Chapter 12 Alkenes Alkynes and Aromatic Compounds 12


- Slides: 21

Chapter 12 Alkenes, Alkynes, and Aromatic Compounds 12. 3 Addition Reactions General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 1

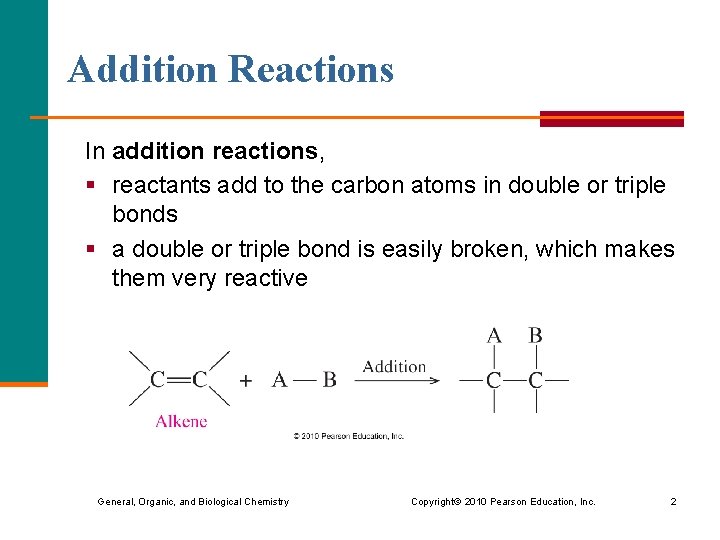
Addition Reactions In addition reactions, § reactants add to the carbon atoms in double or triple bonds § a double or triple bond is easily broken, which makes them very reactive General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 2

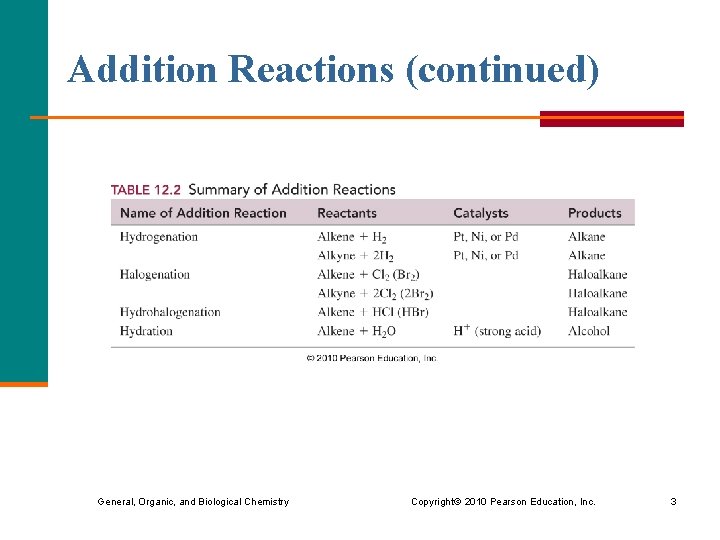
Addition Reactions (continued) General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 3

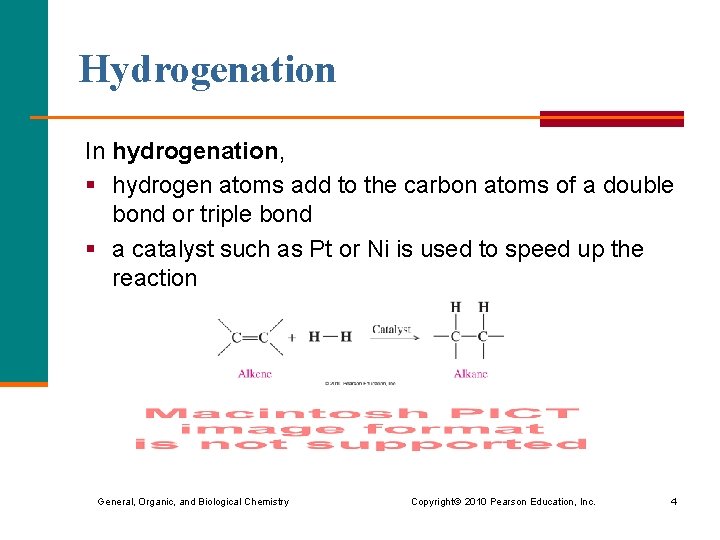
Hydrogenation In hydrogenation, § hydrogen atoms add to the carbon atoms of a double bond or triple bond § a catalyst such as Pt or Ni is used to speed up the reaction General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 4

