Chapter 11 Refrigeration Cycles 1 OUTLINE Refrigerators And



- Slides: 40

Chapter 11 Refrigeration Cycles 1

OUTLINE • • Refrigerators And Heat Pumps The Reversed Carnot Cycle The Ideal Vapor compression Refrigeration Cycle Actual Vapor compression Refrigeration Cycle Selecting The Right Refrigerant Heat Pump Systems Gas Refrigeration Cycles Absorption Refrigeration Systems 2

OBJECTIVE • Introduce the concepts of refrigerators and heat pumps and the measure of their performance. • Analyze the ideal vapor-compression refrigeration cycle. • Analyze the actual vapor-compression refrigeration cycle. • Review the factors involved in selecting the right refrigerant for an application. • Discuss the operation of refrigeration and heat pump systems. • Analyze gas refrigeration systems. • Introduce the concepts of absorption-refrigeration systems. 3

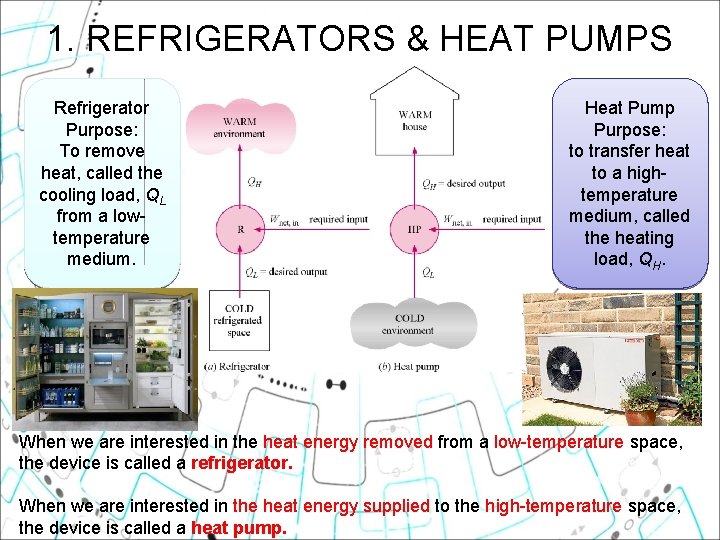
1. REFRIGERATORS & HEAT PUMPS Refrigerator Purpose: To remove heat, called the cooling load, QL from a lowtemperature medium. Heat Pump Purpose: to transfer heat to a hightemperature medium, called the heating load, QH. When we are interested in the heat energy removed from a low-temperature space, the device is called a refrigerator. When we are interested in the heat energy supplied to the high-temperature space, the device is called a heat pump.

The performance of refrigerators and heat pumps is expressed in terms of coefficient of performance (COP), defined as Both COPR and COPHP can be larger than 1. Under the same operating conditions, the COPs are related by

Refrigerators, air conditioners, and heat pumps are rated with a SEER number or seasonal adjusted energy efficiency ratio. The SEER is defined as the Btu/hr of heat transferred per watt of work energy input. The Btu is the British thermal unit and is equivalent to 778 ft-lbf of work (1 W = 3. 4122 Btu/hr). An EER of 10 yields a COP of 2. 9. Refrigeration systems are also rated in terms of tons of refrigeration. One ton of refrigeration is equivalent to 12, 000 Btu/hr or 211 k. J/min. 6

