Chapter 12 Chemical Bonding How do bonds work
Chapter 12: Chemical Bonding

How do bonds work? Bonding is all about electrons. Atoms either share or donate electrons to each other in a bond in order to have eight electrons
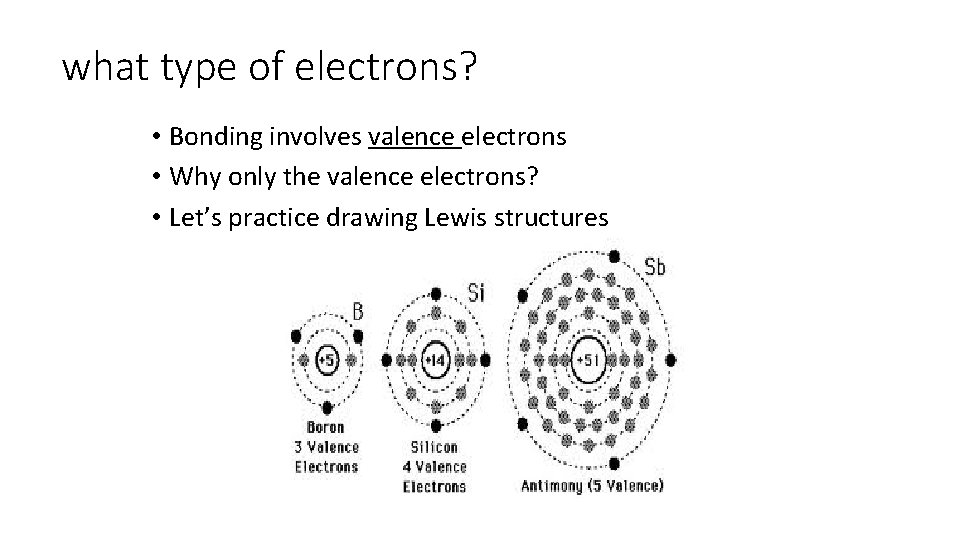
what type of electrons? • Bonding involves valence electrons • Why only the valence electrons? • Let’s practice drawing Lewis structures
What determines the type of bond? Bond type is determined by differences in electronegativity. Electronegativity – The ability of an atom to attract electrons in a bond. In other words how greedy an atom is for electrons.
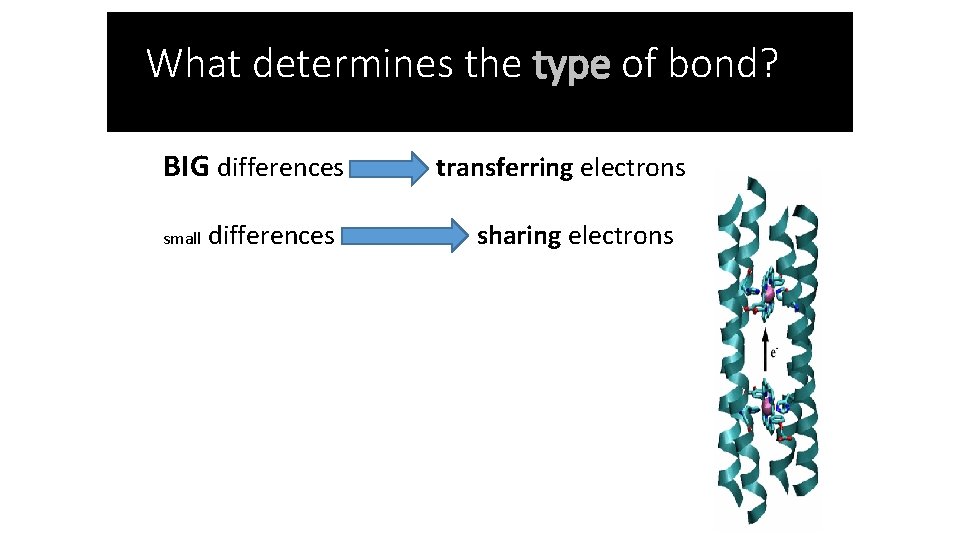
What determines the type of bond? BIG differences small differences transferring electrons sharing electrons

• Ionic Bonding – a bond between a metal and a nonmetal where one atom accepts/gains an electron and the other donates/loses an electron • The more electronegative, nonmetal element ACCEPTS electron(s) • The less electronegative, metal element DONATES electron(s) • This is due to a BIG difference in electronegativity.
