Chapter 12 Chemical Kinetics Section 12 1 Reaction


![Section 12. 2 Rate Laws: An Introduction Rate Law Rate = k[NO 2]n § Section 12. 2 Rate Laws: An Introduction Rate Law Rate = k[NO 2]n §](https://slidetodoc.com/presentation_image_h2/74898869e2f9fdb0515306664a857671/image-7.jpg)
![Section 12. 2 Rate Laws: An Introduction Rate Law Rate = k[NO 2]n § Section 12. 2 Rate Laws: An Introduction Rate Law Rate = k[NO 2]n §](https://slidetodoc.com/presentation_image_h2/74898869e2f9fdb0515306664a857671/image-9.jpg)





![Section 12. 4 The Integrated Rate Law First-Order § Rate = k[A] § Integrated: Section 12. 4 The Integrated Rate Law First-Order § Rate = k[A] § Integrated:](https://slidetodoc.com/presentation_image_h2/74898869e2f9fdb0515306664a857671/image-17.jpg)
![Section 12. 4 The Integrated Rate Law Plot of ln[N 2 O 5] vs Section 12. 4 The Integrated Rate Law Plot of ln[N 2 O 5] vs](https://slidetodoc.com/presentation_image_h2/74898869e2f9fdb0515306664a857671/image-18.jpg)

![First-order reaction A product # of half-lives [A] = [A]0/n 1 2 2 4 First-order reaction A product # of half-lives [A] = [A]0/n 1 2 2 4](https://slidetodoc.com/presentation_image_h2/74898869e2f9fdb0515306664a857671/image-24.jpg)
![Section 12. 4 The Integrated Rate Law Second-Order § Rate = k[A]2 § Integrated: Section 12. 4 The Integrated Rate Law Second-Order § Rate = k[A]2 § Integrated:](https://slidetodoc.com/presentation_image_h2/74898869e2f9fdb0515306664a857671/image-25.jpg)
![Second-Order Reactions A product D[A] rate = Dt rate M/s = = 1/M • Second-Order Reactions A product D[A] rate = Dt rate M/s = = 1/M •](https://slidetodoc.com/presentation_image_h2/74898869e2f9fdb0515306664a857671/image-26.jpg)
![Section 12. 4 The Integrated Rate Law Plot of ln[C 4 H 6] vs Section 12. 4 The Integrated Rate Law Plot of ln[C 4 H 6] vs](https://slidetodoc.com/presentation_image_h2/74898869e2f9fdb0515306664a857671/image-27.jpg)

![Section 12. 4 The Integrated Rate Law Zero-Order § Rate = k[A]0 = k Section 12. 4 The Integrated Rate Law Zero-Order § Rate = k[A]0 = k](https://slidetodoc.com/presentation_image_h2/74898869e2f9fdb0515306664a857671/image-30.jpg)
![Section 12. 4 The Integrated Rate Law Plot of [A] vs Time Copyright © Section 12. 4 The Integrated Rate Law Plot of [A] vs Time Copyright ©](https://slidetodoc.com/presentation_image_h2/74898869e2f9fdb0515306664a857671/image-31.jpg)



![Rate Laws and Elementary Steps Unimolecular reaction A products rate = k [A] Bimolecular Rate Laws and Elementary Steps Unimolecular reaction A products rate = k [A] Bimolecular](https://slidetodoc.com/presentation_image_h2/74898869e2f9fdb0515306664a857671/image-41.jpg)













- Slides: 66

Chapter 12 Chemical Kinetics

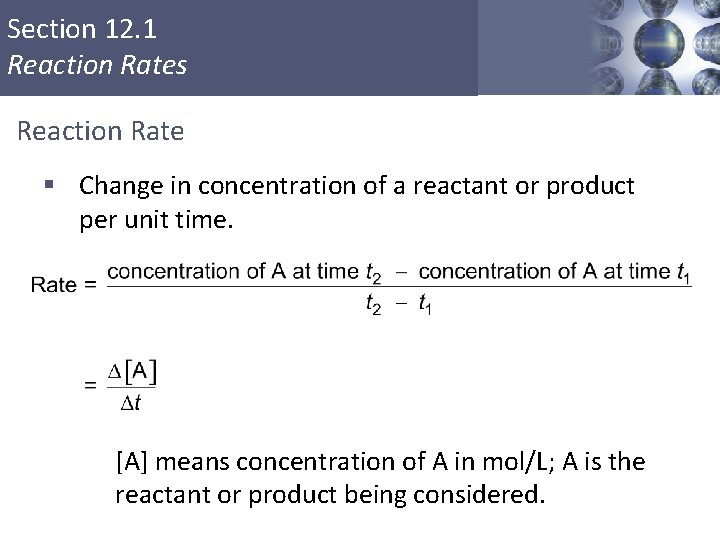
Section 12. 1 Reaction Rates Reaction Rate § Change in concentration of a reactant or product per unit time. [A] means concentration of A in mol/L; A is the reactant or product being considered. Copyright © Cengage Learning. All rights reserved 2

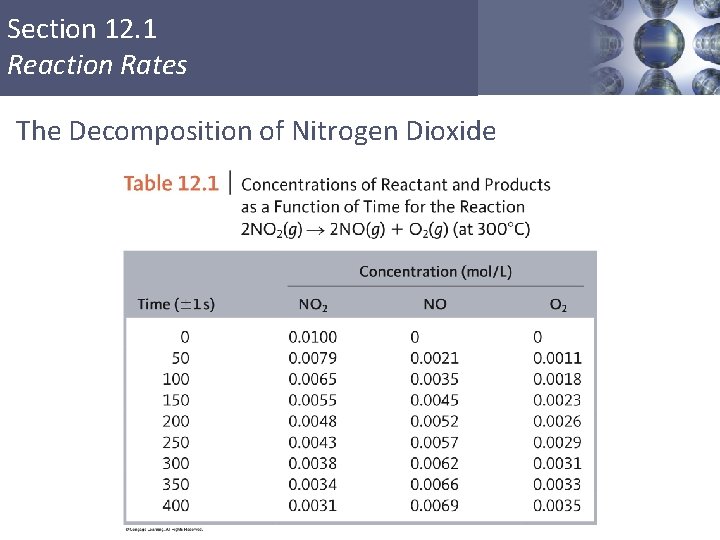
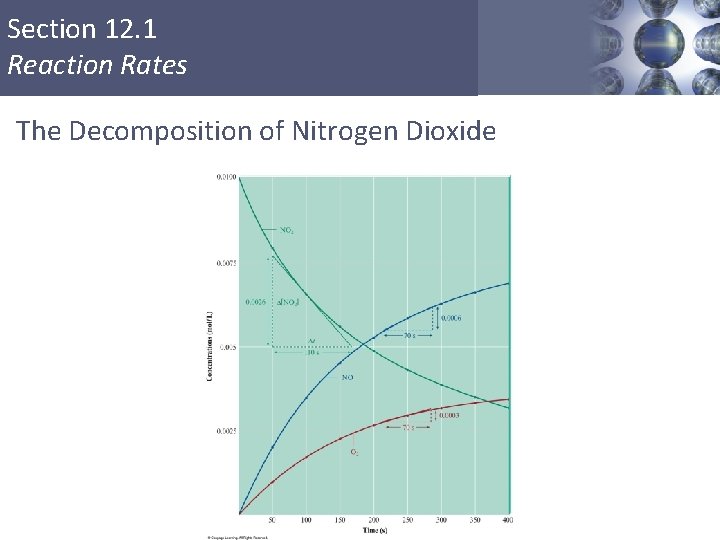
Section 12. 1 Reaction Rates The Decomposition of Nitrogen Dioxide Copyright © Cengage Learning. All rights reserved 3

