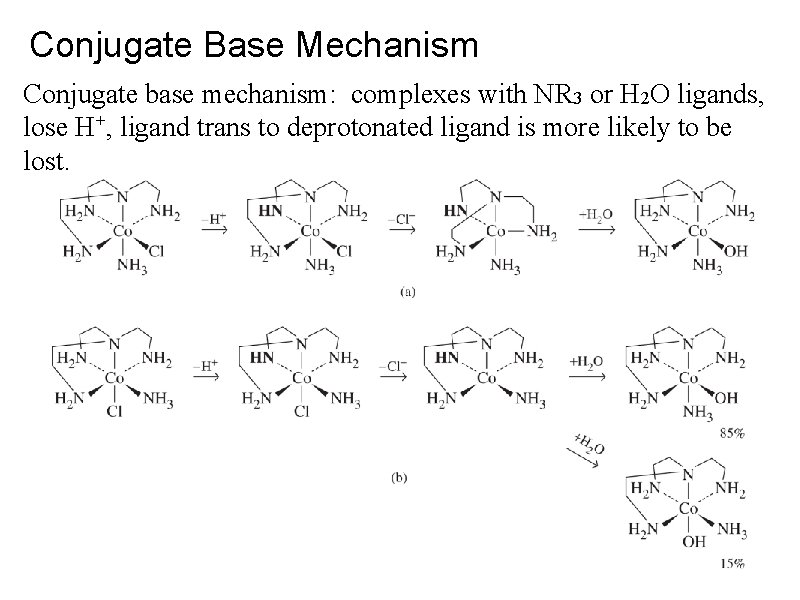
Conjugate Base Mechanism Conjugate base mechanism: complexes with NR 3 or H 2 O ligands, lose H+, ligand trans to deprotonated ligand is more likely to be lost.

Reaction Modeling using Excel Programming

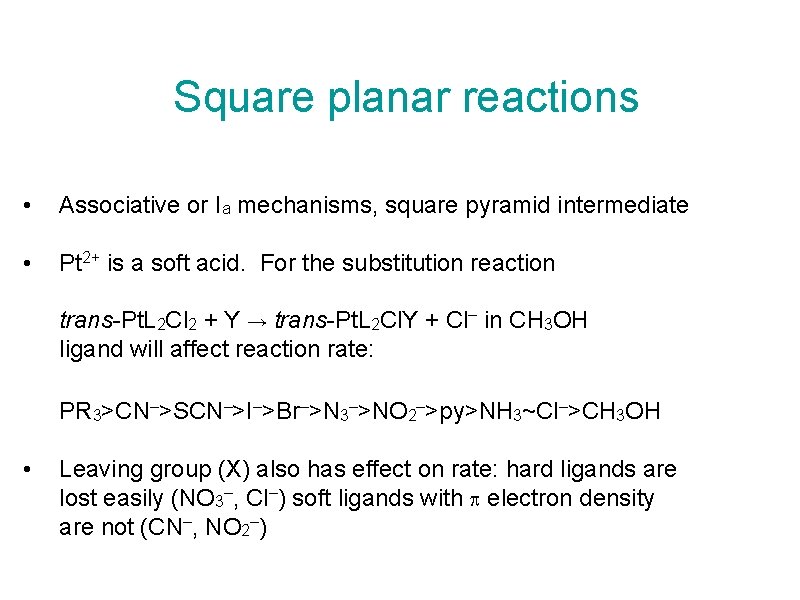
Square planar reactions • Associative or Ia mechanisms, square pyramid intermediate • Pt 2+ is a soft acid. For the substitution reaction trans-Pt. L 2 Cl 2 + Y → trans-Pt. L 2 Cl. Y + Cl– in CH 3 OH ligand will affect reaction rate: PR 3>CN–>SCN–>I–>Br–>N 3–>NO 2–>py>NH 3~Cl–>CH 3 OH • Leaving group (X) also has effect on rate: hard ligands are lost easily (NO 3–, Cl–) soft ligands with electron density are not (CN–, NO 2–)