Hydrogenation of Oils Adding H 2 to double bonds in vegetable oils produces § compounds with higher melting points § solids at room temperature, such as margarine, soft margarine, and shortening General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 5

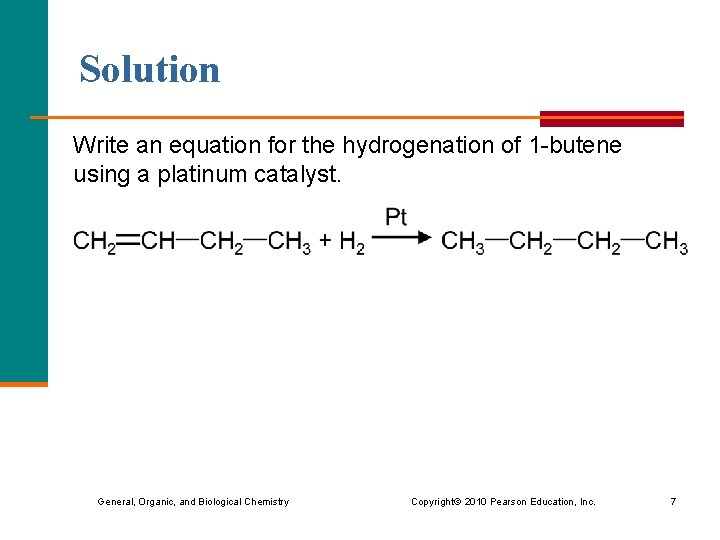
Learning Check Write an equation for the hydrogenation of 1 -butene using a platinum catalyst. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 6

Solution Write an equation for the hydrogenation of 1 -butene using a platinum catalyst. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 7

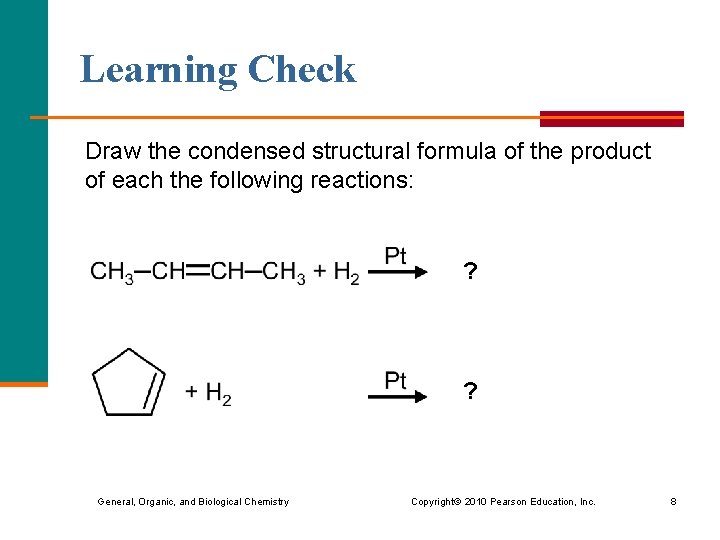
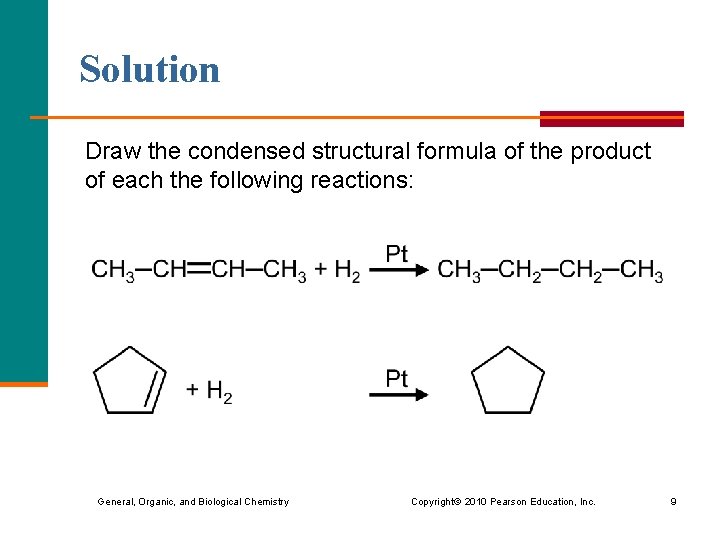
Learning Check Draw the condensed structural formula of the product of each the following reactions: ? ? General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 8

