Chapter 12 Atomic Xray Spectrometry Xrays are short







- Slides: 29

Chapter 12 Atomic X-ray Spectrometry X-rays are short wavelength electromagnetic radiation produced by the deceleration of high energy electrons or by electronic transitions of electrons in the inner orbitals of atoms. l = 0. 1 Å ~ 25 Å X-ray emission X-ray fluorescence (XRF) X-ray absorption (XAS) Diffraction of X-ray 歐亞書局 2021/3/8 1

12 A Fundamental Principles 12 A-1 Emission of X-rays: continuum (white radiation) and line spectra. Continuum radiation results from collision between the electrons of the beam and the atoms of the target material (a series of collisions). Short-wavelength limit (l 0): depends on accelerating voltage, but independent of target material. (0. 35 Å at 35 k. V for both tungsten and molybdenum) hn 0 = hc/l 0 = Ve (Duane-Hunt law) l 0 (Å) = 12398/V FIGURE 12 -1 Distribution of continuum radiation from an X-ray tube with a tungsten target. The numbers above the curves indicate the accelerating voltages. 歐亞書局 Ch 12 Atomic X-ray Spectrometry P. 275

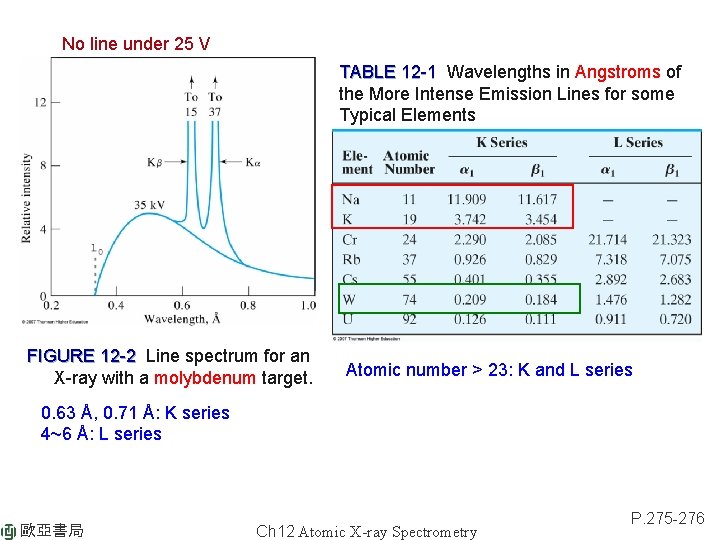
No line under 25 V TABLE 12 -1 Wavelengths in Angstroms of the More Intense Emission Lines for some Typical Elements FIGURE 12 -2 Line spectrum for an X-ray with a molybdenum target. Atomic number > 23: K and L series 0. 63 Å, 0. 71 Å: K series 4~6 Å: L series 歐亞書局 Ch 12 Atomic X-ray Spectrometry P. 275 -276

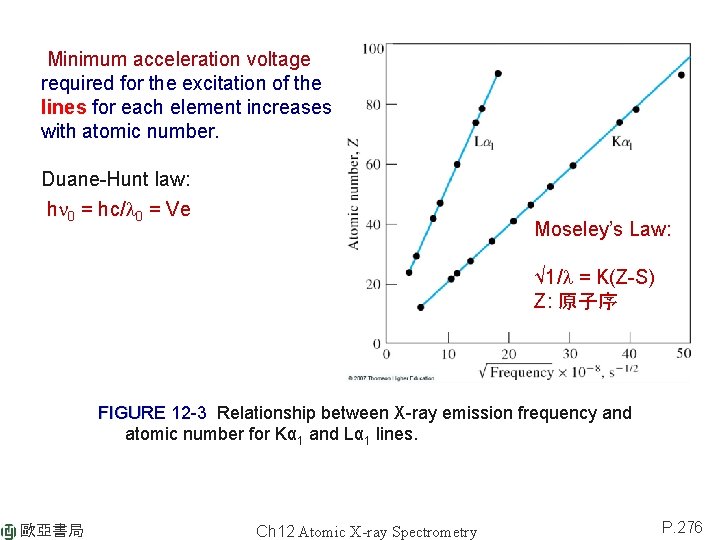
Minimum acceleration voltage required for the excitation of the lines for each element increases with atomic number. Duane-Hunt law: hn 0 = hc/l 0 = Ve Moseley’s Law: √ 1/l = K(Z-S) Z: 原子序 FIGURE 12 -3 Relationship between X-ray emission frequency and atomic number for Kα 1 and Lα 1 lines. 歐亞書局 Ch 12 Atomic X-ray Spectrometry P. 276