Section 12. 1 Reaction Rates The Decomposition of Nitrogen Dioxide Copyright © Cengage Learning. All rights reserved 4

Section 12. 1 Reaction Rates Instantaneous Rate § Value of the rate at a particular time. § Can be obtained by computing the slope of a line tangent to the curve at that point. Copyright © Cengage Learning. All rights reserved 5

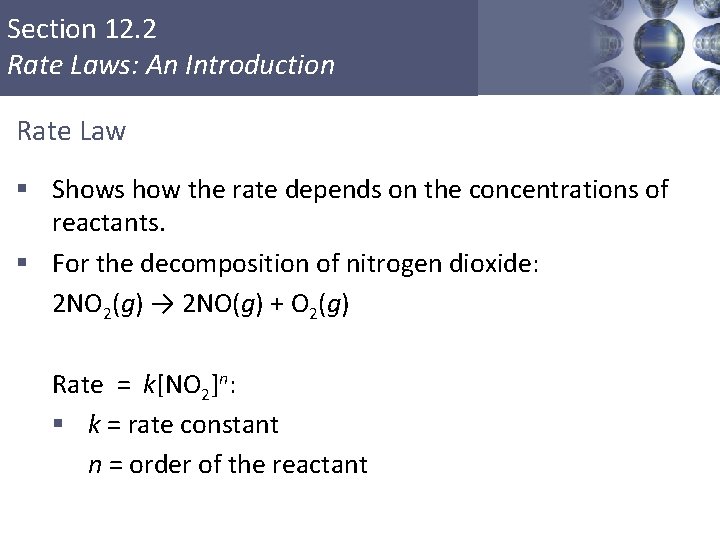
Section 12. 2 Rate Laws: An Introduction Rate Law § Shows how the rate depends on the concentrations of reactants. § For the decomposition of nitrogen dioxide: 2 NO 2(g) → 2 NO(g) + O 2(g) Rate = k[NO 2]n: § k = rate constant § n = order of the reactant Copyright © Cengage Learning. All rights reserved 6
![Section 12 2 Rate Laws An Introduction Rate Law Rate kNO 2n Section 12. 2 Rate Laws: An Introduction Rate Law Rate = k[NO 2]n §](https://slidetodoc.com/presentation_image_h2/74898869e2f9fdb0515306664a857671/image-7.jpg)
Section 12. 2 Rate Laws: An Introduction Rate Law Rate = k[NO 2]n § The concentrations of the products do not appear in the rate law because the reaction rate is being studied under conditions where the reverse reaction does not contribute to the overall rate. Copyright © Cengage Learning. All rights reserved 7

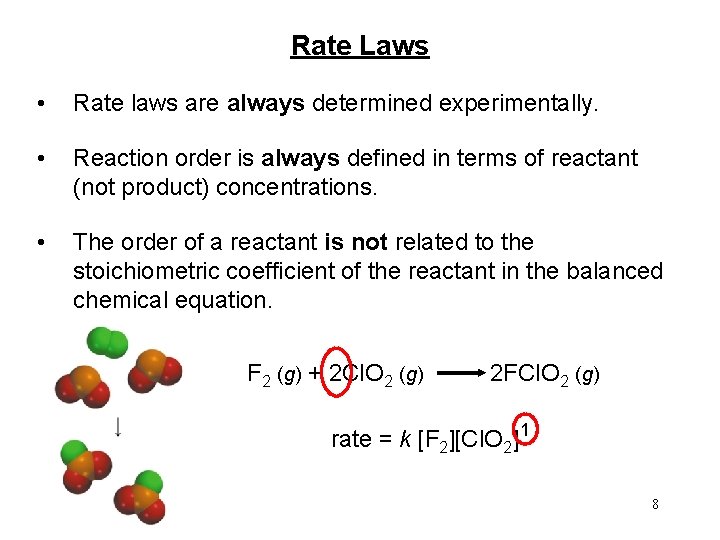
Rate Laws • Rate laws are always determined experimentally. • Reaction order is always defined in terms of reactant (not product) concentrations. • The order of a reactant is not related to the stoichiometric coefficient of the reactant in the balanced chemical equation. F 2 (g) + 2 Cl. O 2 (g) 2 FCl. O 2 (g) rate = k [F 2][Cl. O 2] 1 8
![Section 12 2 Rate Laws An Introduction Rate Law Rate kNO 2n Section 12. 2 Rate Laws: An Introduction Rate Law Rate = k[NO 2]n §](https://slidetodoc.com/presentation_image_h2/74898869e2f9fdb0515306664a857671/image-9.jpg)
Section 12. 2 Rate Laws: An Introduction Rate Law Rate = k[NO 2]n § The value of the exponent n must be determined by experiment; it cannot be written from the balanced equation. Copyright © Cengage Learning. All rights reserved 9

Section 12. 2 Rate Laws: An Introduction Types of Rate Laws § Differential Rate Law (rate law) – shows how the rate of a reaction depends on concentrations. § Integrated Rate Law – shows how the concentrations of species in the reaction depend on time. Copyright © Cengage Learning. All rights reserved 10

Section 12. 2 Rate Laws: An Introduction Rate Laws: A Summary § Because we typically consider reactions only under conditions where the reverse reaction is unimportant, our rate laws will involve only concentrations of reactants. § Because the differential and integrated rate laws for a given reaction are related in a well–defined way, the experimental determination of either of the rate laws is sufficient. Copyright © Cengage Learning. All rights reserved 11

Section 12. 2 Rate Laws: An Introduction Rate Laws: A Summary § Experimental convenience usually dictates which type of rate law is determined experimentally. § Knowing the rate law for a reaction is important mainly because we can usually infer the individual steps involved in the reaction from the specific form of the rate law. Copyright © Cengage Learning. All rights reserved 12

Section 12. 3 Determining the Form of the Rate Law § Determine experimentally the power to which each reactant concentration must be raised in the rate law. Copyright © Cengage Learning. All rights reserved 13