Solution Draw the condensed structural formula of the product of each the following reactions: General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 9

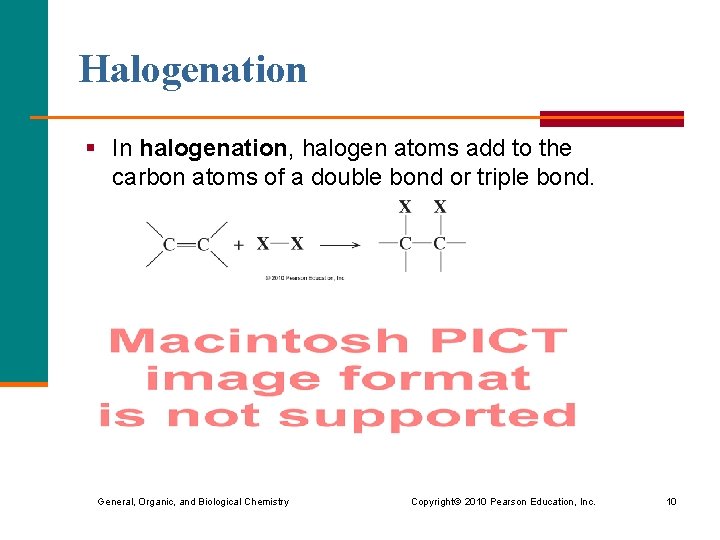
Halogenation § In halogenation, halogen atoms add to the carbon atoms of a double bond or triple bond. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 10

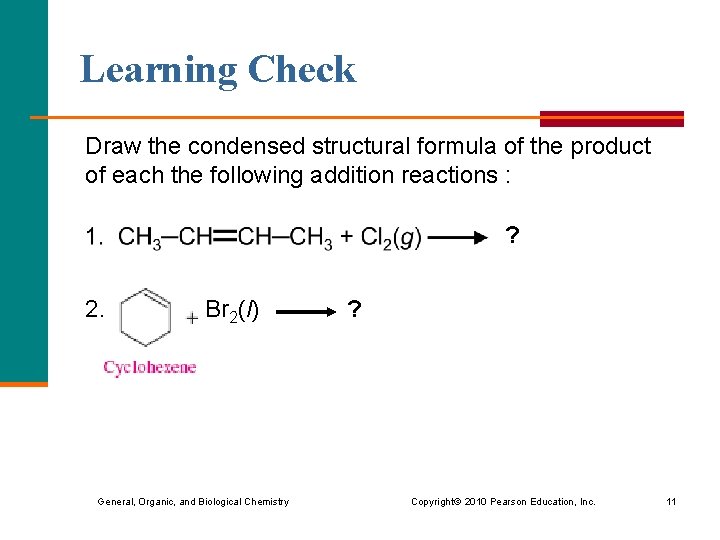
Learning Check Draw the condensed structural formula of the product of each the following addition reactions : ? 2. Br 2(l) General, Organic, and Biological Chemistry ? Copyright © 2010 Pearson Education, Inc. 11