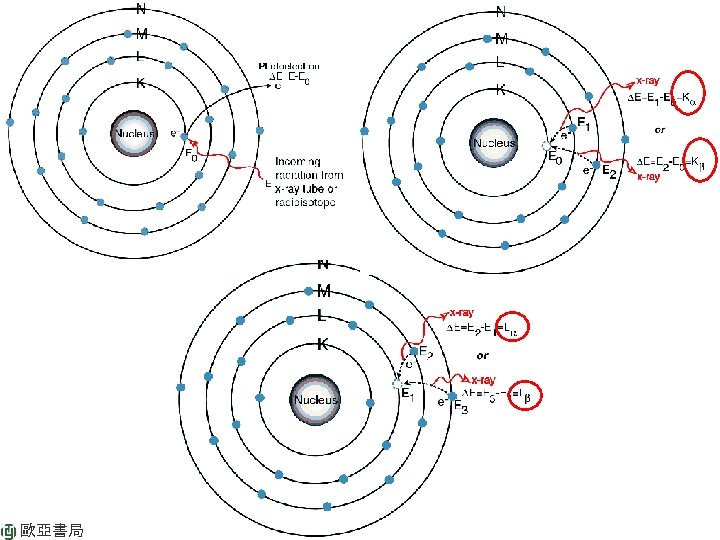
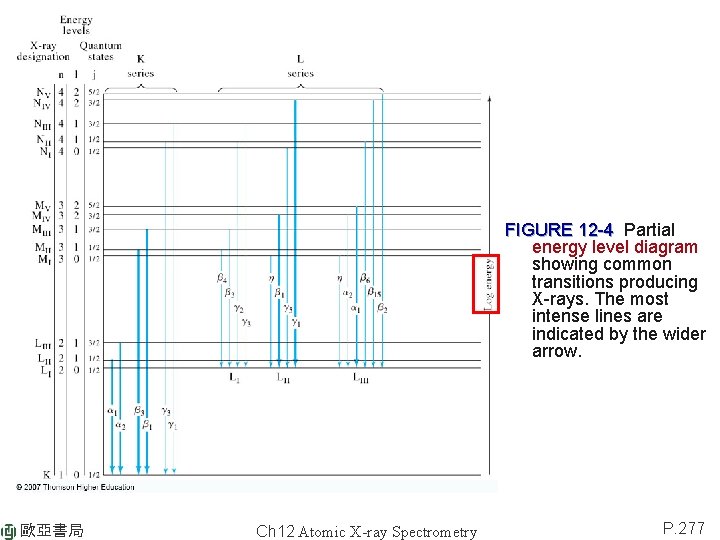
FIGURE 12 -4 Partial energy level diagram showing common transitions producing X-rays. The most intense lines are indicated by the wider arrow. 歐亞書局 Ch 12 Atomic X-ray Spectrometry P. 277

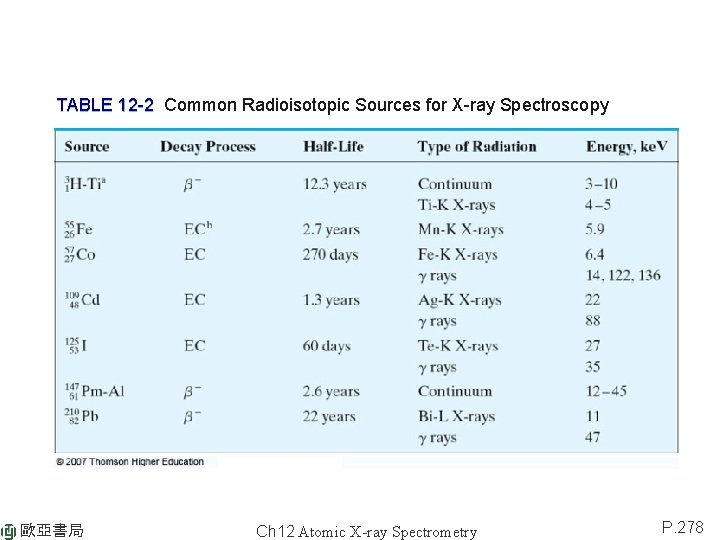
TABLE 12 -2 Common Radioisotopic Sources for X-ray Spectroscopy 歐亞書局 Ch 12 Atomic X-ray Spectrometry P. 278

12 A-2 Absorption spectra (ejecting one of the innermost electrons) Absorption process: Absorption of X-ray cause ejection of one of the innermost electrons from an atom, which results in the production of an excited ion. Beer’s Law: ln(P 0/P) = mx m: linear absorption coefficient x: sample thickness Absorption edge ln(P 0/P) = m. M rx r: density m. M: mass absorption coefficient (independent of the physical and chemical state of the element) FIGUER 12 -5 X-ray absorption spectra for lead and silver. 歐亞書局

Review: n n n 歐亞書局 1) How X-rays are generated? 2) Explain the origins of continuum and line spectrum of X-rays. 3) Explain short-wavelength limit (lo) and absorption edge?