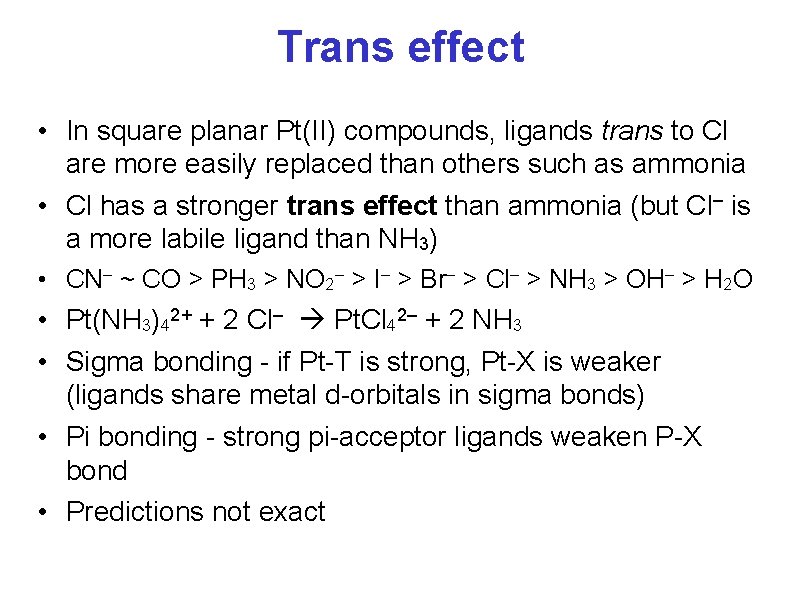
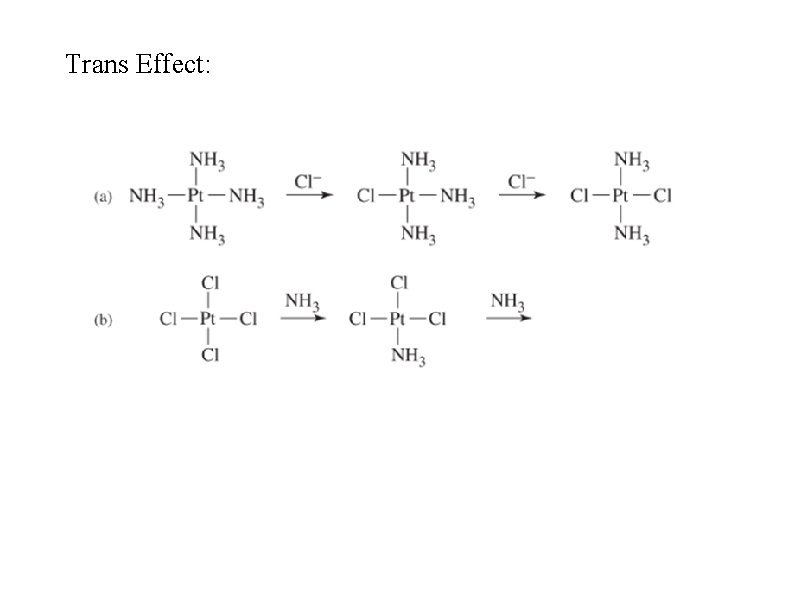
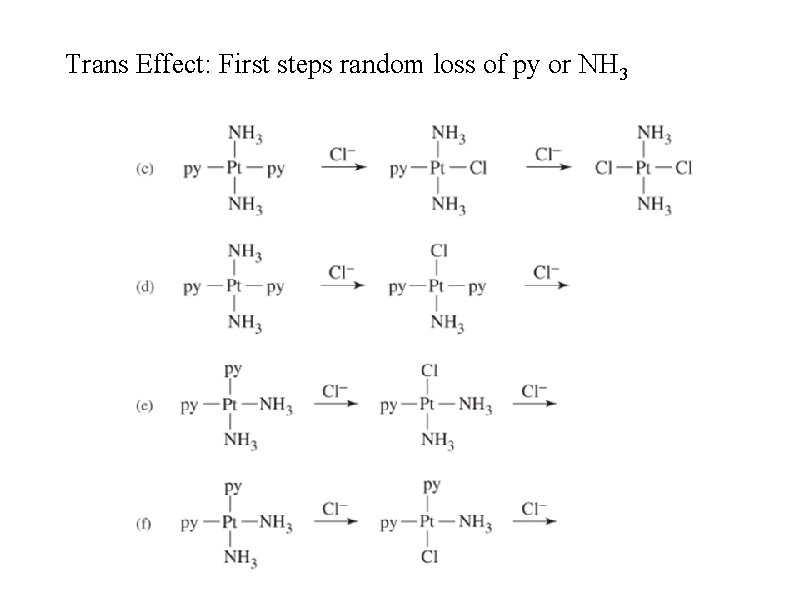
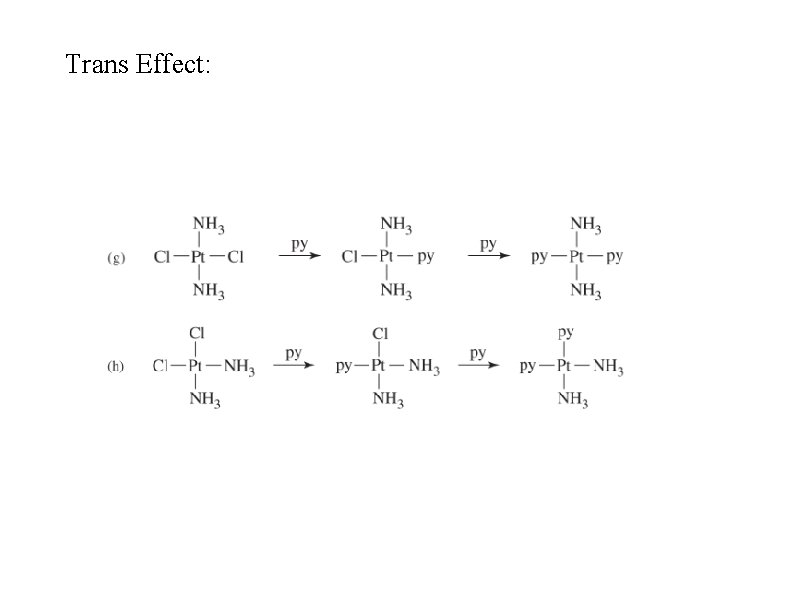
Trans effect • In square planar Pt(II) compounds, ligands trans to Cl are more easily replaced than others such as ammonia • Cl has a stronger trans effect than ammonia (but Cl– is a more labile ligand than NH 3) • CN– ~ CO > PH 3 > NO 2– > I– > Br– > Cl– > NH 3 > OH– > H 2 O • Pt(NH 3)42+ + 2 Cl– Pt. Cl 42– + 2 NH 3 • Sigma bonding - if Pt-T is strong, Pt-X is weaker (ligands share metal d-orbitals in sigma bonds) • Pi bonding - strong pi-acceptor ligands weaken P-X bond • Predictions not exact