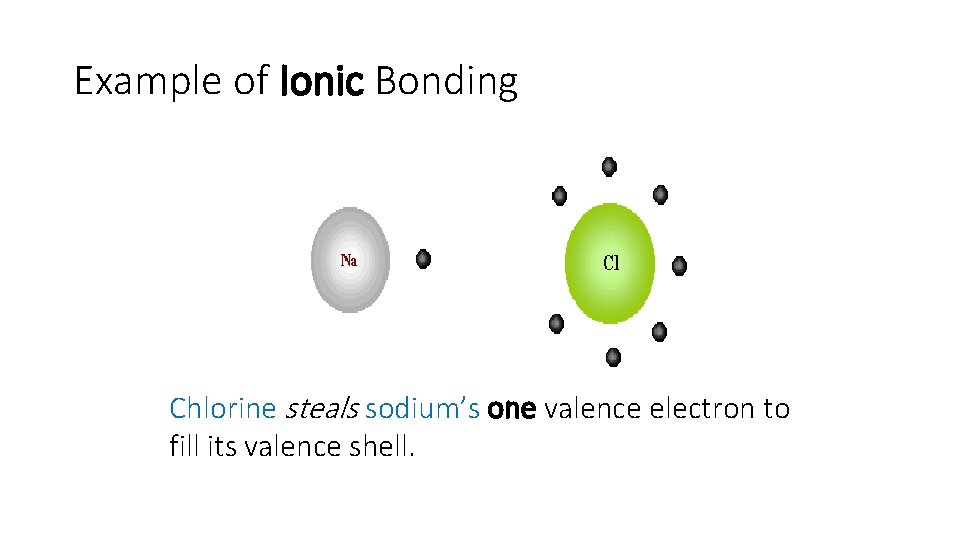
Example of Ionic Bonding Chlorine steals sodium’s one valence electron to fill its valence shell.
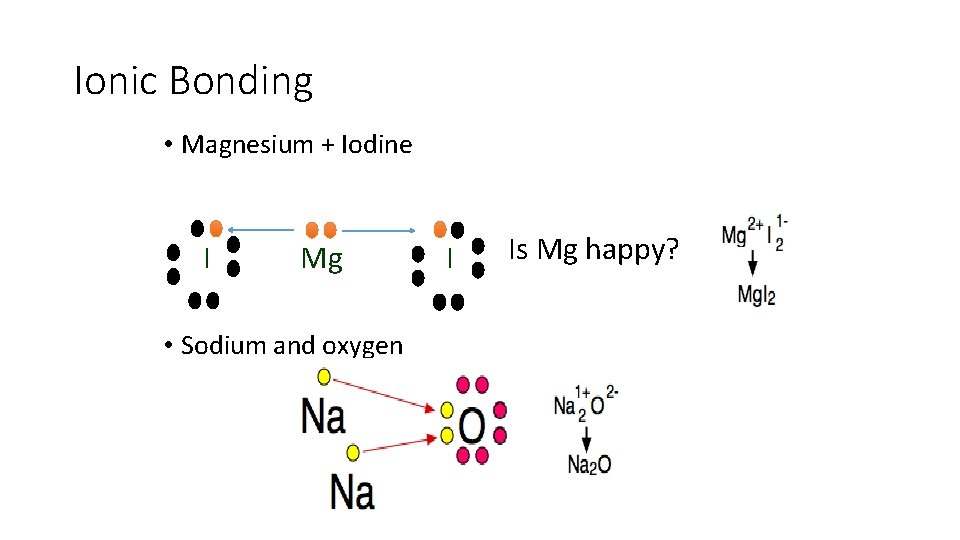
Ionic Bonding • Magnesium + Iodine I Mg • Sodium and oxygen I Is Mg happy?