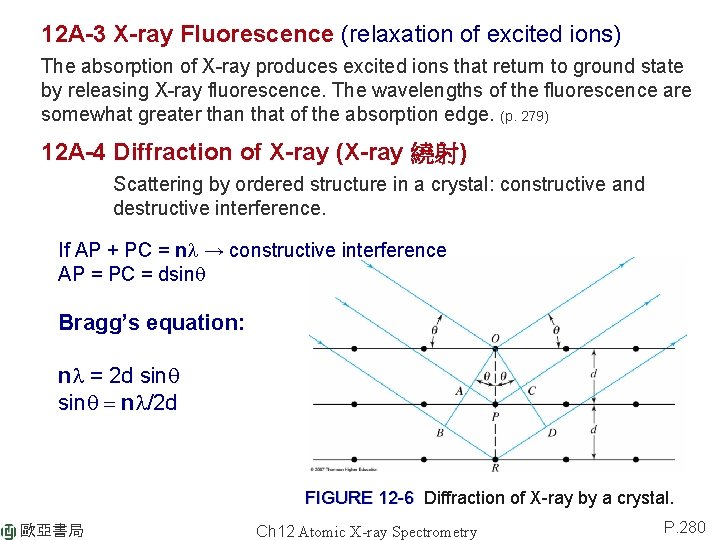
12 A-3 X-ray Fluorescence (relaxation of excited ions) The absorption of X-ray produces excited ions that return to ground state by releasing X-ray fluorescence. The wavelengths of the fluorescence are somewhat greater than that of the absorption edge. (p. 279) 12 A-4 Diffraction of X-ray (X-ray 繞射) Scattering by ordered structure in a crystal: constructive and destructive interference. If AP + PC = nl → constructive interference AP = PC = dsinq Bragg’s equation: nl = 2 d sinq = nl/2 d FIGURE 12 -6 Diffraction of X-ray by a crystal. 歐亞書局 Ch 12 Atomic X-ray Spectrometry P. 280

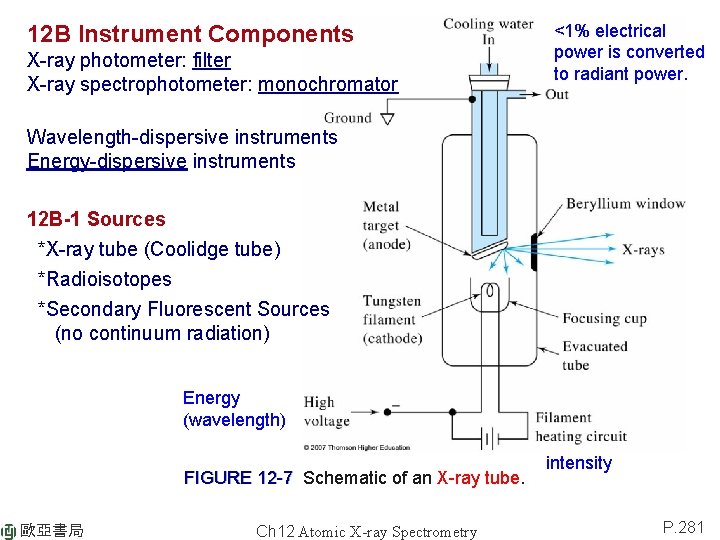
12 B Instrument Components X-ray photometer: filter X-ray spectrophotometer: monochromator <1% electrical power is converted to radiant power. Wavelength-dispersive instruments Energy-dispersive instruments 12 B-1 Sources *X-ray tube (Coolidge tube) *Radioisotopes *Secondary Fluorescent Sources (no continuum radiation) Energy (wavelength) FIGURE 12 -7 Schematic of an X-ray tube. 歐亞書局 Ch 12 Atomic X-ray Spectrometry intensity P. 281

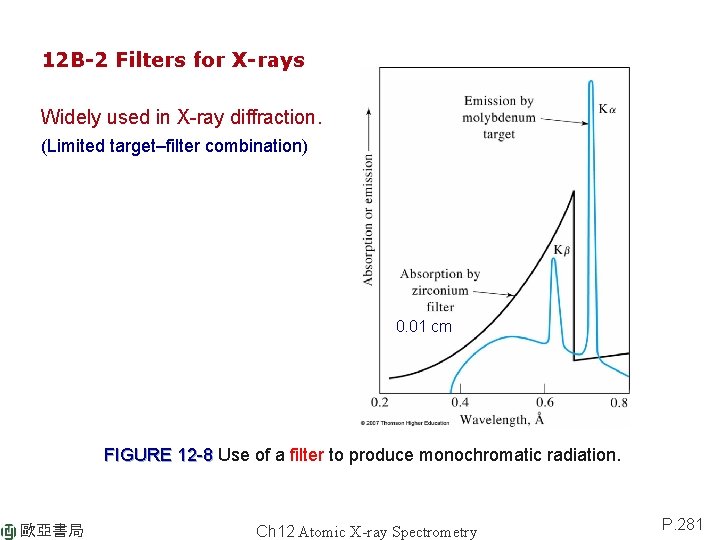
12 B-2 Filters for X-rays Widely used in X-ray diffraction. (Limited target–filter combination) 0. 01 cm FIGURE 12 -8 Use of a filter to produce monochromatic radiation. 歐亞書局 Ch 12 Atomic X-ray Spectrometry P. 281