Section 12. 3 Determining the Form of the Rate Law Method of Initial Rates § The value of the initial rate is determined for each experiment at the same value of t as close to t = 0 as possible. § Several experiments are carried out using different initial concentrations of each of the reactants, and the initial rate is determined for each run. § The results are then compared to see how the initial rate depends on the initial concentrations of each of the reactants. Copyright © Cengage Learning. All rights reserved 14

Section 12. 3 Determining the Form of the Rate Law Overall Reaction Order § The sum of the exponents in the reaction rate equation. Rate = k[A]n[B]m Overall reaction order = n + m k = rate constant [A] = concentration of reactant A [B] = concentration of reactant B Copyright © Cengage Learning. All rights reserved 15

Section 12. 3 Determining the Form of the Rate Law CONCEPT CHECK! How do exponents (orders) in rate laws compare to coefficients in balanced equations? Why? Copyright © Cengage Learning. All rights reserved 16
![Section 12 4 The Integrated Rate Law FirstOrder Rate kA Integrated Section 12. 4 The Integrated Rate Law First-Order § Rate = k[A] § Integrated:](https://slidetodoc.com/presentation_image_h2/74898869e2f9fdb0515306664a857671/image-17.jpg)
Section 12. 4 The Integrated Rate Law First-Order § Rate = k[A] § Integrated: ln[A] = –kt + ln[A]o [A] = concentration of A at time t k = rate constant t = time [A]o = initial concentration of A Copyright © Cengage Learning. All rights reserved 17
![Section 12 4 The Integrated Rate Law Plot of lnN 2 O 5 vs Section 12. 4 The Integrated Rate Law Plot of ln[N 2 O 5] vs](https://slidetodoc.com/presentation_image_h2/74898869e2f9fdb0515306664a857671/image-18.jpg)
Section 12. 4 The Integrated Rate Law Plot of ln[N 2 O 5] vs Time Copyright © Cengage Learning. All rights reserved 18

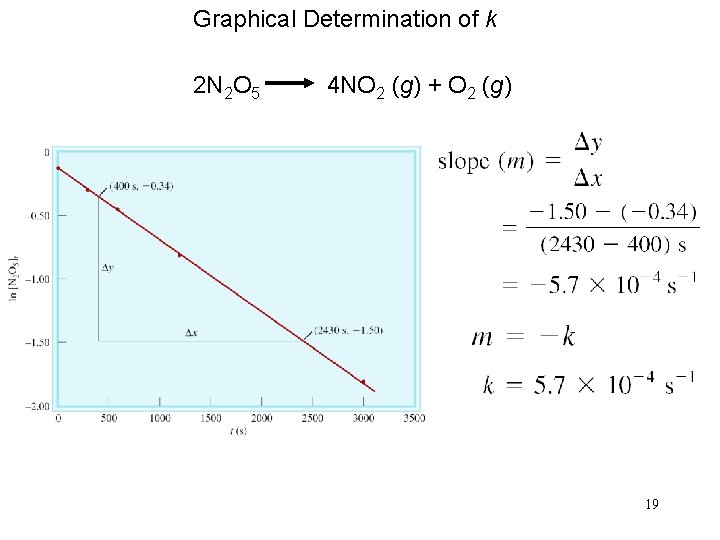
Graphical Determination of k 2 N 2 O 5 4 NO 2 (g) + O 2 (g) 19

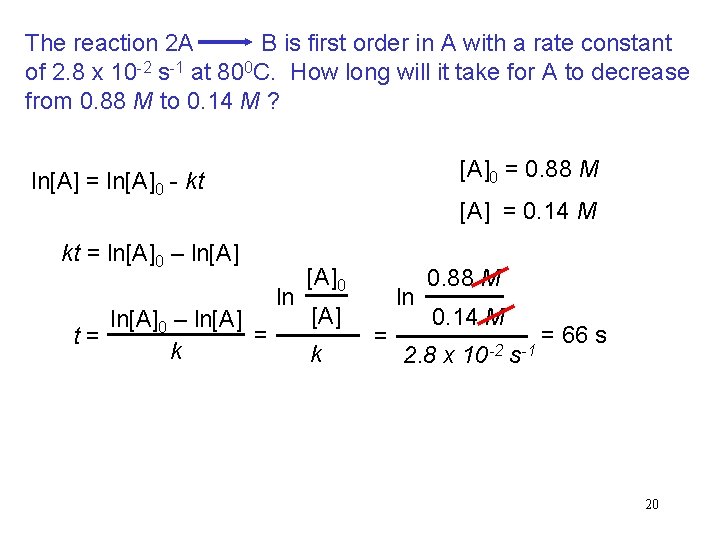
The reaction 2 A B is first order in A with a rate constant of 2. 8 x 10 -2 s-1 at 800 C. How long will it take for A to decrease from 0. 88 M to 0. 14 M ? [A]0 = 0. 88 M ln[A] = ln[A]0 - kt [A] = 0. 14 M kt = ln[A]0 – ln[A] = t= k ln [A]0 [A] k ln = 0. 88 M 0. 14 M 2. 8 x 10 -2 s-1 = 66 s 20

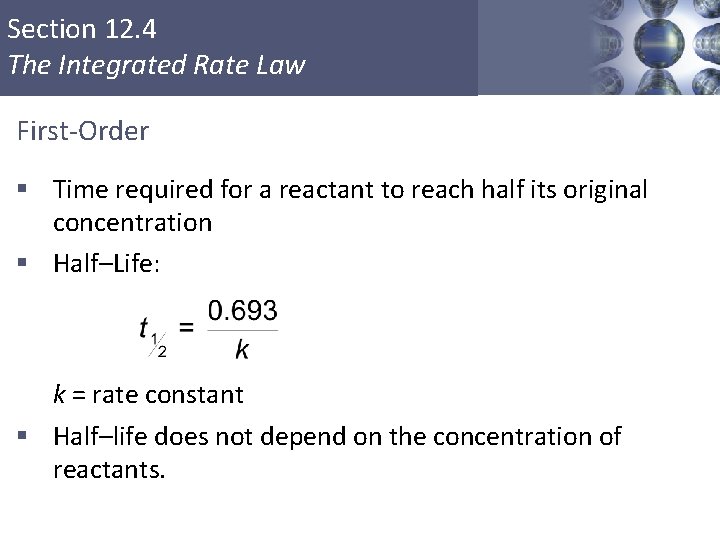
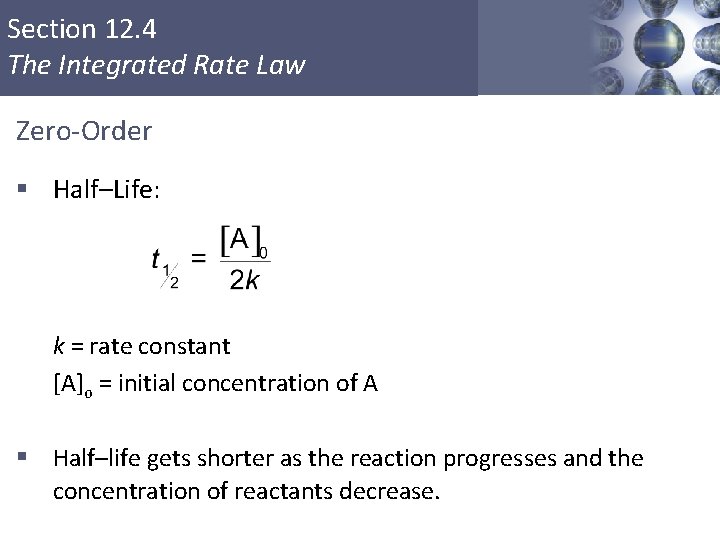
Section 12. 4 The Integrated Rate Law First-Order § Time required for a reactant to reach half its original concentration § Half–Life: k = rate constant § Half–life does not depend on the concentration of reactants. Copyright © Cengage Learning. All rights reserved 21

