Chapter 12 Celsius and Fahrenheit scales are the








- Slides: 15

Chapter 12

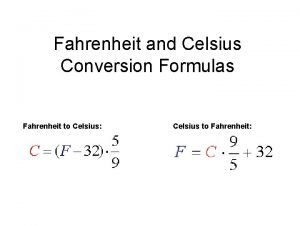
� Celsius and Fahrenheit scales are the two most commonly used scales. � They were both designed with reference to the freezing point and boiling point of water � Positions were marked on a thermometer for these two temperatures and graduates were made in equal increments � Celsius is used more often around the world

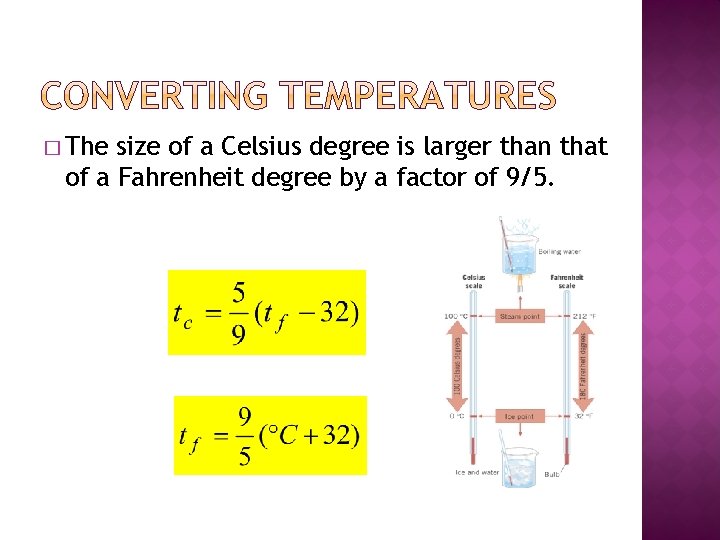
� The size of a Celsius degree is larger than that of a Fahrenheit degree by a factor of 9/5.

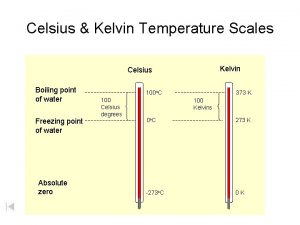
� Introduced by William Thompson (Lord Kelvin) � Not expressed with the word “degrees” � SI base unit for temperature � Based on the concept of absolute zero.

� Measure changes in thermometric properties � Several types exist including �Thermocouple �Electric resistance thermometers �Thermograph or thermogram � Type needed depends on goal of research and necessary procedure � Here, we will use a regular mercury or alcohol thermometer.

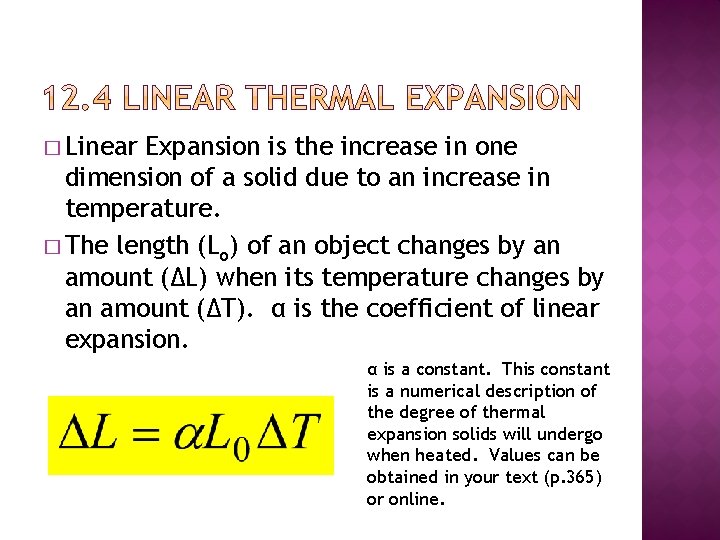
� Linear Expansion is the increase in one dimension of a solid due to an increase in temperature. � The length (Lo) of an object changes by an amount (∆L) when its temperature changes by an amount (∆T). α is the coefficient of linear expansion. α is a constant. This constant is a numerical description of the degree of thermal expansion solids will undergo when heated. Values can be obtained in your text (p. 365) or online.

� Thermal Stress – buildings using steel supports must account for expansion in order to prevent “buckling” of steel beams � Bimetallic Strip – used in coffee pots, thermostats, etc. � Expansion of Holes – A hole in a piece of solid material expands when heated and contracts when cooled, just as if it were filled with the material that surrounds it. � Holes with larger coefficients of linear expansion expand more than those in materials with smaller coefficients.

� It makes sense that if the length of an object expands when heated, the volume would too. � The volume (V 0) of an object changes by an amount (∆V) when its temperature changes by an amount (∆T). β is the coefficient of volume expansion.

� If water at 0°C is heated, its volume decreases until the temperature reaches 4°C. � Above 4°C, water behaves normally and volume will increase as temperature increases. � The density of water is greatest at 4°C. Click here to find out why volume of ice is greater than volume of water.