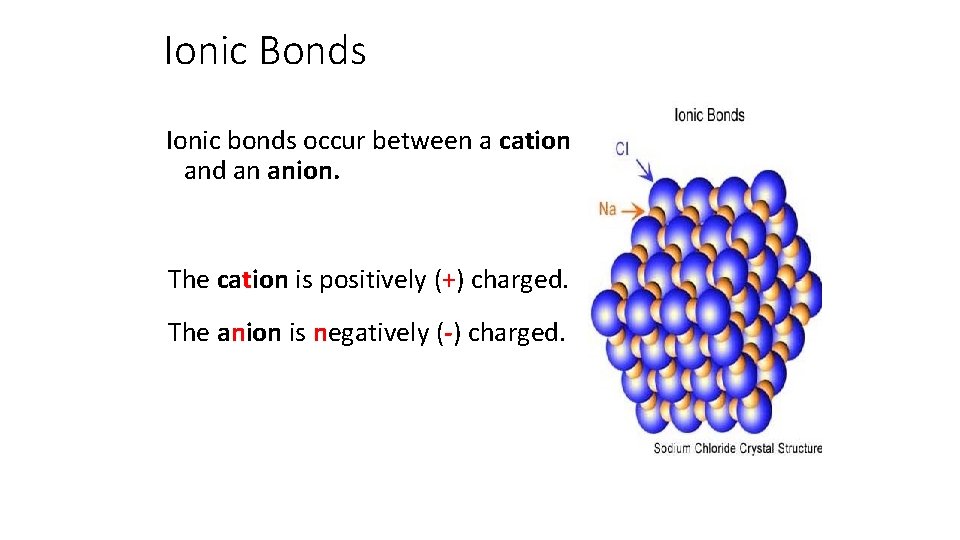
Ionic Bonds Ionic bonds occur between a cation and an anion. The cation is positively (+) charged. The anion is negatively (-) charged.

Properties of Ionic Bonds • Electrons are transferred between a metal and a non-metal. • Ionic solids are crystal solids at room temperature. • High melting points • Good conductors when dissolved. • Have a positive and negative charge.
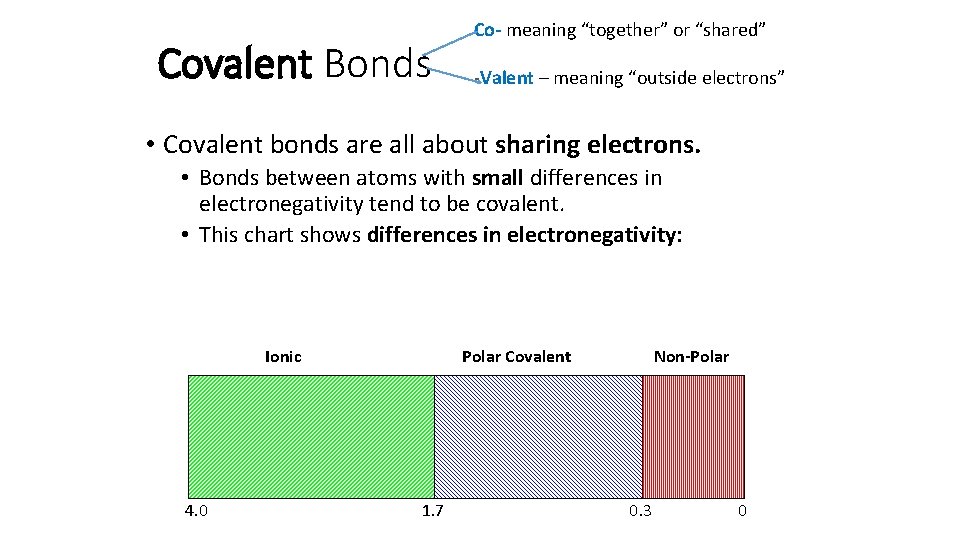
Covalent Bonds Co- meaning “together” or “shared” -Valent – meaning “outside electrons” • Covalent bonds are all about sharing electrons. • Bonds