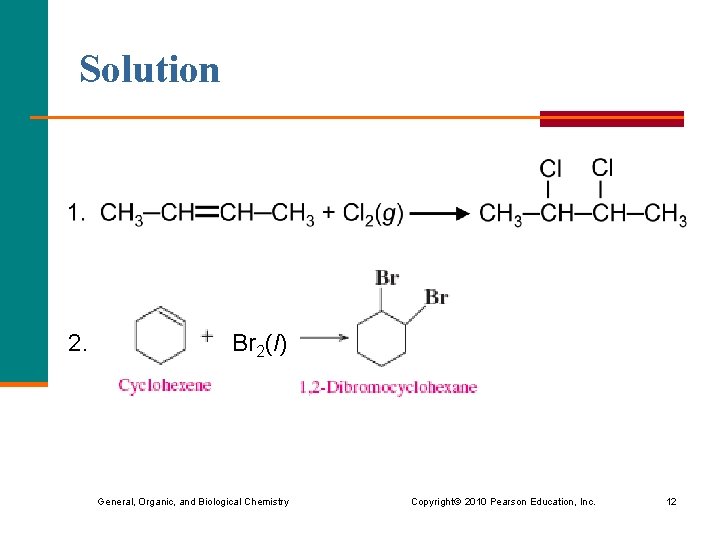
Solution 2. Br 2(l) General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 12

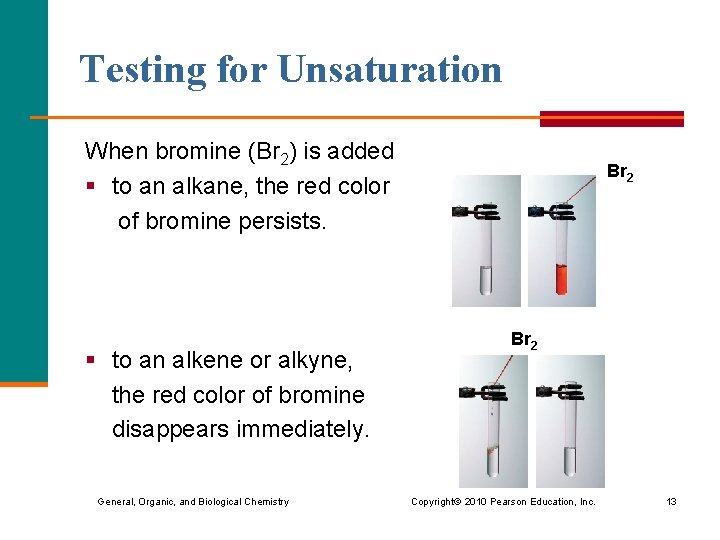
Testing for Unsaturation When bromine (Br 2) is added § to an alkane, the red color of bromine persists. § to an alkene or alkyne, the red color of bromine disappears immediately. General, Organic, and Biological Chemistry Br 2 Copyright © 2010 Pearson Education, Inc. 13

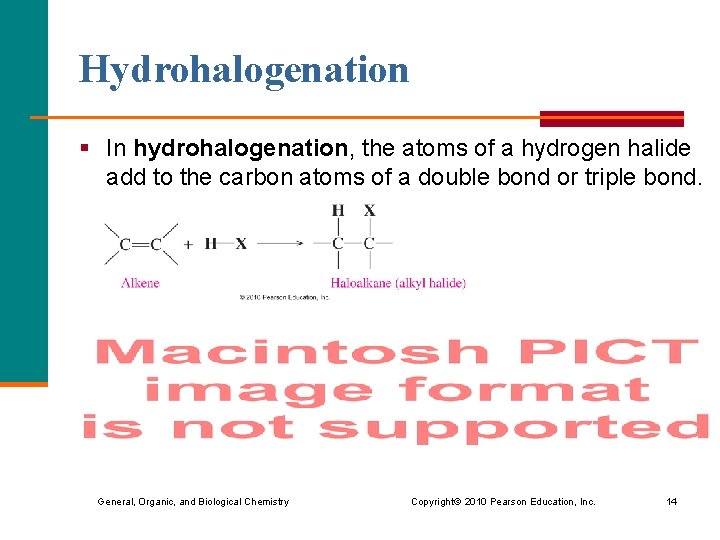
Hydrohalogenation § In hydrohalogenation, the atoms of a hydrogen halide add to the carbon atoms of a double bond or triple bond. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 14

Markovnikov’s Rule When an unsymmetrical alkene undergoes hydrohalogenation, § the H in HX adds to the carbon in the double bond that has the greater number of H atoms General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 15