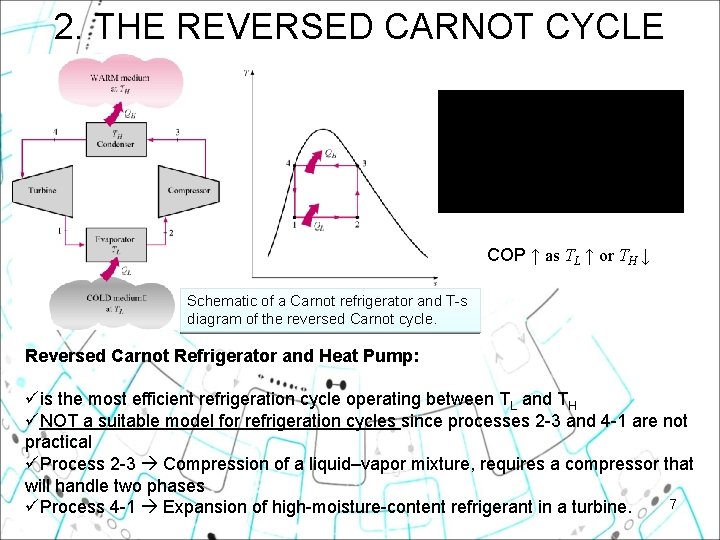
2. THE REVERSED CARNOT CYCLE COP ↑ as TL ↑ or TH ↓ Schematic of a Carnot refrigerator and T-s diagram of the reversed Carnot cycle. Reversed Carnot Refrigerator and Heat Pump: üis the most efficient refrigeration cycle operating between TL and TH üNOT a suitable model for refrigeration cycles since processes 2 -3 and 4 -1 are not practical üProcess 2 -3 Compression of a liquid–vapor mixture, requires a compressor that will handle two phases 7 üProcess 4 -1 Expansion of high-moisture-content refrigerant in a turbine.

Why not use the reversed Carnot refrigeration cycle? • Easier to compress vapor only and not liquid-vapor mixture. • Cheaper to have irreversible expansion through an expansion valve, not turbine 8

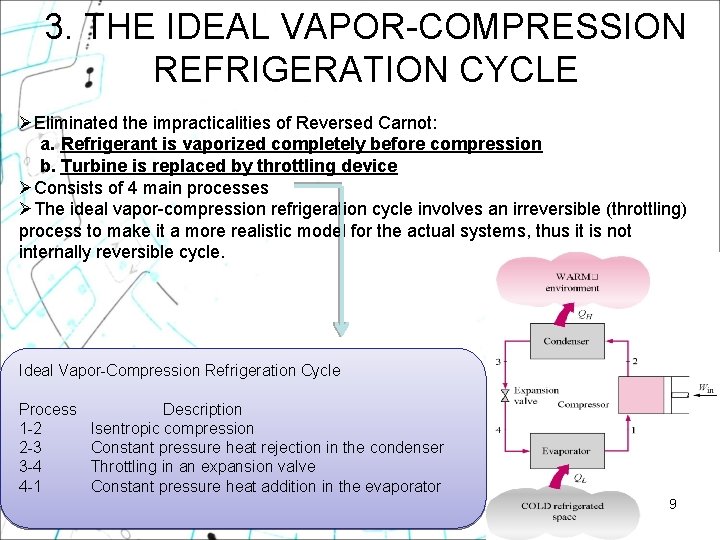
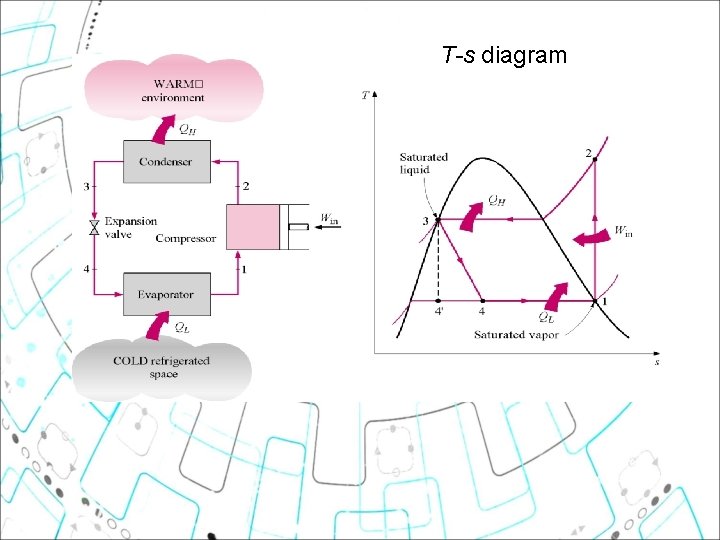
3. THE IDEAL VAPOR-COMPRESSION REFRIGERATION CYCLE ØEliminated the impracticalities of Reversed Carnot: a. Refrigerant is vaporized completely before compression b. Turbine is replaced by throttling device ØConsists of 4 main processes ØThe ideal vapor-compression refrigeration cycle involves an irreversible (throttling) process to make it a more realistic model for the actual systems, thus it is not internally reversible cycle. Ideal Vapor-Compression Refrigeration Cycle Process 1 -2 2 -3 3 -4 4 -1 Description Isentropic compression Constant pressure heat rejection in the condenser Throttling in an expansion valve Constant pressure heat addition in the evaporator 9

