Chapter 11 Structure of solids continued Structure and










- Slides: 22

Chapter 11 Structure of solids continued

Structure and Bonding in Metals • Metals have: – High thermal and electrical conductivity – Are malleable – Are Ductile • The reason for this is they are like small spheres packed together and bonded equally with other metal atoms in all directions.

Body-centered & Face-centered Crystal Lattice

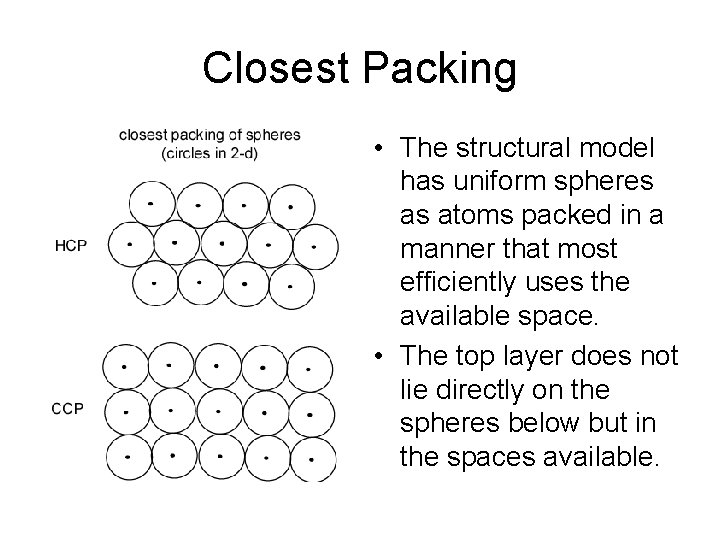
Closest Packing • The structural model has uniform spheres as atoms packed in a manner that most efficiently uses the available space. • The top layer does not lie directly on the spheres below but in the spaces available.

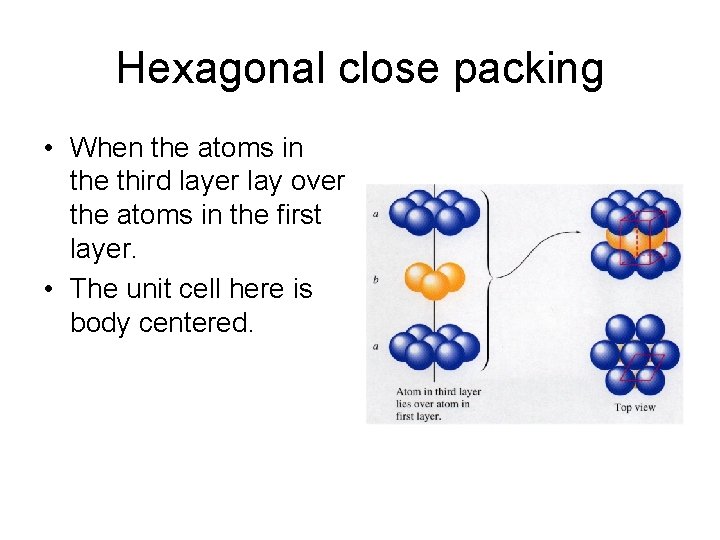
Hexagonal close packing • When the atoms in the third layer lay over the atoms in the first layer. • The unit cell here is body centered.

Other examples in nature of Hexagonal Close Packing

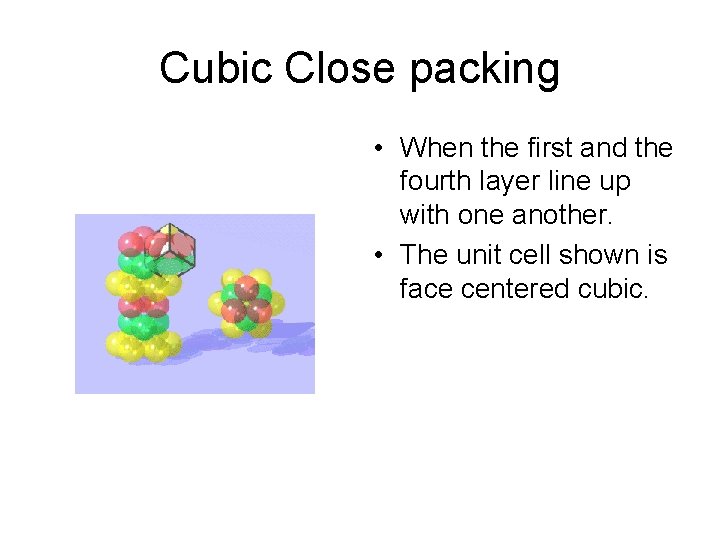
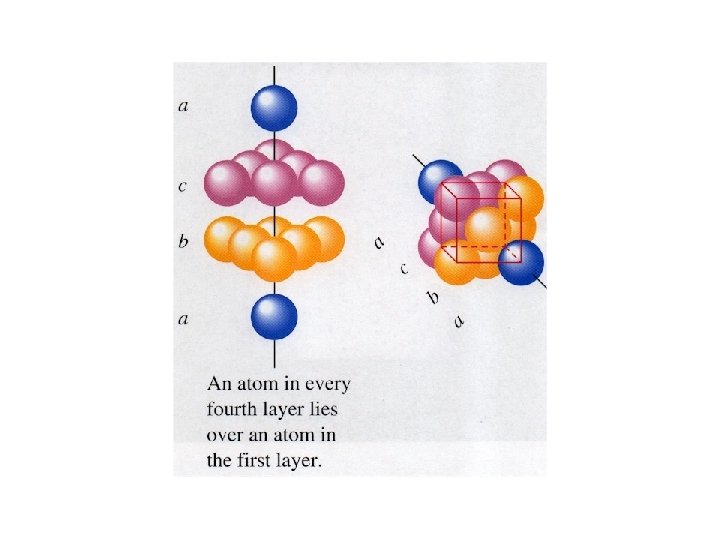
Cubic Close packing • When the first and the fourth layer line up with one another. • The unit cell shown is face centered cubic.