� Heat is energy that flows from a highertemperature object to a lower-temperature object because of the difference in temperatures. � SI Unit: joule (J) � Substances DO NOT contain heat. Instead, they contain internal kinetic energy due to the motion of their molecules or atoms. � Heat is only used to describe the energy in transit from one substance to another.

� The heat (Q) that must be supplied or removed to change the temperature of a substance of mass (m) by an amount (∆T) is • c is the specific heat capacity of the substance. • Basically, this constant describes how quickly or slowly a substance will change temperature 1 kcal = 4186 joules 1 cal = 4. 186 joules

� Remember: In a closed system, energy is neither created nor destroyed. � A calorimeter is a container used to determine the specific heat capacity of a substance. � In following the law of conservation of energy we can say that heat lost = heat gained.

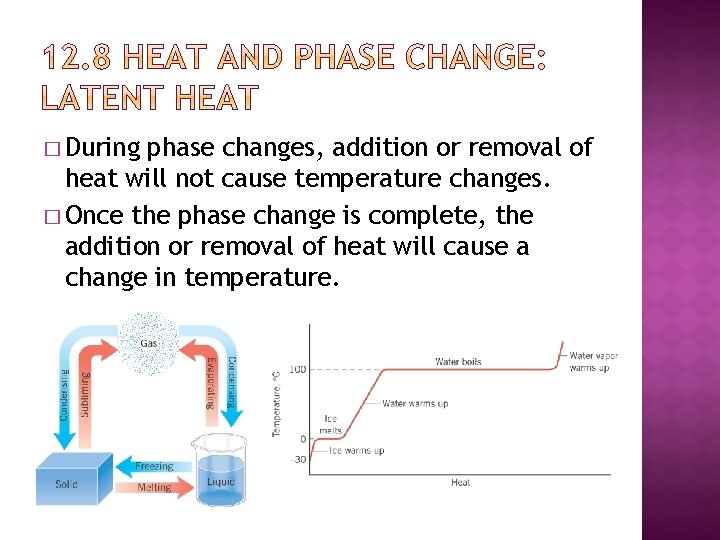
� During phase changes, addition or removal of heat will not cause temperature changes. � Once the phase change is complete, the addition or removal of heat will cause a change in temperature.

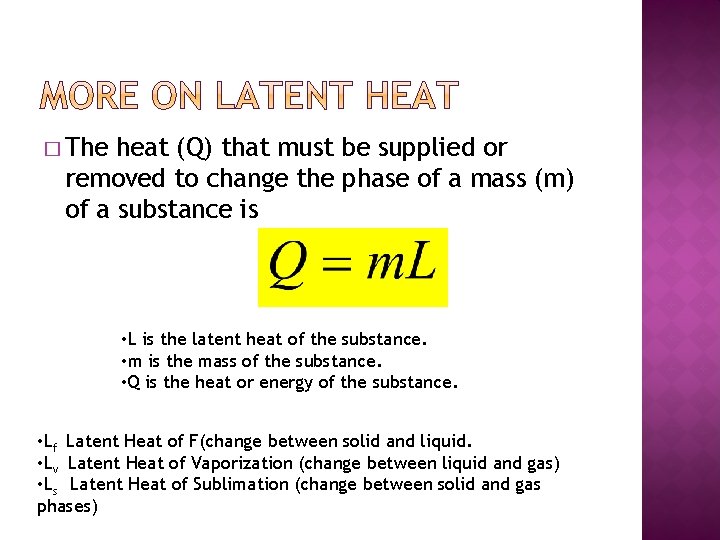
� The heat (Q) that must be supplied or removed to change the phase of a mass (m) of a substance is • L is the latent heat of the substance. • m is the mass of the substance. • Q is the heat or energy of the substance. • Lf Latent Heat of F(change between solid and liquid. • Lv Latent Heat of Vaporization (change between liquid and gas) • Ls Latent Heat of Sublimation (change between solid and gas phases)

�Q is the symbol for heat. Heat is an exchange of energy. � In this chapter you were given several equations for heat (Q). � Remember that Q = Q and these equations can be set equal to one another in many cases. � Substance react differently to changes in temperature and experience different energy exchanges (heat) depending on their chemical composition.
 Mikael ferm
Mikael ferm Gabriel fahrenheit and anders celsius
Gabriel fahrenheit and anders celsius Temperature conversion formula
Temperature conversion formula 12 centigrade to fahrenheit
12 centigrade to fahrenheit 451 degrees fahrenheit
451 degrees fahrenheit 25celsius to fahrenheit
25celsius to fahrenheit Decimal system of measurement
Decimal system of measurement Fahrenheit to celsius
Fahrenheit to celsius Fahrenheit to celsius
Fahrenheit to celsius 70o fahrenheit to celsius
70o fahrenheit to celsius 32 fahrenheit to celsius
32 fahrenheit to celsius Fahrenheit to celsius formula in c
Fahrenheit to celsius formula in c 350 fahrenheit to celsius
350 fahrenheit to celsius 320 fahrenheit to celsius
320 fahrenheit to celsius 320 fahrenheit to celsius
320 fahrenheit to celsius 320 fahrenheit to celsius
320 fahrenheit to celsius