T-s diagram

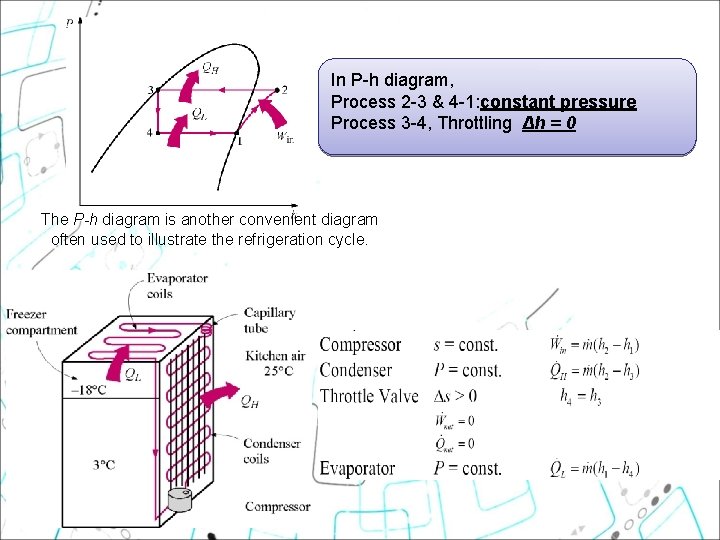
In P-h diagram, Process 2 -3 & 4 -1: constant pressure Process 3 -4, Throttling Δh = 0 The P-h diagram is another convenient diagram often used to illustrate the refrigeration cycle.

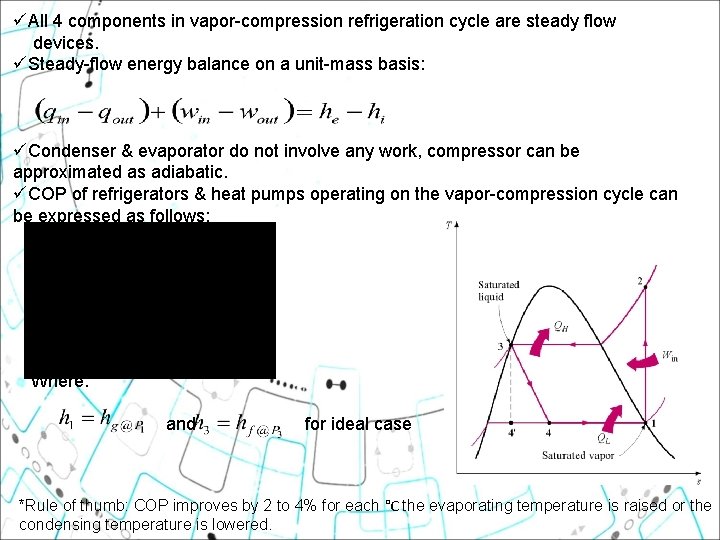
üAll 4 components in vapor-compression refrigeration cycle are steady flow devices. üSteady-flow energy balance on a unit-mass basis: üCondenser & evaporator do not involve any work, compressor can be approximated as adiabatic. üCOP of refrigerators & heat pumps operating on the vapor-compression cycle can be expressed as follows: Where: and for ideal case *Rule of thumb: COP improves by 2 to 4% for each °C the evaporating temperature is raised or the condensing temperature is lowered.