between atoms with small differences in electronegativity tend to be covalent. • This chart shows differences in electronegativity: Ionic 4. 0 Polar Covalent 1. 7 Non-Polar 0. 3 0
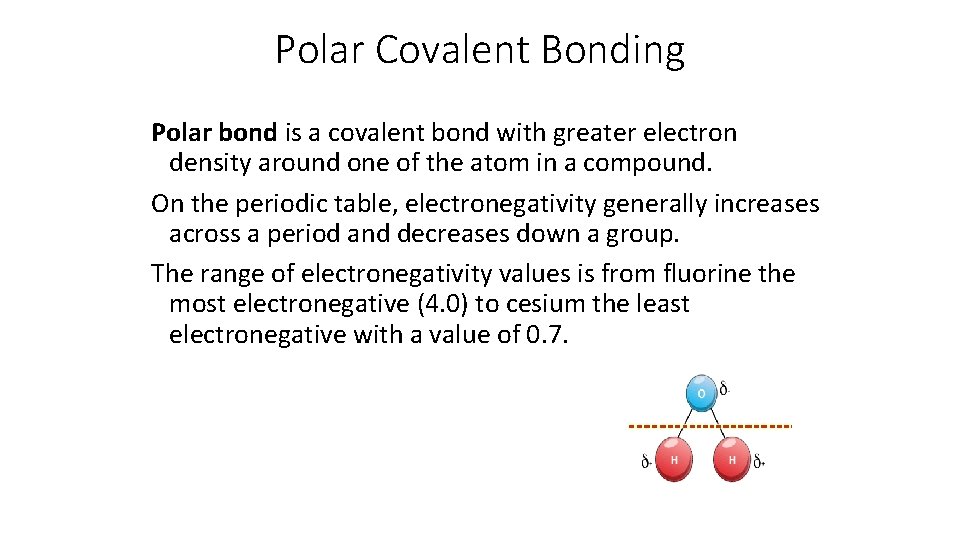
Polar Covalent Bonding Polar bond is a covalent bond with greater electron density around one of the atom in a compound. On the periodic table, electronegativity generally increases across a period and decreases down a group. The range of electronegativity values is from fluorine the most electronegative (4. 0) to cesium the least electronegative with a value of 0. 7.