Trans Effect:

Trans Effect: First steps random loss of py or NH 3

Trans Effect:

Electron Transfer Reactions Inner vs. Outer Sphere Electron Transfer

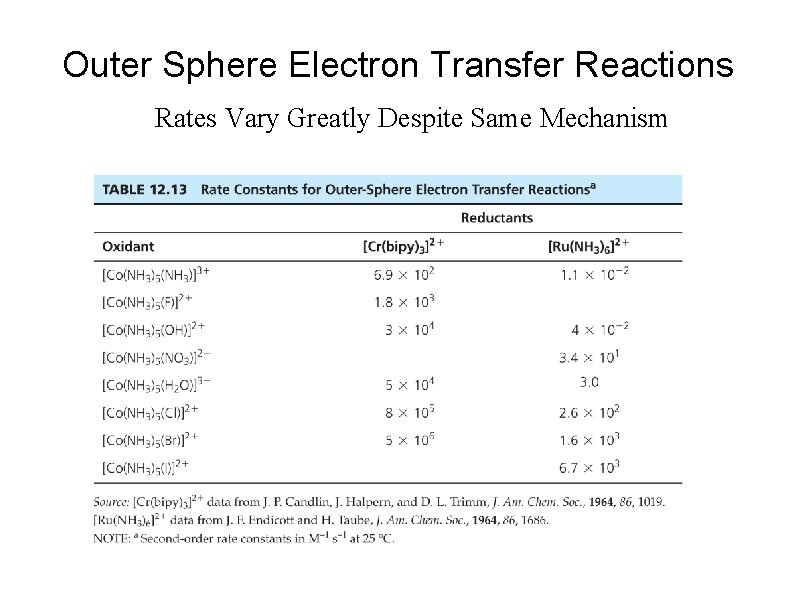
Outer Sphere Electron Transfer Reactions Rates Vary Greatly Despite Same Mechanism