The ordinary household refrigerator is a good example of the application of this cycle. 13

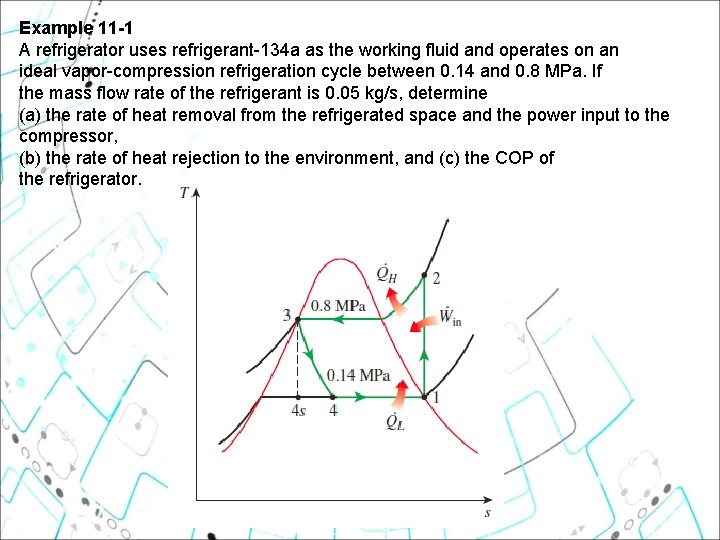
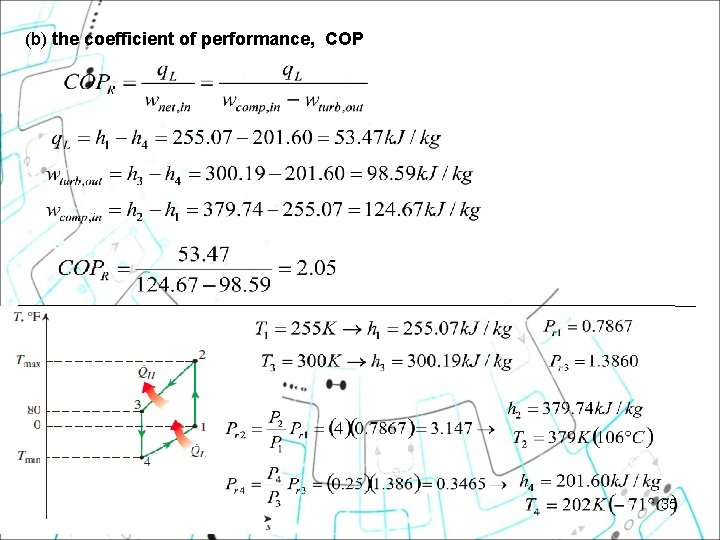
Example 11 1 A refrigerator uses refrigerant-134 a as the working fluid and operates on an ideal vapor-compression refrigeration cycle between 0. 14 and 0. 8 MPa. If the mass flow rate of the refrigerant is 0. 05 kg/s, determine (a) the rate of heat removal from the refrigerated space and the power input to the compressor, (b) the rate of heat rejection to the environment, and (c) the COP of the refrigerator.