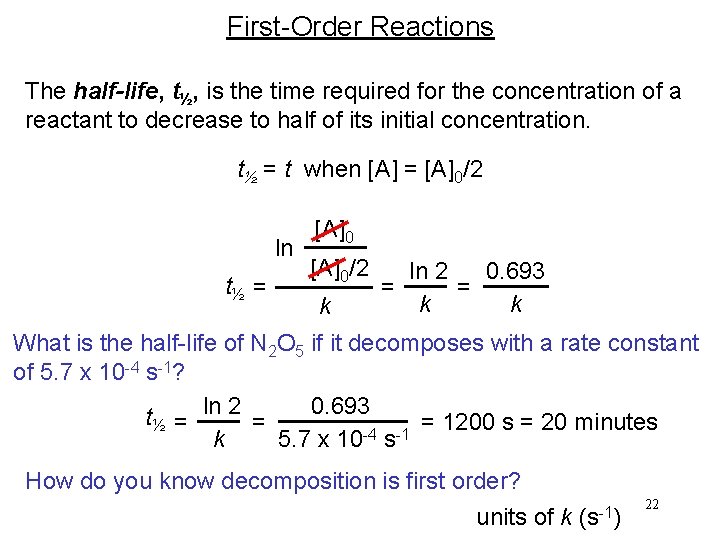
First-Order Reactions The half-life, t½, is the time required for the concentration of a reactant to decrease to half of its initial concentration. t½ = t when [A] = [A]0/2 ln t½ = [A]0/2 k ln 2 0. 693 = = k k What is the half-life of N 2 O 5 if it decomposes with a rate constant of 5. 7 x 10 -4 s-1? 0. 693 t½ = ln 2 = = 1200 s = 20 minutes -4 -1 5. 7 x 10 s k How do you know decomposition is first order? units of k (s-1) 22

Section 12. 4 The Integrated Rate Law A first order reaction is 35% complete at the end of 55 minutes. What is the value of k? k = 7. 8 × 10– 3 min– 1 Copyright © Cengage Learning. All rights reserved 23
![Firstorder reaction A product of halflives A A0n 1 2 2 4 First-order reaction A product # of half-lives [A] = [A]0/n 1 2 2 4](https://slidetodoc.com/presentation_image_h2/74898869e2f9fdb0515306664a857671/image-24.jpg)
First-order reaction A product # of half-lives [A] = [A]0/n 1 2 2 4 3 8 4 16 24
![Section 12 4 The Integrated Rate Law SecondOrder Rate kA2 Integrated Section 12. 4 The Integrated Rate Law Second-Order § Rate = k[A]2 § Integrated:](https://slidetodoc.com/presentation_image_h2/74898869e2f9fdb0515306664a857671/image-25.jpg)
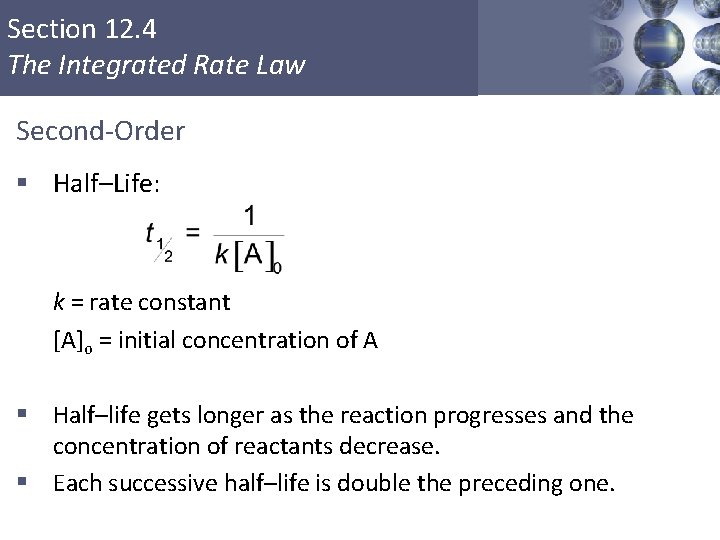
Section 12. 4 The Integrated Rate Law Second-Order § Rate = k[A]2 § Integrated: [A] = concentration of A at time t k = rate constant t = time [A]o = initial concentration of A Copyright © Cengage Learning. All rights reserved 25
![SecondOrder Reactions A product DA rate Dt rate Ms 1M Second-Order Reactions A product D[A] rate = Dt rate M/s = = 1/M •](https://slidetodoc.com/presentation_image_h2/74898869e2f9fdb0515306664a857671/image-26.jpg)
Second-Order Reactions A product D[A] rate = Dt rate M/s = = 1/M • s k= 2 2 M [A] 1 1 = + kt [A]0 rate = k [A]2 D[A] = k [A]2 Dt [A] is the concentration of A at any time t [A]0 is the concentration of A at time t=0 t½ = t when [A] = [A]0/2 1 t½ = k[A]0 26
![Section 12 4 The Integrated Rate Law Plot of lnC 4 H 6 vs Section 12. 4 The Integrated Rate Law Plot of ln[C 4 H 6] vs](https://slidetodoc.com/presentation_image_h2/74898869e2f9fdb0515306664a857671/image-27.jpg)
Section 12. 4 The Integrated Rate Law Plot of ln[C 4 H 6] vs Time and Plot of 1/[C 4 H 6] vs Time

Section 12. 4 The Integrated Rate Law Second-Order § Half–Life: k = rate constant [A]o = initial concentration of A § Half–life gets longer as the reaction progresses and the concentration of reactants decrease. § Each successive half–life is double the preceding one. Copyright © Cengage Learning. All rights reserved 28