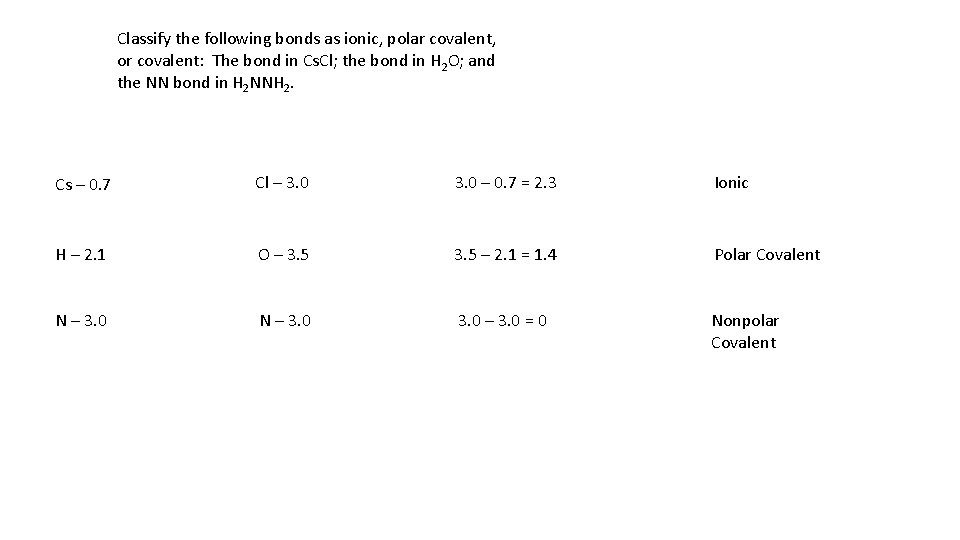
Classify the following bonds as ionic, polar covalent, or covalent: The bond in Cs. Cl; the bond in H 2 O; and the NN bond in H 2 NNH 2. Cs – 0. 7 Cl – 3. 0 – 0. 7 = 2. 3 Ionic H – 2. 1 O – 3. 5 – 2. 1 = 1. 4 Polar Covalent N – 3. 0 – 3. 0 = 0 Nonpolar Covalent
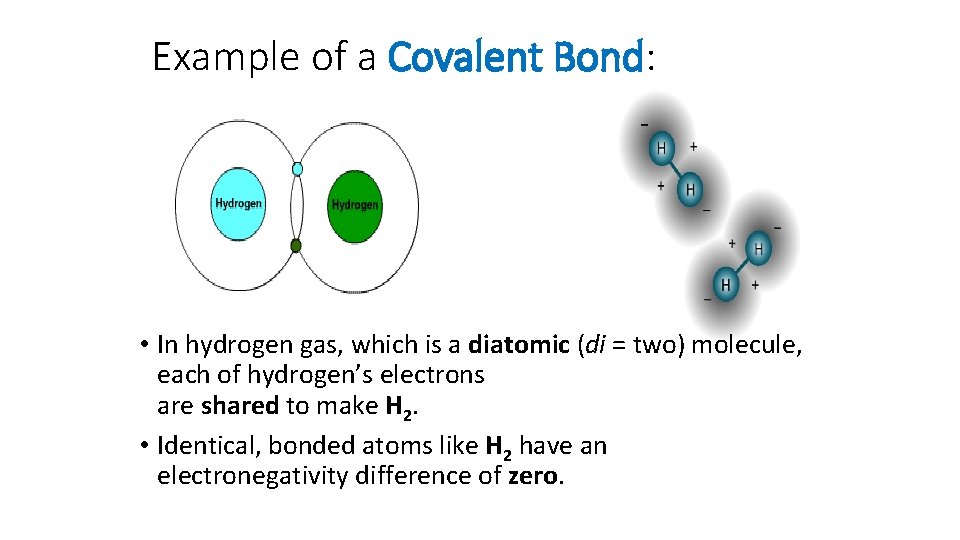
Example of a Covalent Bond: • In hydrogen gas, which is a diatomic (di = two) molecule, each of hydrogen’s electrons are shared to make H 2. • Identical, bonded atoms like H 2 have an electronegativity difference of zero.
Properties of Covalent Bonds • Covalent bonds happen between atoms on the same side of the periodic table. • They tend to have: • Lower melting and boiling points • Can exist at any state/phase at room temp. • Poor conductivity (electrons can’t move easily)
![Na+: [Ne] Al 3+: [Ne] O 2 -: [Ne] F-: [Ne] N 3 -: Na+: [Ne] Al 3+: [Ne] O 2 -: [Ne] F-: [Ne] N 3 -:](http://slidetodoc.com/presentation_image_h2/6d21505d7afa26bd137bd3b676e74cde/image-16.jpg)
Na+: [Ne] Al 3+: [Ne] O 2 -: [Ne] F-: [Ne] N 3 -: [Ne] Na+, Al 3+, F-, O 2 -, and N 3 - are all isoelectronic with Ne What neutral atom is isoelectronic with H- ? same electron configuration as He 8. 2
Lewis Structure Shows how valence electrons are arranged among atoms in a molecule& attains stability by having the noble gas electron configuration. Hydrogen forms stable molecules where it shares two electrons(Duet Rule). Most elements form stable molecules by attaining eight electrons (Octet Rule).