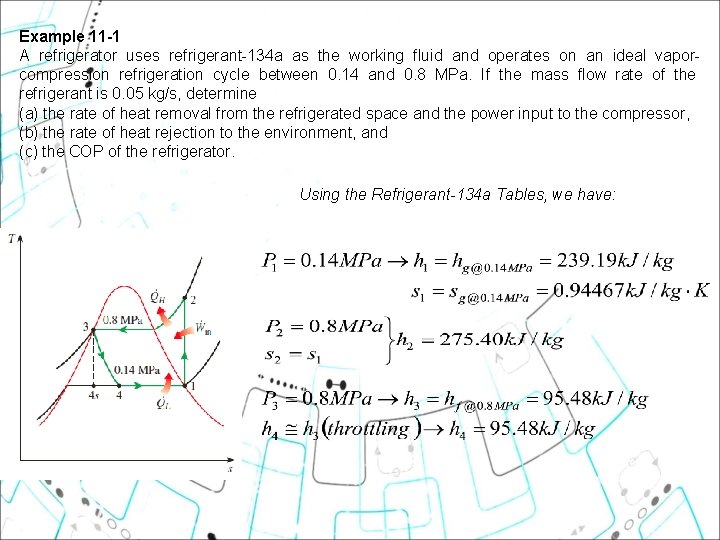
Example 11 1 A refrigerator uses refrigerant-134 a as the working fluid and operates on an ideal vaporcompression refrigeration cycle between 0. 14 and 0. 8 MPa. If the mass flow rate of the refrigerant is 0. 05 kg/s, determine (a) the rate of heat removal from the refrigerated space and the power input to the compressor, (b) the rate of heat rejection to the environment, and (c) the COP of the refrigerator. Using the Refrigerant-134 a Tables, we have:

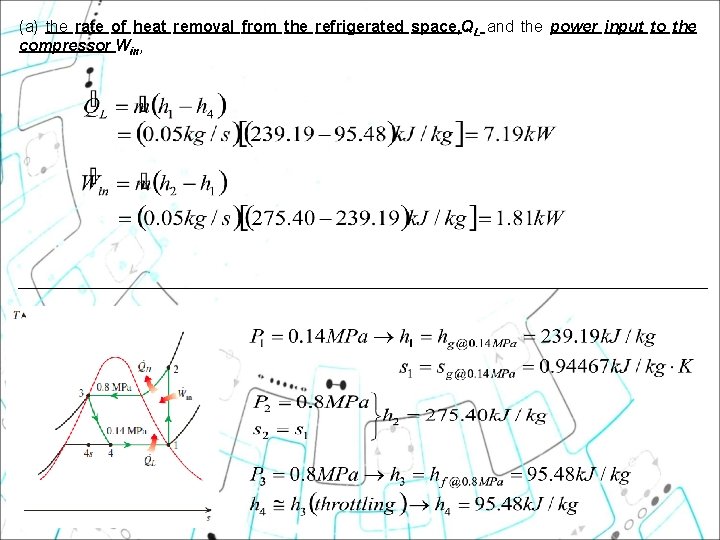
(a) the rate of heat removal from the refrigerated space, QL and the power input to the compressor Win,

(b) the rate of heat rejection to the environment, QH

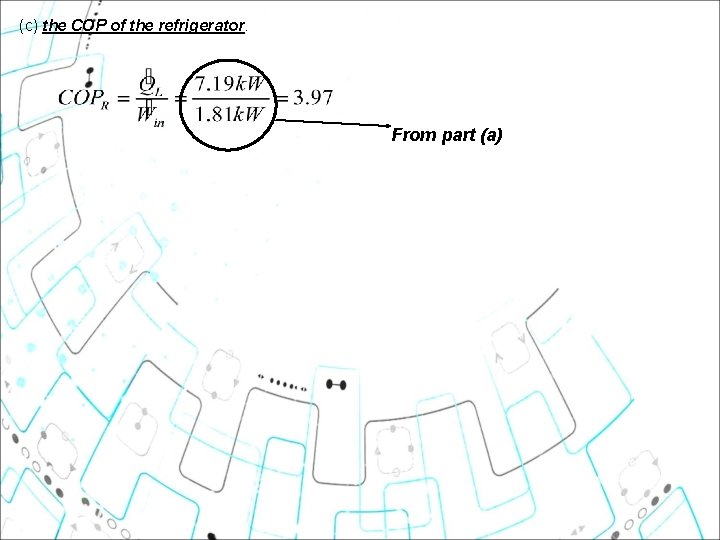
(c) the COP of the refrigerator. From part (a)