Section 12. 4 The Integrated Rate Law EXERCISE! For a reaction a. A Products, [A]0 = 5. 0 M, and the first two half-lives are 25 and 50 minutes, respectively. a) Write the rate law for this reaction. rate = k[A]2 b) Calculate k. k = 8. 0 × 10 -3 M– 1 min– 1 c) Calculate [A] at t = 525 minutes. [A] = 0. 23 M Copyright © Cengage Learning. All rights reserved 29
![Section 12 4 The Integrated Rate Law ZeroOrder Rate kA0 k Section 12. 4 The Integrated Rate Law Zero-Order § Rate = k[A]0 = k](https://slidetodoc.com/presentation_image_h2/74898869e2f9fdb0515306664a857671/image-30.jpg)
Section 12. 4 The Integrated Rate Law Zero-Order § Rate = k[A]0 = k § Integrated: [A] = –kt + [A]o [A] = concentration of A at time t k = rate constant t = time [A]o = initial concentration of A Copyright © Cengage Learning. All rights reserved 30
![Section 12 4 The Integrated Rate Law Plot of A vs Time Copyright Section 12. 4 The Integrated Rate Law Plot of [A] vs Time Copyright ©](https://slidetodoc.com/presentation_image_h2/74898869e2f9fdb0515306664a857671/image-31.jpg)
Section 12. 4 The Integrated Rate Law Plot of [A] vs Time Copyright © Cengage Learning. All rights reserved 31

Section 12. 4 The Integrated Rate Law Zero-Order § Half–Life: k = rate constant [A]o = initial concentration of A § Half–life gets shorter as the reaction progresses and the concentration of reactants decrease. Copyright © Cengage Learning. All rights reserved 32

Section 12. 4 The Integrated Rate Law CONCEPT CHECK! How can you tell the difference among 0 th, 1 st, and 2 nd order rate laws from their graphs? Copyright © Cengage Learning. All rights reserved 33

Section 12. 4 The Integrated Rate Laws To play movie you must be in Slide Show Mode PC Users: Please wait for content to load, then click to play Mac Users: CLICK HERE Copyright © Cengage Learning. All rights reserved 34

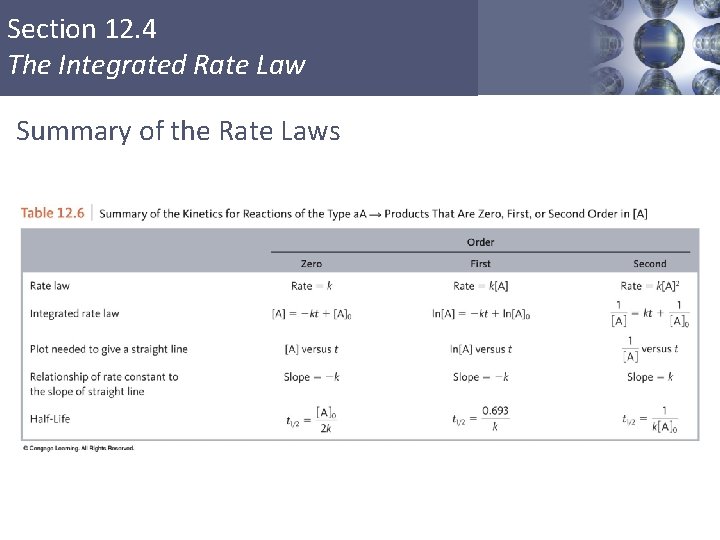
Section 12. 4 The Integrated Rate Law Summary of the Rate Laws Copyright © Cengage Learning. All rights reserved 35

Section 12. 4 The Integrated Rate Law EXERCISE! Consider the reaction a. A Products. [A]0 = 5. 0 M and k = 1. 0 × 10– 2 (assume the units are appropriate for each case). Calculate [A] after 30. 0 seconds have passed, assuming the reaction is: a) Zero order b) First order c) Second order Copyright © Cengage Learning. All rights reserved 4. 7 M 3. 7 M 2. 0 M 36

Section 12. 5 Reaction Mechanisms Reaction Mechanism § Most chemical reactions occur by a series of elementary steps. § An intermediate is formed in one step and used up in a subsequent step and thus is never seen as a product in the overall balanced reaction. Copyright © Cengage Learning. All rights reserved 37

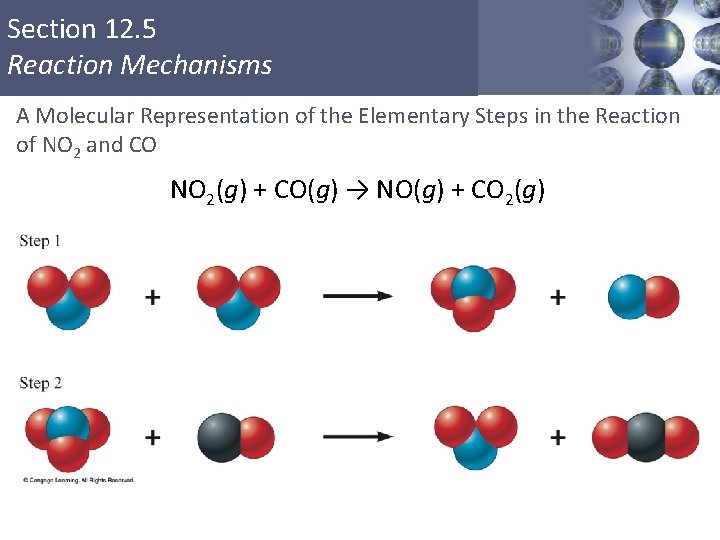
Section 12. 5 Reaction Mechanisms A Molecular Representation of the Elementary Steps in the Reaction of NO 2 and CO NO 2(g) + CO(g) → NO(g) + CO 2(g) Copyright © Cengage Learning. All rights reserved 38

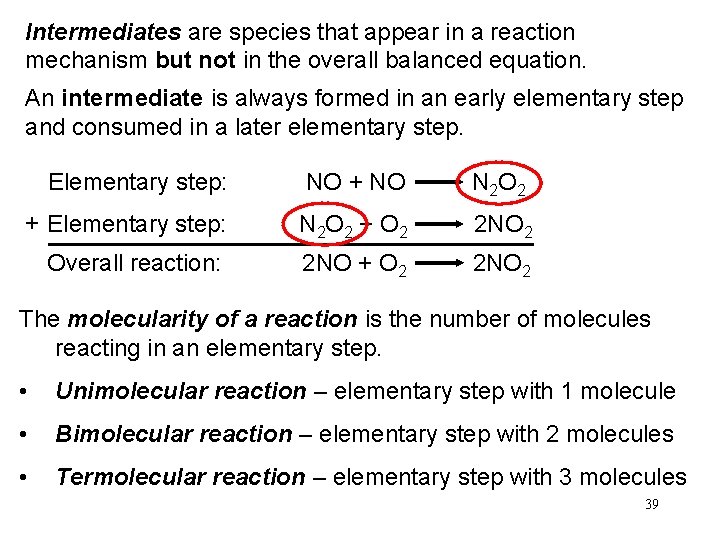
Intermediates are species that appear in a reaction mechanism but not in the overall balanced equation. An intermediate is always formed in an early elementary step and consumed in a later elementary step. Elementary step: NO + NO N 2 O 2 + Elementary step: N 2 O 2 + O 2 2 NO 2 Overall reaction: 2 NO + O 2 2 NO 2 The molecularity of a reaction is the number of molecules reacting in an elementary step. • Unimolecular reaction – elementary step with 1 molecule • Bimolecular reaction – elementary step with 2 molecules • Termolecular reaction – elementary step with 3 molecules 39