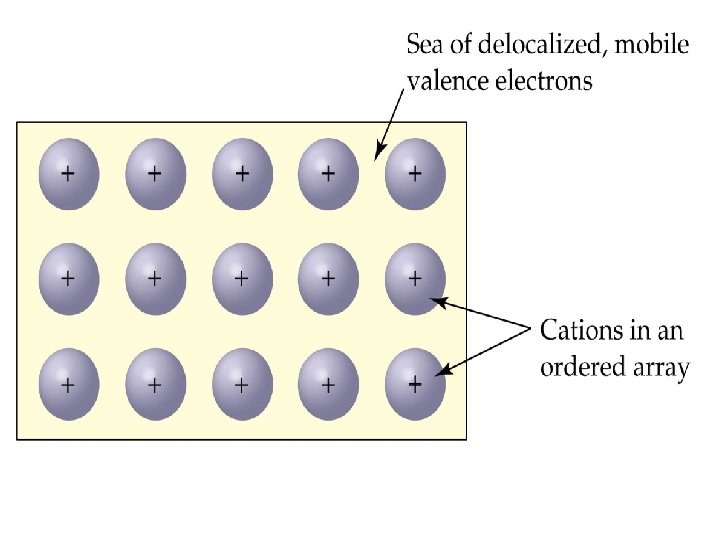
Bonding Model for Metals • Metals qualities are best explained by the electron sea model. • This envisions a regular array of organized cations surrounded by delocalized sea of electrons. • This allows the movement of electrical current, and the metal ions can be easily moved around as a metal is hammered into a shape.


Metal Strength • Sodium, potassium and lithium are soft metals that may be cut with a spoon! They have only one valence electron each. • Chromium and iron are much harder metals each with 6 and 8 valence electrons respectively. • What about mercury?

Discussion • Mercury hangs on to its valence 6 s electrons very tightly. Mercury-mercury bonding is very weak because its valence electrons are not shared readily. (In fact mercury is the only metal that doesn't form diatomic molecules in the gas phase). • Hg 200. 59 [Kr] 4 d 10 4 f 14 5 s 2 5 p 6 5 d 10 6 s 2

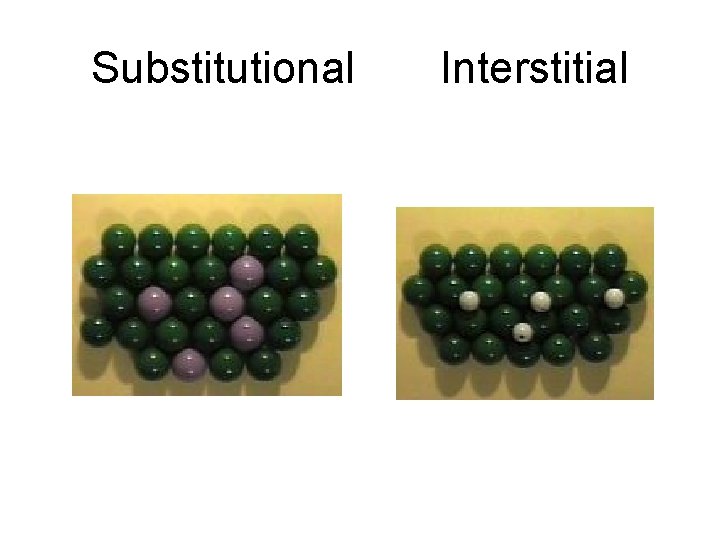
Other notes: • Metal alloys are a substance that contains a mixture of elements and has metallic properties. • There are two types of alloys: – Substitutional alloy – Interstitial alloy

Substitutional Interstitial

Bonding in Molecular Solids • Molecular solids are held together by intermolecular forces. • London forces, Dipole-dipole and hydrogen bonding. • The properties of the molecular solids depends not only on the strength of these forces but also on the ability of the molecules to closely pack. • Examples: Ar, CO 2, and H 2 O