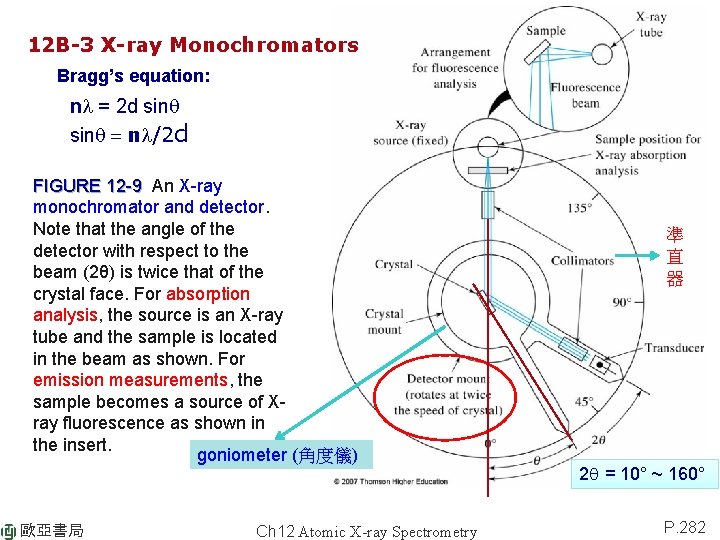
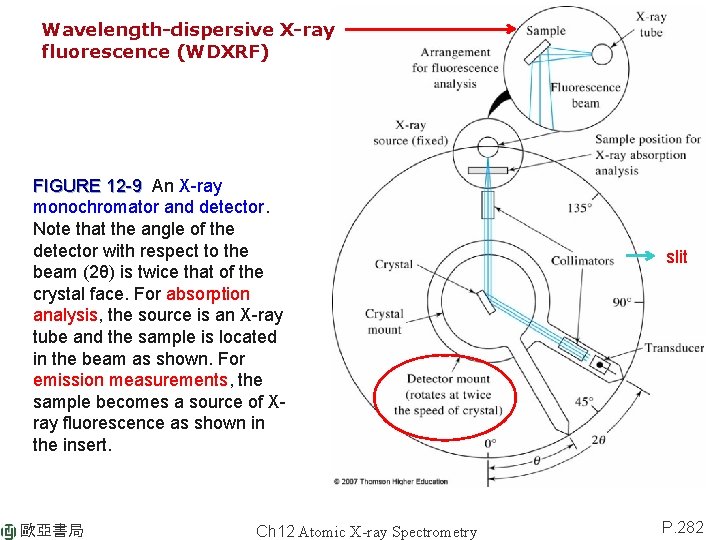
12 B-3 X-ray Monochromators Bragg’s equation: nl = 2 d sinq = nl/2 d FIGURE 12 -9 An X-ray monochromator and detector. Note that the angle of the detector with respect to the beam (2θ) is twice that of the crystal face. For absorption analysis, the source is an X-ray tube and the sample is located in the beam as shown. For emission measurements, the sample becomes a source of Xray fluorescence as shown in the insert. goniometer (角度儀) 歐亞書局 Ch 12 Atomic X-ray Spectrometry 準 直 器 2 q = 10° ~ 160° P. 282

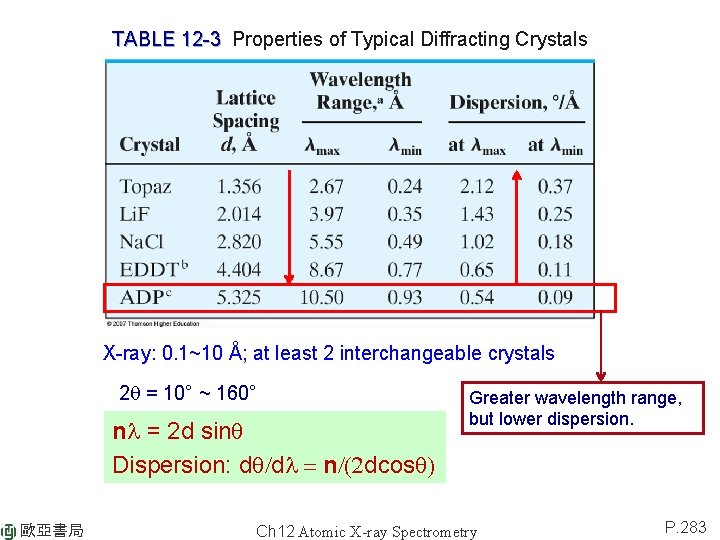
TABLE 12 -3 Properties of Typical Diffracting Crystals X-ray: 0. 1~10 Å; at least 2 interchangeable crystals 2 q = 10° ~ 160° nl = 2 d sinq Dispersion: dq/dl = n/(2 dcosq) 歐亞書局 Greater wavelength range, but lower dispersion. Ch 12 Atomic X-ray Spectrometry P. 283