Section 12. 5 Reaction Mechanisms Elementary Steps (Molecularity) § Unimolecular – reaction involving one molecule; first order. § Bimolecular – reaction involving the collision of two species; second order. § Termolecular – reaction involving the collision of three species; third order. Very rare. Copyright © Cengage Learning. All rights reserved 40
![Rate Laws and Elementary Steps Unimolecular reaction A products rate k A Bimolecular Rate Laws and Elementary Steps Unimolecular reaction A products rate = k [A] Bimolecular](https://slidetodoc.com/presentation_image_h2/74898869e2f9fdb0515306664a857671/image-41.jpg)
Rate Laws and Elementary Steps Unimolecular reaction A products rate = k [A] Bimolecular reaction A+B products rate = k [A][B] Bimolecular reaction A+A products rate = k [A]2 Writing plausible reaction mechanisms: • The sum of the elementary steps must give the overall balanced equation for the reaction. • The rate-determining step should predict the same rate law that is determined experimentally. The rate-determining step is the slowest step in the sequence of steps leading to product formation. 41

Section 12. 5 Reaction Mechanisms Rate-Determining Step § A reaction is only as fast as its slowest step. § The rate-determining step (slowest step) determines the rate law and the molecularity of the overall reaction. Copyright © Cengage Learning. All rights reserved 42

Section 12. 5 Reaction Mechanisms Reaction Mechanism Requirements § The sum of the elementary steps must give the overall balanced equation for the reaction. § The mechanism must agree with the experimentally determined rate law. Copyright © Cengage Learning. All rights reserved 43

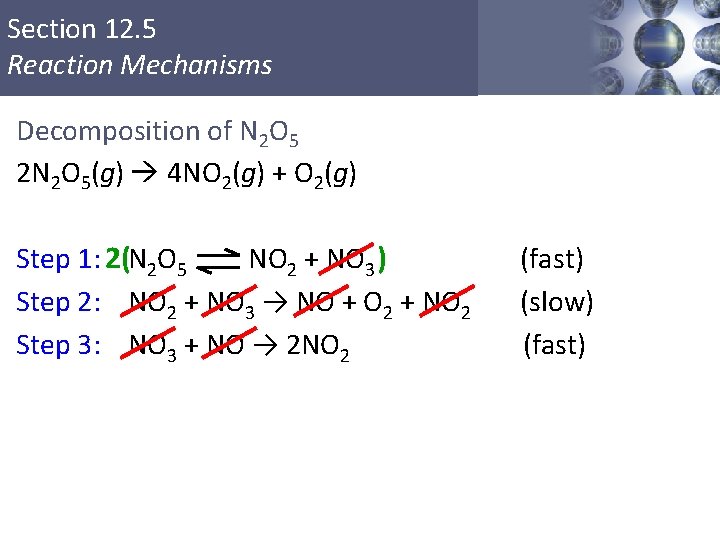
Section 12. 5 Reaction Mechanisms Decomposition of N 2 O 5 To play movie you must be in Slide Show Mode PC Users: Please wait for content to load, then click to play Mac Users: CLICK HERE Copyright © Cengage Learning. All rights reserved 44

Section 12. 5 Reaction Mechanisms Decomposition of N 2 O 5 2 N 2 O 5(g) 4 NO 2(g) + O 2(g) Step 1: 2(N 2 O 5 NO 2 + NO 3 ) Step 2: NO 2 + NO 3 → NO + O 2 + NO 2 Step 3: NO 3 + NO → 2 NO 2 Copyright © Cengage Learning. All rights reserved (fast) (slow) (fast) 45

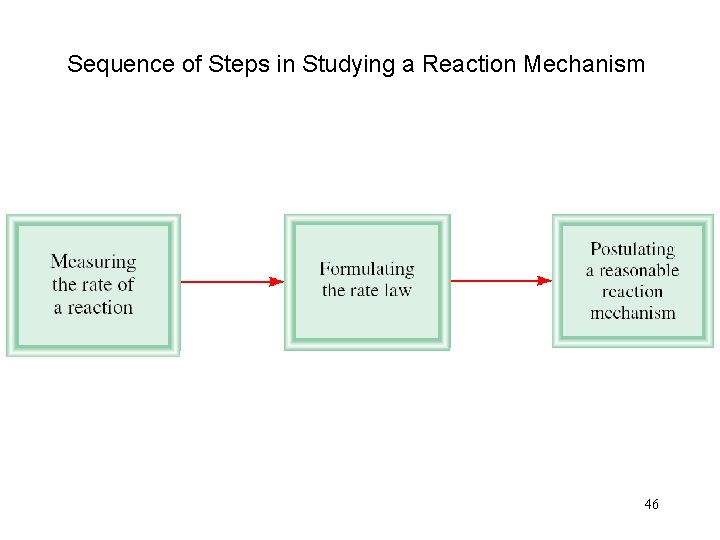
Sequence of Steps in Studying a Reaction Mechanism 46

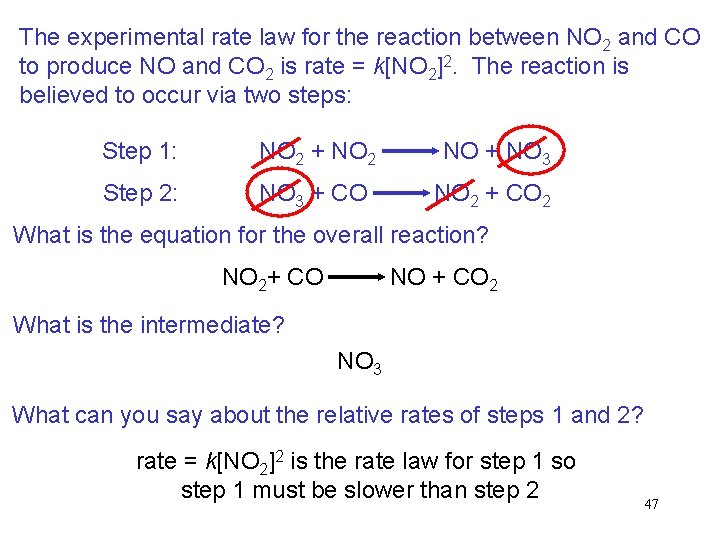
The experimental rate law for the reaction between NO 2 and CO to produce NO and CO 2 is rate = k[NO 2]2. The reaction is believed to occur via two steps: Step 1: NO 2 + NO 2 NO + NO 3 Step 2: NO 3 + CO NO 2 + CO 2 What is the equation for the overall reaction? NO 2+ CO NO + CO 2 What is the intermediate? NO 3 What can you say about the relative rates of steps 1 and 2? rate = k[NO 2]2 is the rate law for step 1 so step 1 must be slower than step 2 47