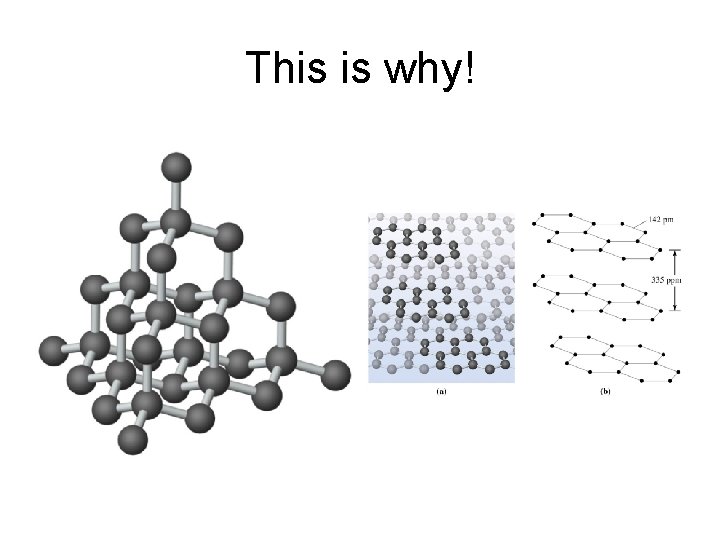
Network Solids • Many atomic solids form strong directional covalent bonds. This allows the formation of “giant” molecules. • Silicon and Carbon form some of the most important network solids. • Diamond and graphite are both made of carbon. Yet diamond is a poor conductor and graphite can conduct electricity.

Why? • Diamond is carbon bound in a tetrahedral shape to other carbons (sp 3). This localizes the electrons and prevents conduction. • Graphite is layers of 6 carbon rings with some delocalized electrons between the sheets of rings. Aka. sp 2 hybridization with pi-bonds.

This is why!

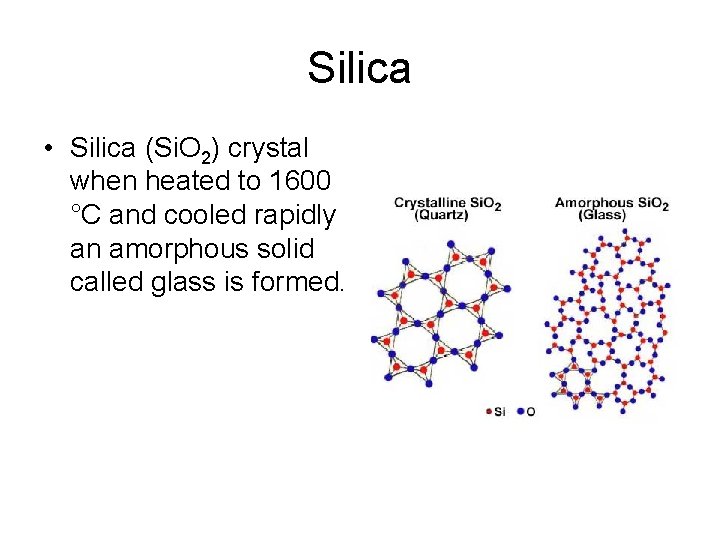
Silica • Silica (Si. O 2) crystal when heated to 1600 °C and cooled rapidly an amorphous solid called glass is formed.

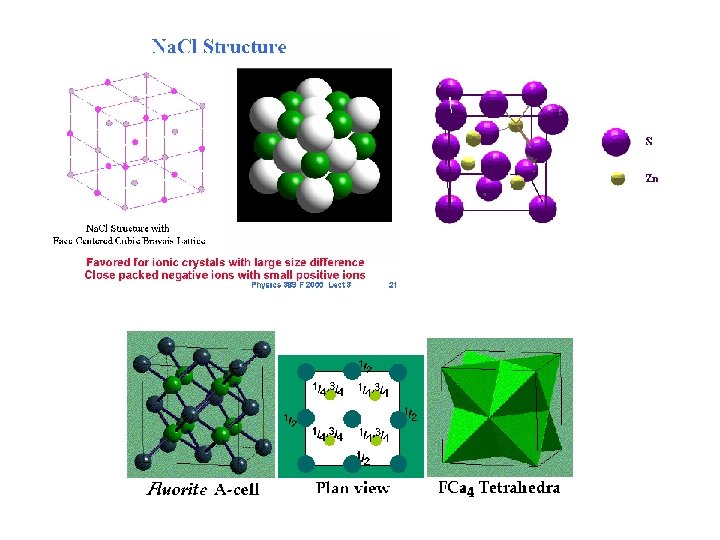
Ionic Solids • These are stable high melting substances held together by strong static forces between oppositely charged ions. • Most are binary solids and can be modeled by closest packing spheres. • The smaller cations fit in the holes created by closely packing the anions. • The packing is done to maximize the oppositely charged particles and minimize the repulsions by ions with the same charge.

Shapes • There are three types of holes in closest packed structures. • Trigonal holes formed by three sphere in the same layer • Tetrahedral holes formed when a sphere sits in the dimple of three spheres in an adjacent layer. • Octahedral holes are formed by two sets of three spheres of the closest packed structure. • The relative size of the wholes is : Trigonal<tetrahedral<octahedral