Covalent Bonds Single Covalent Bond: A covalent bond in which two atoms share one pair of electrons. Ex: H 2, F 2 Double Covalent Bond: A covalent bond in which two atoms share two pairs of electrons. Ex: O 2 Triple Covalent Bond: A covalent bond in which two atoms share three pairs of electrons. Ex: N 2

Steps for Writing Lewis Structures 1. Sum the valence electrons from all the atoms. • • If it is an anion, add one electron for each negative charge. If it is a cation, subtract one electron for each positive charge. 2. Draw skeletal structure of compound showing what atoms are bonded to each other. Keep the least electronegative element in the center. 3. Atoms usually have noble gas configurations. Arrange the remaining electrons to satisfy the octet rule or duet rule for hydrogen. 4. If the structure contains too many electrons, form double or triple bonds on the central atom.
Lewis Dot Structure Ex: Draw the Lewis Dot Structures for 1) CCl 4 2) CO 2 3) BCl 3 4) H 2 O 5) NH 4+
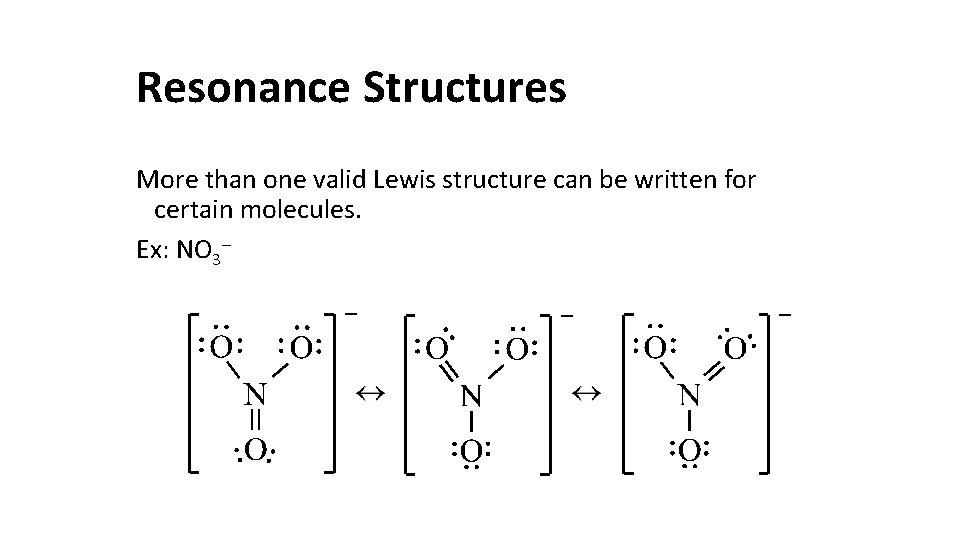
Resonance Structures More than one valid Lewis structure can be written for certain molecules. Ex: NO 3–
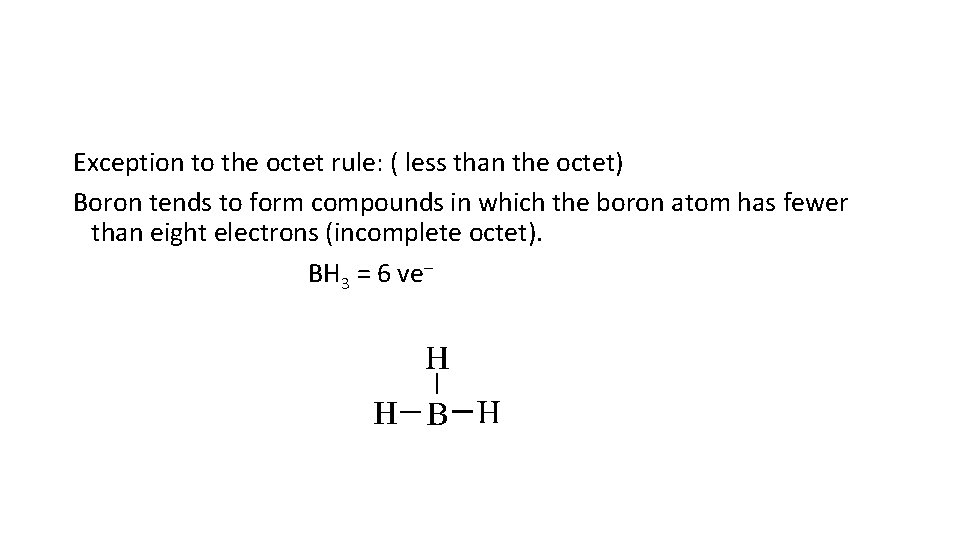
Exception to the octet rule: ( less than the octet) Boron tends to form compounds in which the boron atom has fewer than eight electrons (incomplete octet). BH 3 = 6 ve–
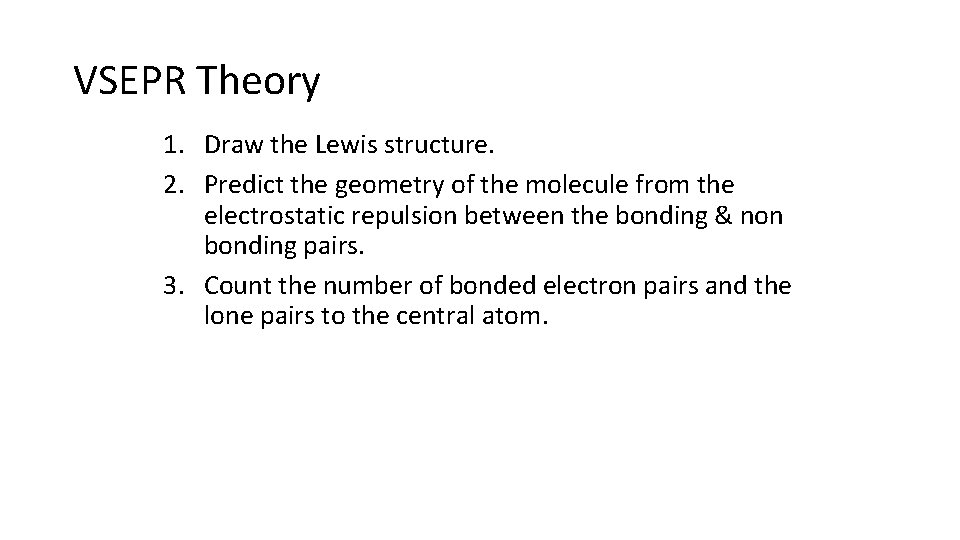
VSEPR Theory 1. Draw the Lewis structure. 2. Predict the geometry of the molecule from the electrostatic repulsion between the bonding & non bonding pairs. 3. Count the number of bonded electron pairs and the lone pairs to the central atom.

VSEPR Theory The theory depicts the orientation of bonds and how molecules form. Orientations vary based on the number of non-bonded or lone pair electrons present in the molecule. Angles also change due to the number of lone pairs of electrons.