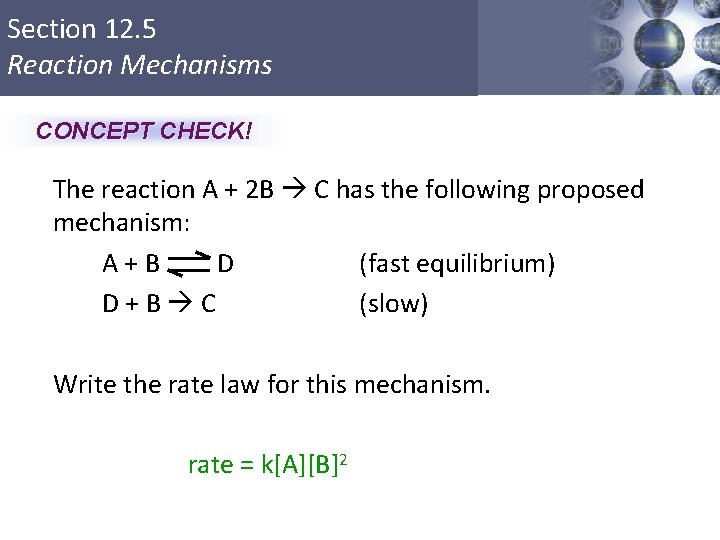
Section 12. 5 Reaction Mechanisms CONCEPT CHECK! The reaction A + 2 B C has the following proposed mechanism: A+B D (fast equilibrium) D+B C (slow) Write the rate law for this mechanism. rate = k[A][B]2 Copyright © Cengage Learning. All rights reserved 48

Section 12. 6 A Model for Chemical Kinetics Collision Model § Molecules must collide to react. § Main Factors: § Activation energy, Ea § Temperature § Molecular orientations Copyright © Cengage Learning. All rights reserved 49

Section 12. 6 A Model for Chemical Kinetics Activation Energy, Ea § Energy that must be overcome to produce a chemical reaction. Copyright © Cengage Learning. All rights reserved 50

Section 12. 6 A Model for Chemical Kinetics Transition States and Activation Energy To play movie you must be in Slide Show Mode PC Users: Please wait for content to load, then click to play Mac Users: CLICK HERE Copyright © Cengage Learning. All rights reserved 51

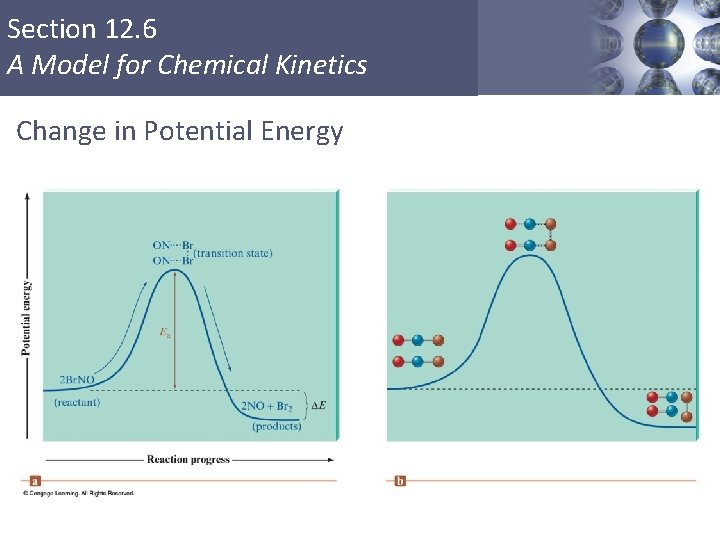
Section 12. 6 A Model for Chemical Kinetics Change in Potential Energy Copyright © Cengage Learning. All rights reserved 52

Section 12. 6 A Model for Chemical Kinetics For Reactants to Form Products § Collision must involve enough energy to produce the reaction (must equal or exceed the activation energy). § Relative orientation of the reactants must allow formation of any new bonds necessary to produce products. Copyright © Cengage Learning. All rights reserved 53

Section 12. 6 A Model for Chemical Kinetics The Gas Phase Reaction of NO and Cl 2 To play movie you must be in Slide Show Mode PC Users: Please wait for content to load, then click to play Mac Users: CLICK HERE Copyright © Cengage Learning. All rights reserved 54

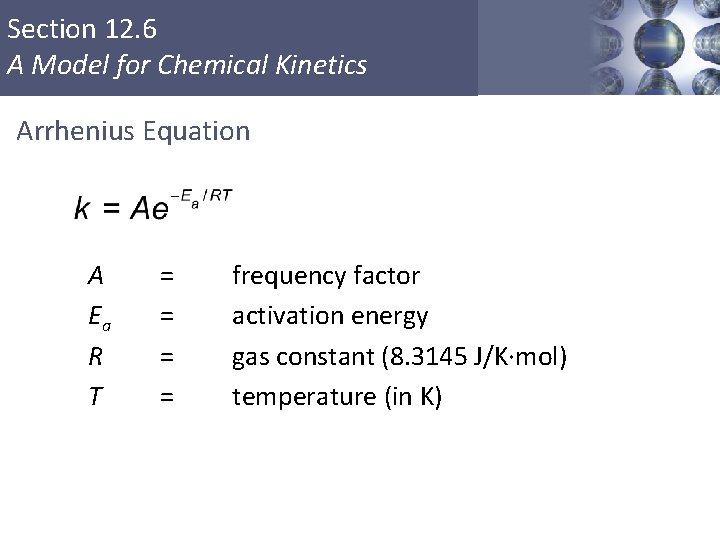
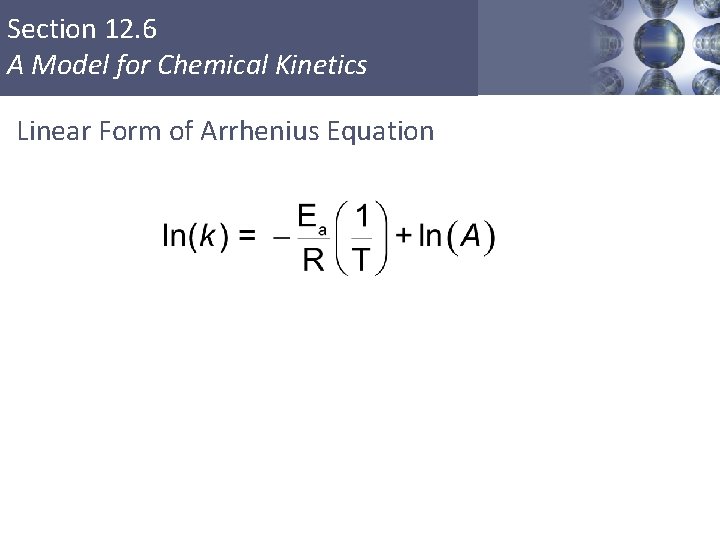
Section 12. 6 A Model for Chemical Kinetics Arrhenius Equation A Ea R T = = Copyright © Cengage Learning. All rights reserved frequency factor activation energy gas constant (8. 3145 J/K·mol) temperature (in K) 55