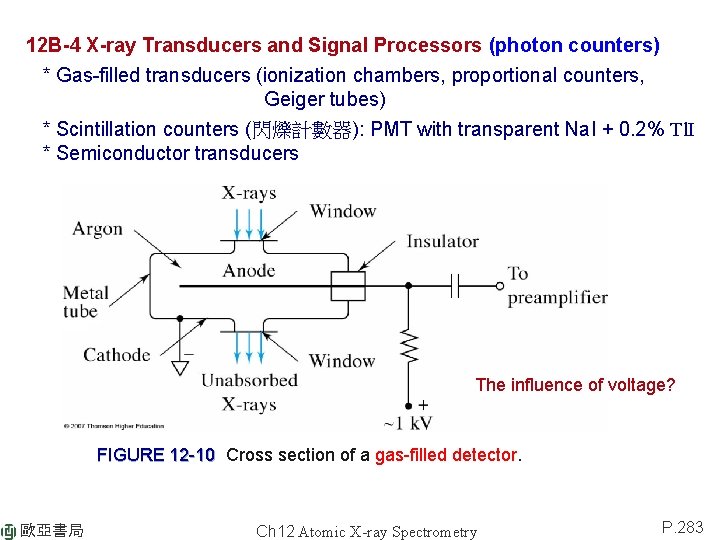
12 B-4 X-ray Transducers and Signal Processors (photon counters) * Gas-filled transducers (ionization chambers, proportional counters, Geiger tubes) * Scintillation counters (閃爍計數器): PMT with transparent Na. I + 0. 2% Tl. I * Semiconductor transducers The influence of voltage? FIGURE 12 -10 Cross section of a gas-filled detector. 歐亞書局 Ch 12 Atomic X-ray Spectrometry P. 283

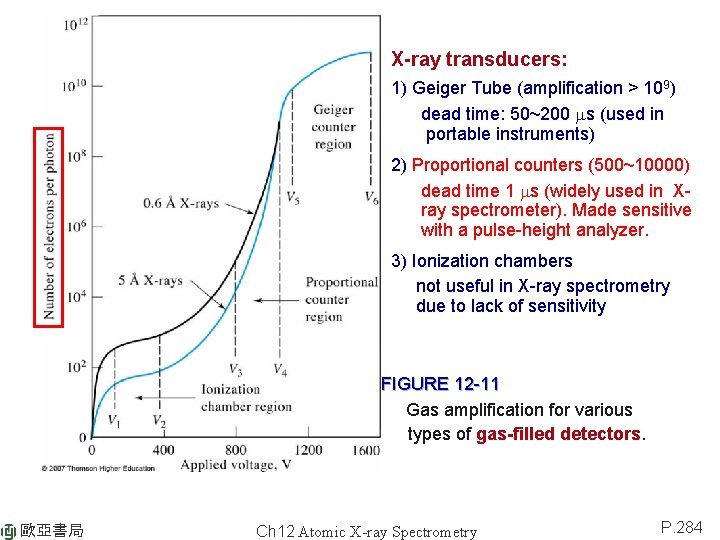
X-ray transducers: 1) Geiger Tube (amplification > 109) dead time: 50~200 ms (used in portable instruments) 2) Proportional counters (500~10000) dead time 1 ms (widely used in Xray spectrometer). Made sensitive with a pulse-height analyzer. 3) Ionization chambers not useful in X-ray spectrometry due to lack of sensitivity FIGURE 12 -11 Gas amplification for various types of gas-filled detectors. 歐亞書局 Ch 12 Atomic X-ray Spectrometry P. 284

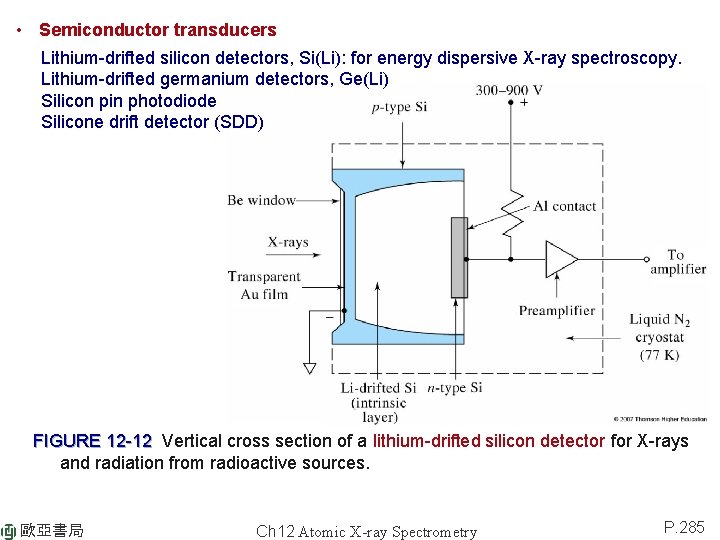
• Semiconductor transducers Lithium-drifted silicon detectors, Si(Li): for energy dispersive X-ray spectroscopy. Lithium-drifted germanium detectors, Ge(Li) Silicon pin photodiode Silicone drift detector (SDD) FIGURE 12 -12 Vertical cross section of a lithium-drifted silicon detector for X-rays and radiation from radioactive sources. 歐亞書局 Ch 12 Atomic X-ray Spectrometry P. 285