Nature of Outer Sphere Activation Barrier

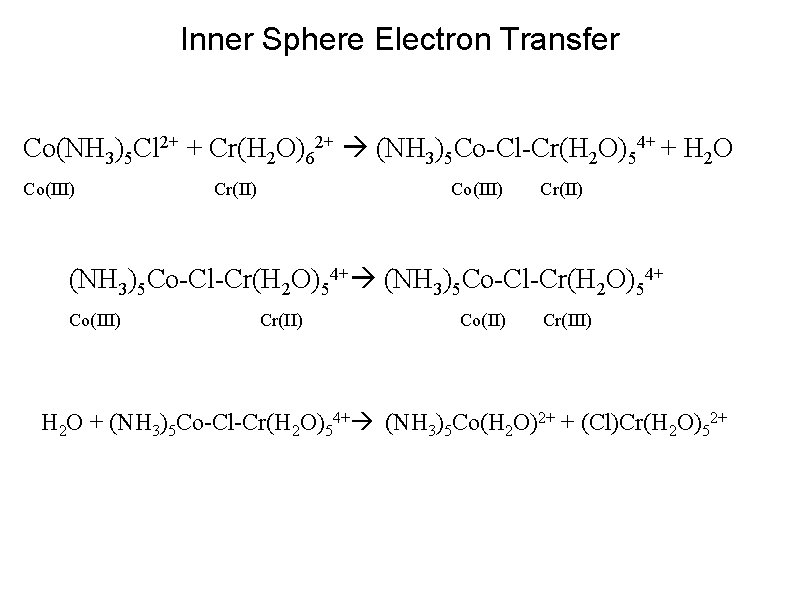
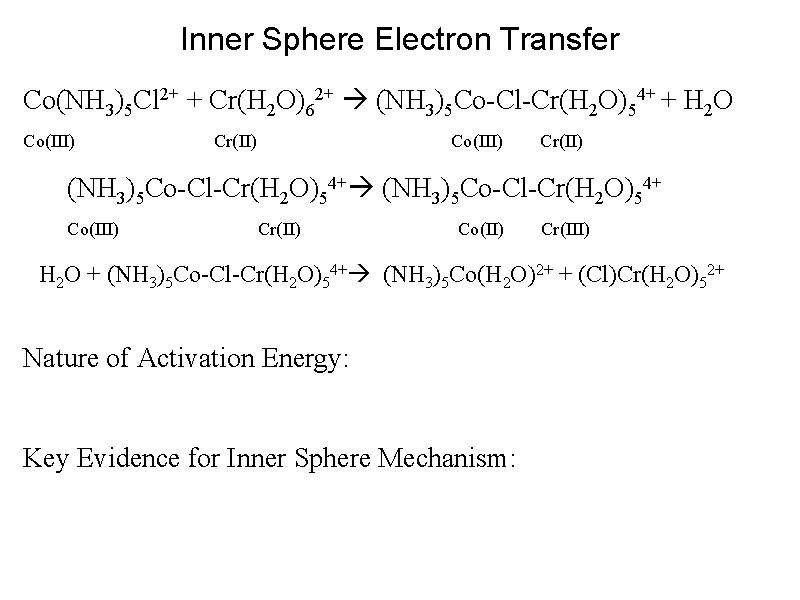
Inner Sphere Electron Transfer Co(NH 3)5 Cl 2+ + Cr(H 2 O)62+ (NH 3)5 Co-Cl-Cr(H 2 O)54+ + H 2 O Co(III) Cr(II) Co(III) Cr(II) (NH 3)5 Co-Cl-Cr(H 2 O)54+ Co(III) Cr(II) Co(II) Cr(III) H 2 O + (NH 3)5 Co-Cl-Cr(H 2 O)54+ (NH 3)5 Co(H 2 O)2+ + (Cl)Cr(H 2 O)52+

Inner Sphere Electron Transfer Co(NH 3)5 Cl 2+ + Cr(H 2 O)62+ (NH 3)5 Co-Cl-Cr(H 2 O)54+ + H 2 O Co(III) Cr(II) Co(III) Cr(II) (NH 3)5 Co-Cl-Cr(H 2 O)54+ Co(III) Cr(II) Co(II) Cr(III) H 2 O + (NH 3)5 Co-Cl-Cr(H 2 O)54+ (NH 3)5 Co(H 2 O)2+ + (Cl)Cr(H 2 O)52+ Nature of Activation Energy: Key Evidence for Inner Sphere Mechanism:
![Example Co IICN53 Co IIINH 35 X 2 Products Those with bridging Example [Co. II(CN)5]3 - + Co. III(NH 3)5 X 2+ Products Those with bridging](https://slidetodoc.com/presentation_image_h/78b24a3ddf53b9e7b1515060e7075380/image-34.jpg)
Example [Co. II(CN)5]3 - + Co. III(NH 3)5 X 2+ Products Those with bridging ligands give product [Co(CN)5 X]2+.