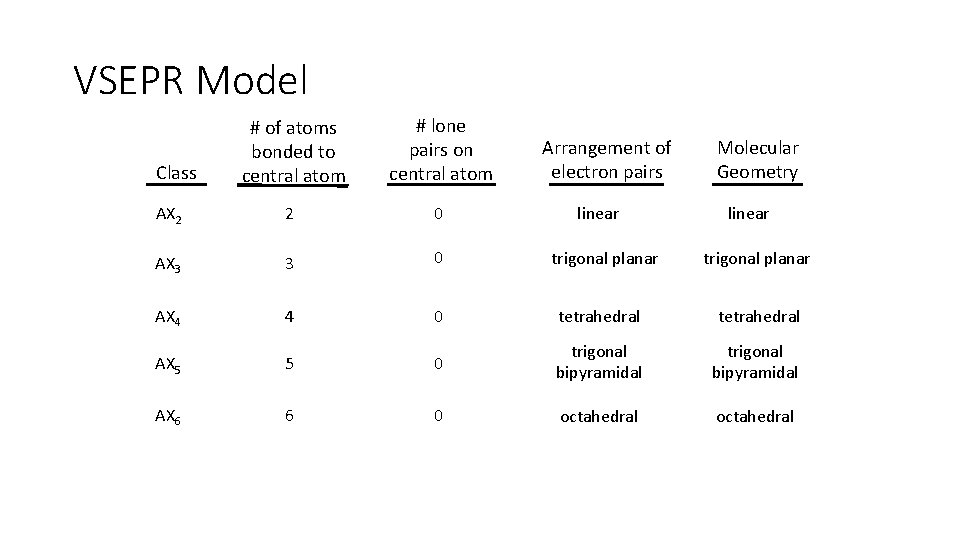
VSEPR Model # of atoms bonded to central atom # lone pairs on central atom AX 2 2 0 AX 3 3 0 AX 4 4 0 tetrahedral AX 5 5 0 trigonal bipyramidal AX 6 6 0 octahedral Class Arrangement of electron pairs linear trigonal planar Molecular Geometry linear trigonal planar
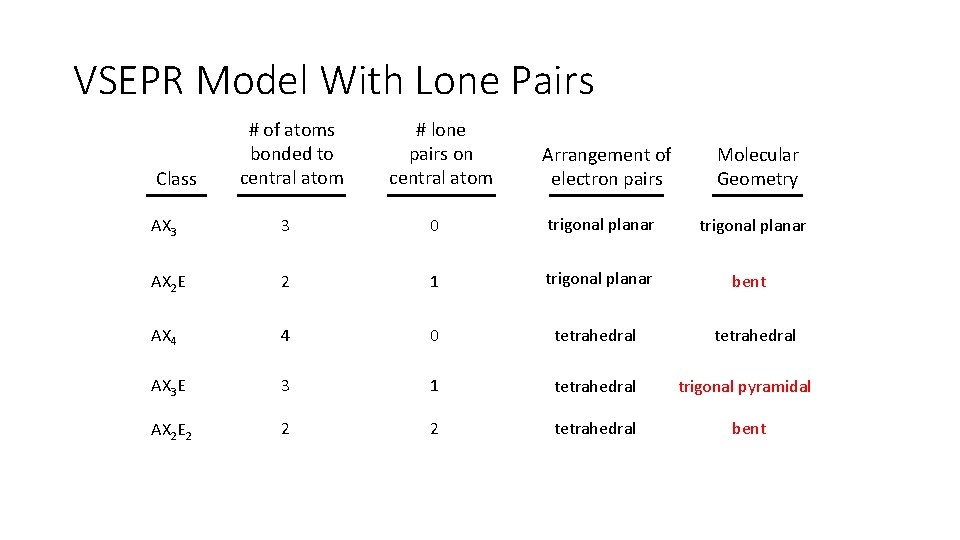
VSEPR Model With Lone Pairs Class # of atoms bonded to central atom # lone pairs on central atom Arrangement of electron pairs Molecular Geometry AX 3 3 0 trigonal planar AX 2 E 2 1 trigonal planar bent AX 4 4 0 tetrahedral AX 3 E 3 1 tetrahedral trigonal pyramidal AX 2 E 2 2 2 tetrahedral bent tetrahedral
- Slides: 26