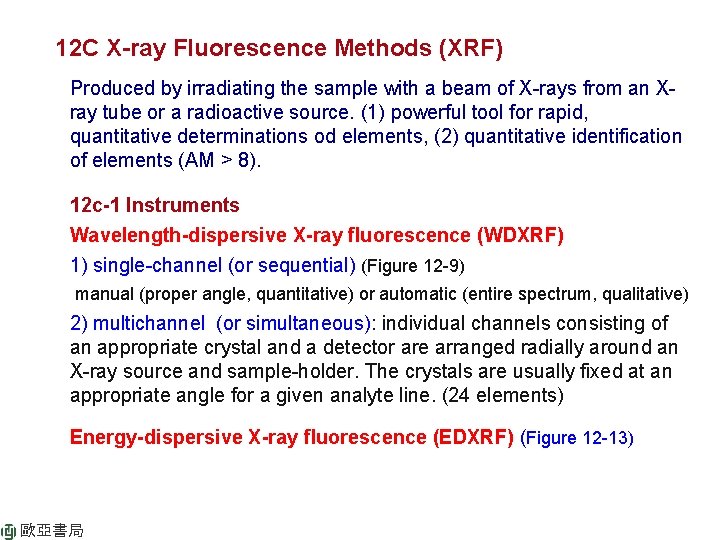
12 C X-ray Fluorescence Methods (XRF) Produced by irradiating the sample with a beam of X-rays from an Xray tube or a radioactive source. (1) powerful tool for rapid, quantitative determinations od elements, (2) quantitative identification of elements (AM > 8). 12 c-1 Instruments Wavelength-dispersive X-ray fluorescence (WDXRF) 1) single-channel (or sequential) (Figure 12 -9) manual (proper angle, quantitative) or automatic (entire spectrum, qualitative) 2) multichannel (or simultaneous): individual channels consisting of an appropriate crystal and a detector are arranged radially around an X-ray source and sample-holder. The crystals are usually fixed at an appropriate angle for a given analyte line. (24 elements) Energy-dispersive X-ray fluorescence (EDXRF) (Figure 12 -13) 歐亞書局

Wavelength-dispersive X-ray fluorescence (WDXRF) FIGURE 12 -9 An X-ray monochromator and detector. Note that the angle of the detector with respect to the beam (2θ) is twice that of the crystal face. For absorption analysis, the source is an X-ray tube and the sample is located in the beam as shown. For emission measurements, the sample becomes a source of Xray fluorescence as shown in the insert. 歐亞書局 Ch 12 Atomic X-ray Spectrometry slit P. 282

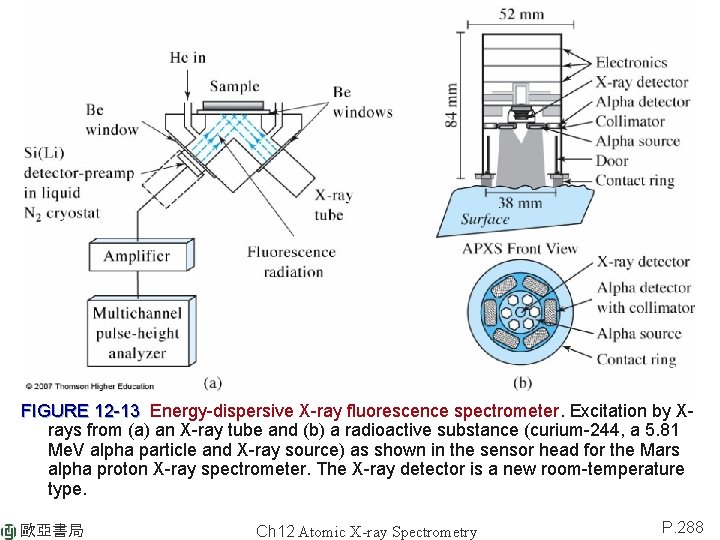
FIGURE 12 -13 Energy-dispersive X-ray fluorescence spectrometer. Excitation by Xrays from (a) an X-ray tube and (b) a radioactive substance (curium-244, a 5. 81 Me. V alpha particle and X-ray source) as shown in the sensor head for the Mars alpha proton X-ray spectrometer. The X-ray detector is a new room-temperature type. 歐亞書局 Ch 12 Atomic X-ray Spectrometry P. 288