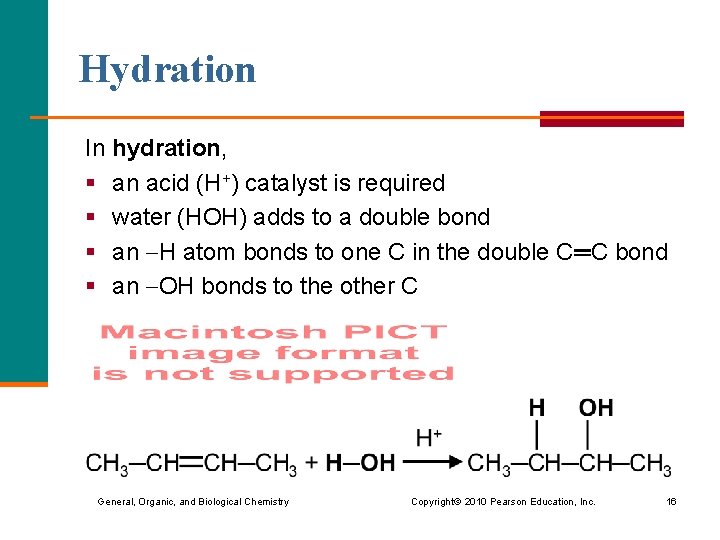
Hydration In hydration, § an acid (H+) catalyst is required § water (HOH) adds to a double bond § an H atom bonds to one C in the double C═C bond § an OH bonds to the other C General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 16

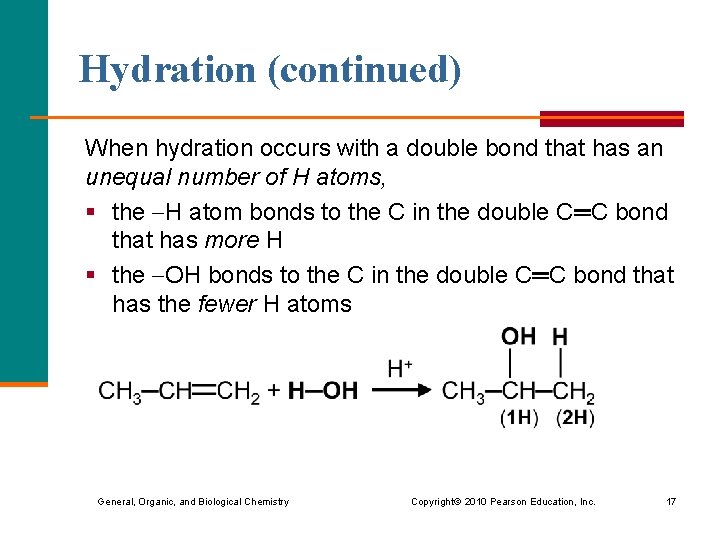
Hydration (continued) When hydration occurs with a double bond that has an unequal number of H atoms, § the H atom bonds to the C in the double C═C bond that has more H § the OH bonds to the C in the double C═C bond that has the fewer H atoms General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 17