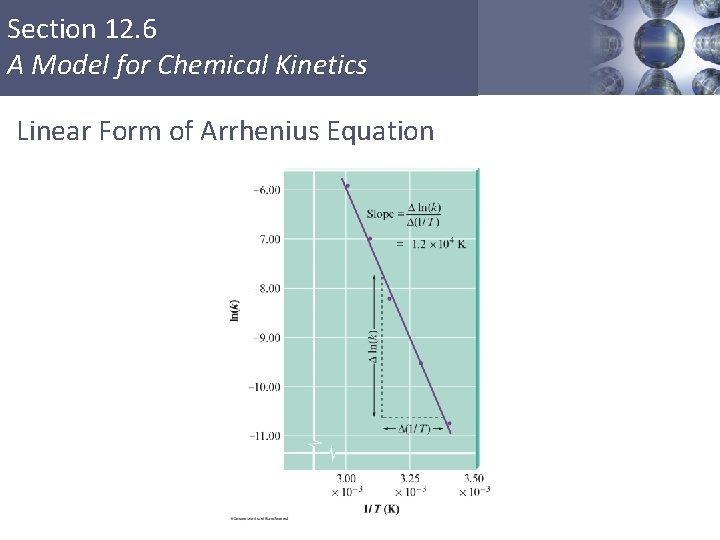
Section 12. 6 A Model for Chemical Kinetics Linear Form of Arrhenius Equation Copyright © Cengage Learning. All rights reserved 56

Section 12. 6 A Model for Chemical Kinetics Linear Form of Arrhenius Equation Copyright © Cengage Learning. All rights reserved 57

Section 12. 6 A Model for Chemical Kinetics EXERCISE! Chemists commonly use a rule of thumb that an increase of 10 K in temperature doubles the rate of a reaction. What must the activation energy be for this statement to be true for a temperature increase from 25°C to 35°C? Ea = 53 k. J Copyright © Cengage Learning. All rights reserved 58

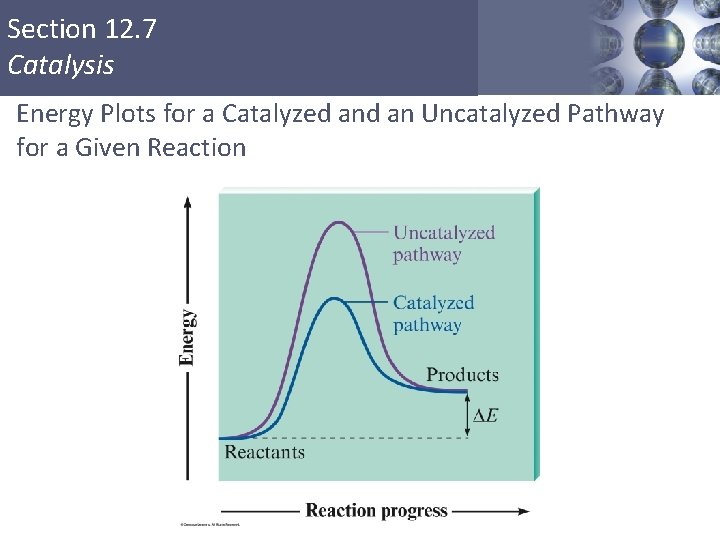
Section 12. 7 Catalysis Catalyst § A substance that speeds up a reaction without being consumed itself. § Provides a new pathway for the reaction with a lower activation energy. Copyright © Cengage Learning. All rights reserved 59

Section 12. 7 Catalysis Energy Plots for a Catalyzed an Uncatalyzed Pathway for a Given Reaction Copyright © Cengage Learning. All rights reserved 60

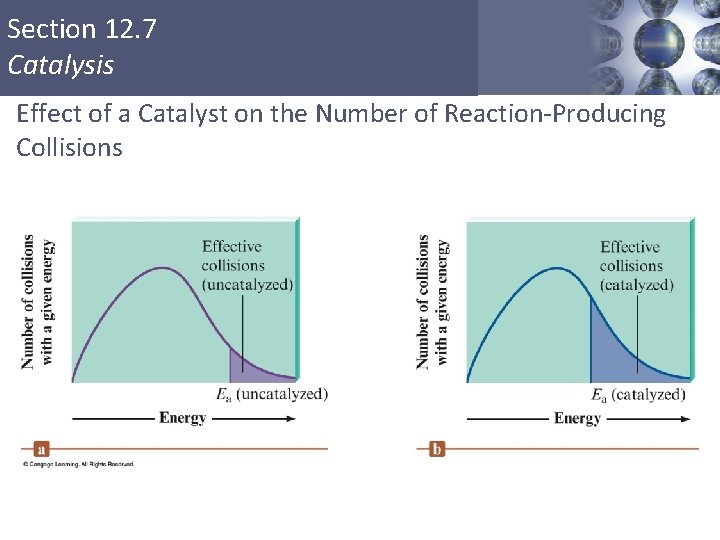
Section 12. 7 Catalysis Effect of a Catalyst on the Number of Reaction-Producing Collisions Copyright © Cengage Learning. All rights reserved 61

Section 12. 7 Catalysis Heterogeneous Catalyst § Most often involves gaseous reactants being adsorbed on the surface of a solid catalyst. § Adsorption – collection of one substance on the surface of another substance. Copyright © Cengage Learning. All rights reserved 62

Section 12. 7 Catalysis Heterogeneous Catalysis To play movie you must be in Slide Show Mode PC Users: Please wait for content to load, then click to play Mac Users: CLICK HERE Copyright © Cengage Learning. All rights reserved 63

Section 12. 7 Catalysis Heterogeneous Catalyst 1. 2. 3. 4. Adsorption and activation of the reactants. Migration of the adsorbed reactants on the surface. Reaction of the adsorbed substances. Escape, or desorption, of the products. Copyright © Cengage Learning. All rights reserved 64

Section 12. 7 Catalysis Homogeneous Catalyst § Exists in the same phase as the reacting molecules. § Enzymes are nature’s catalysts. Copyright © Cengage Learning. All rights reserved 65

Section 12. 7 Catalysis Homogeneous Catalysis To play movie you must be in Slide Show Mode PC Users: Please wait for content to load, then click to play Mac Users: CLICK HERE Copyright © Cengage Learning. All rights reserved 66