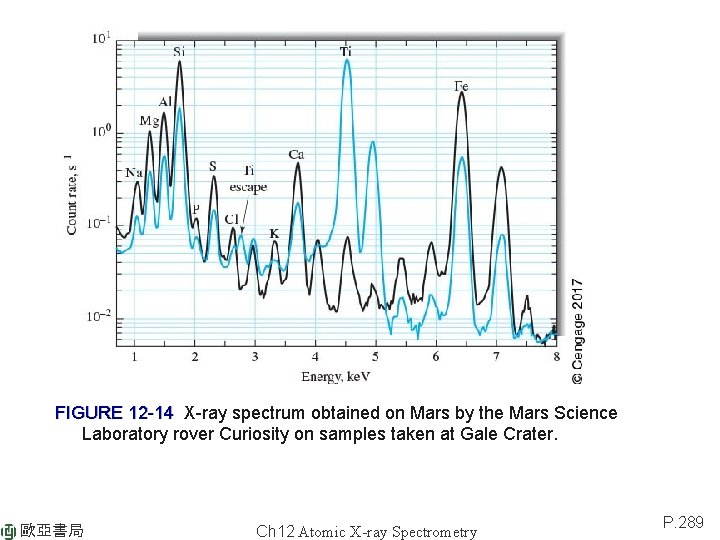
FIGURE 12 -14 X-ray spectrum obtained on Mars by the Mars Science Laboratory rover Curiosity on samples taken at Gale Crater. 歐亞書局 Ch 12 Atomic X-ray Spectrometry P. 289

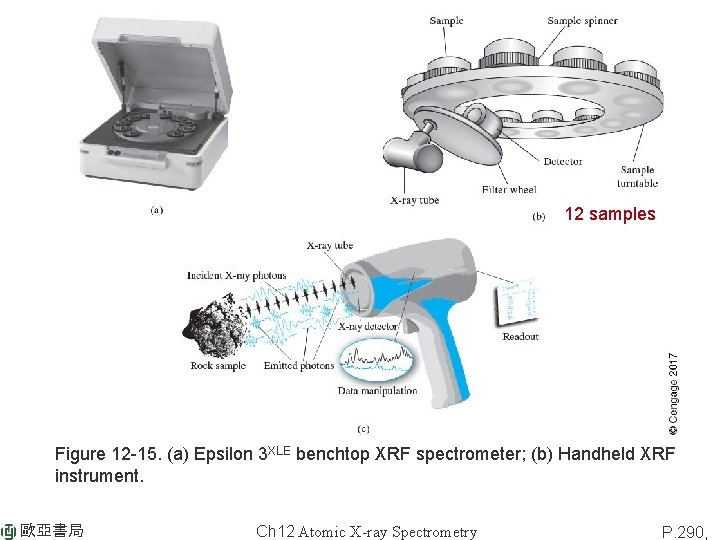
12 samples Figure 12 -15. (a) Epsilon 3 XLE benchtop XRF spectrometer; (b) Handheld XRF instrument. 歐亞書局 Ch 12 Atomic X-ray Spectrometry P. 290,

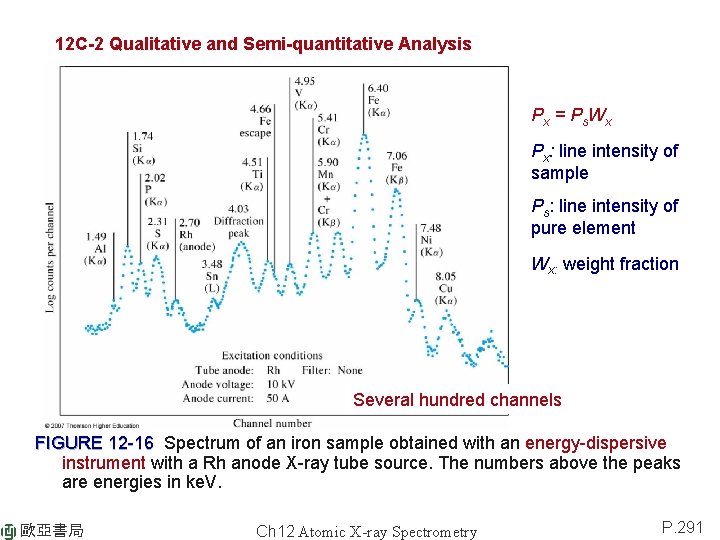
12 C-2 Qualitative and Semi-quantitative Analysis Px = P s Wx Px: line intensity of sample Ps: line intensity of pure element Wx: weight fraction Several hundred channels FIGURE 12 -16 Spectrum of an iron sample obtained with an energy-dispersive instrument with a Rh anode X-ray tube source. The numbers above the peaks are energies in ke. V. 歐亞書局 Ch 12 Atomic X-ray Spectrometry P. 291

12 C-3 Quantitative Analysis Px = P s W x Need 1) availability of calibration standards and 2) suitable methods for dealing with matrix effects. Matrix effects: absorption effect and enhancement effect. Fig 12 -17 a) X-ray fluorescence spectrum of a rice sample. (Fig. 12 -15) b) Calibration curve obtained from nine rice samples. P. 294 歐亞書局