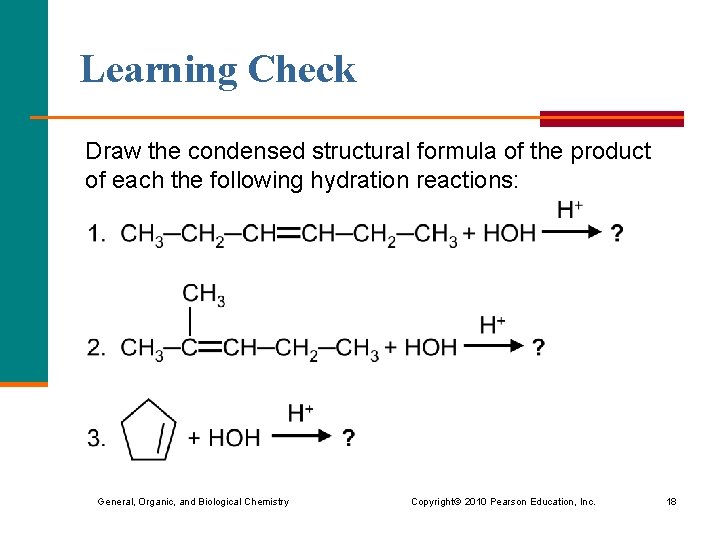
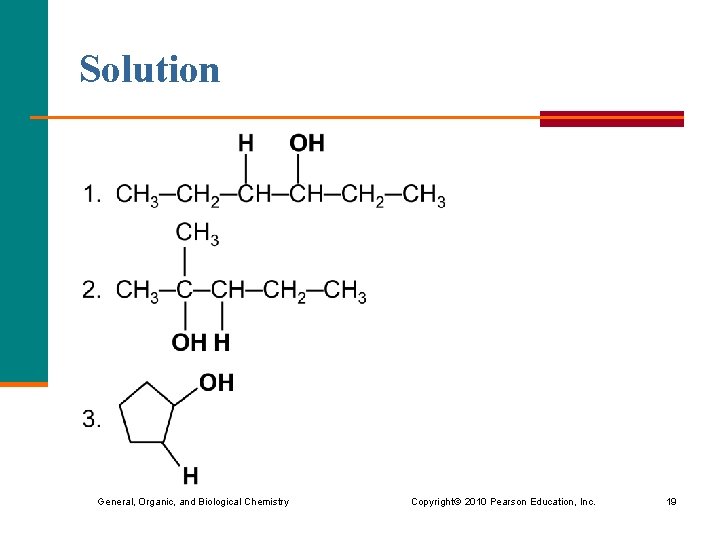
Learning Check Draw the condensed structural formula of the product of each the following hydration reactions: General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 18

Solution General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 19

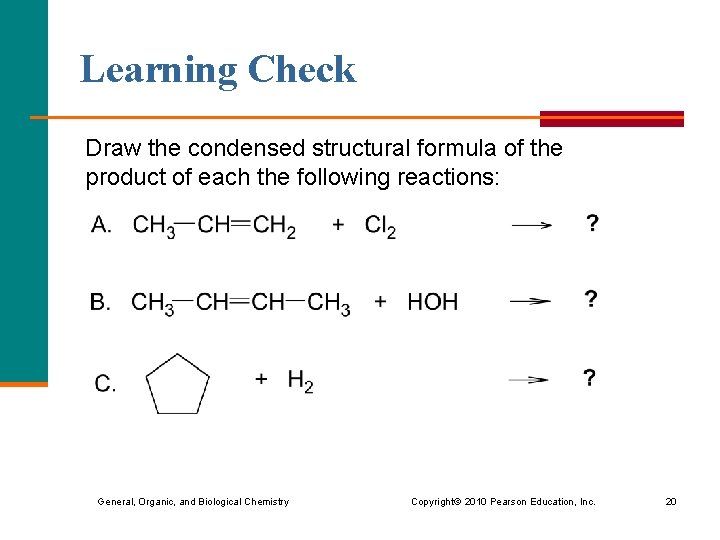
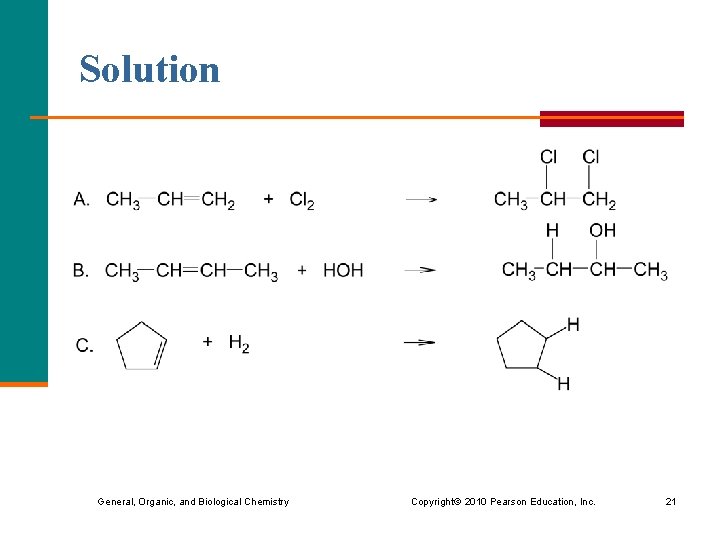
Learning Check Draw the condensed structural formula of the product of each the following reactions: General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 20

Solution General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 21