Review: n n n 1) When fluorescence is to be excited by radiation from an X-ray tube, what is the minimum operating voltage required? For example, what’s the minimum tube voltage needed to generate K line of lead? 2) Describe main components of X-ray monochromator. 3) Why an X-ray monochromator needs at least two diffracting crystals? Which one has better (angular) dispersion? 歐亞書局

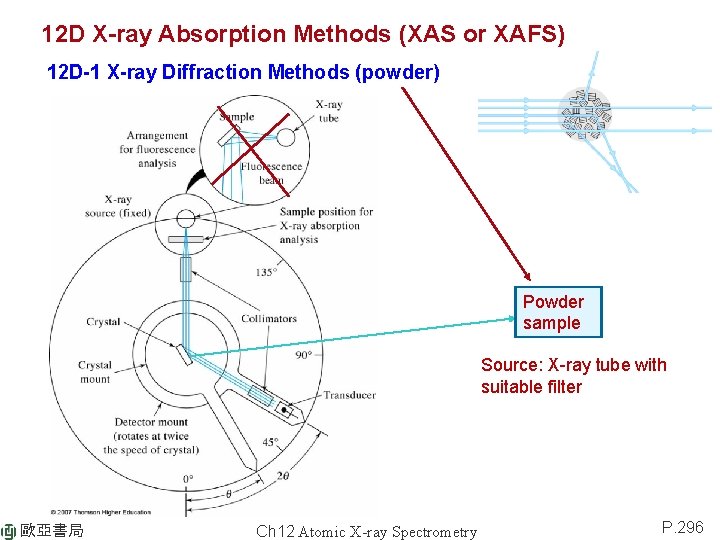
12 D X-ray Absorption Methods (XAS or XAFS) 12 D-1 X-ray Diffraction Methods (powder) Powder sample Source: X-ray tube with suitable filter 歐亞書局 Ch 12 Atomic X-ray Spectrometry P. 296

Powder Diffraction Pattern FIGURE 12 -18 Schematic of (a) a Debye-Scherrer powder camera; (b) the film strip after development. D 2, D 1, and T indicate positions of the film in the camera. 歐亞書局

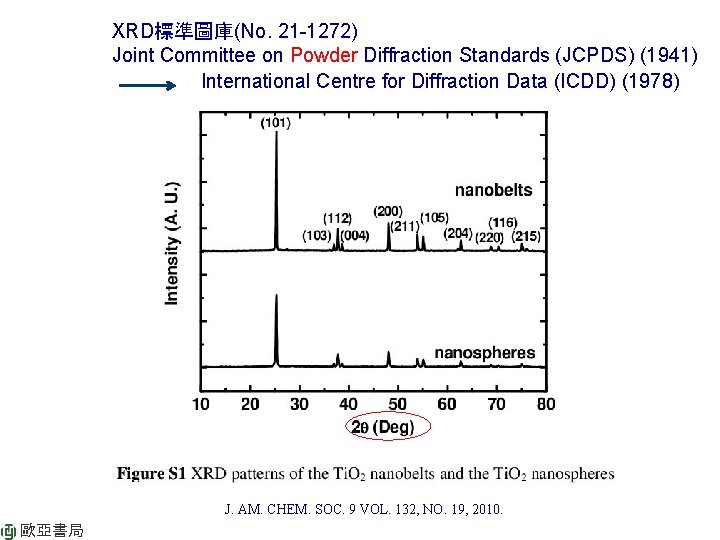
XRD標準圖庫(No. 21 -1272) Joint Committee on Powder Diffraction Standards (JCPDS) (1941) International Centre for Diffraction Data (ICDD) (1978) J. AM. CHEM. SOC. 9 VOL. 132, NO. 19, 2010. 歐亞書局

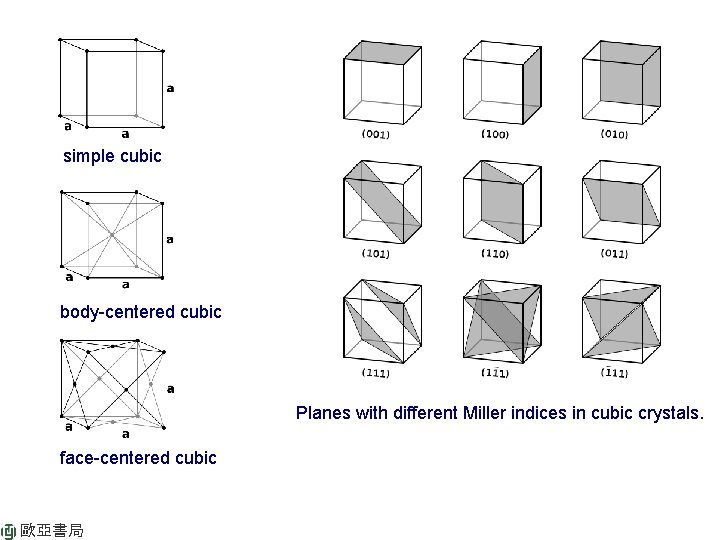
simple cubic body-centered cubic Planes with different Miller indices in cubic crystals. face-centered cubic 歐亞書局