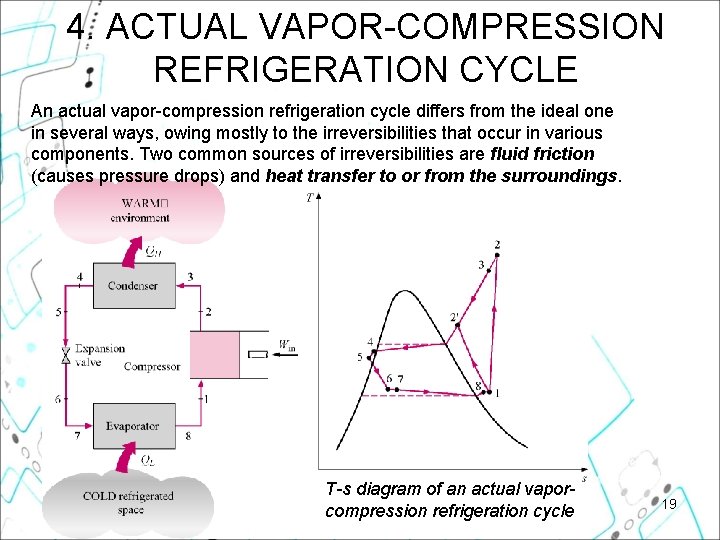
4. ACTUAL VAPOR-COMPRESSION REFRIGERATION CYCLE An actual vapor-compression refrigeration cycle differs from the ideal one in several ways, owing mostly to the irreversibilities that occur in various components. Two common sources of irreversibilities are fluid friction (causes pressure drops) and heat transfer to or from the surroundings. T-s diagram of an actual vaporcompression refrigeration cycle 19

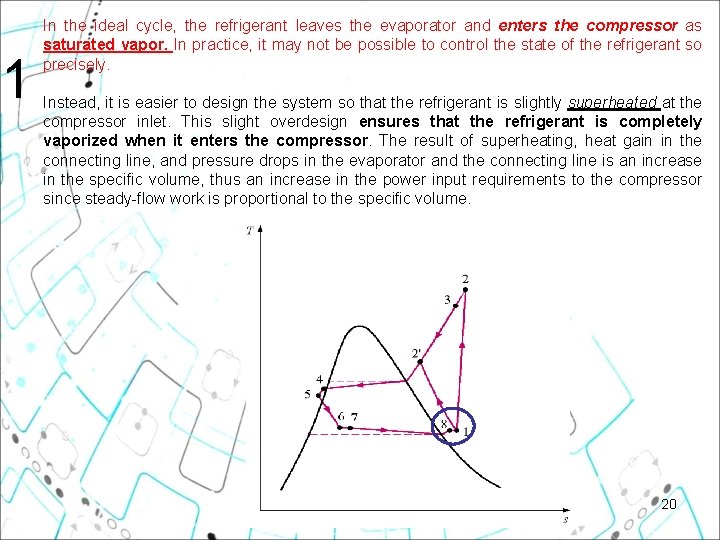
1 In the ideal cycle, the refrigerant leaves the evaporator and enters the compressor as saturated vapor. In practice, it may not be possible to control the state of the refrigerant so precisely. Instead, it is easier to design the system so that the refrigerant is slightly superheated at the compressor inlet. This slight overdesign ensures that the refrigerant is completely vaporized when it enters the compressor. The result of superheating, heat gain in the connecting line, and pressure drops in the evaporator and the connecting line is an increase in the specific volume, thus an increase in the power input requirements to the compressor since steady-flow work is proportional to the specific volume. 20

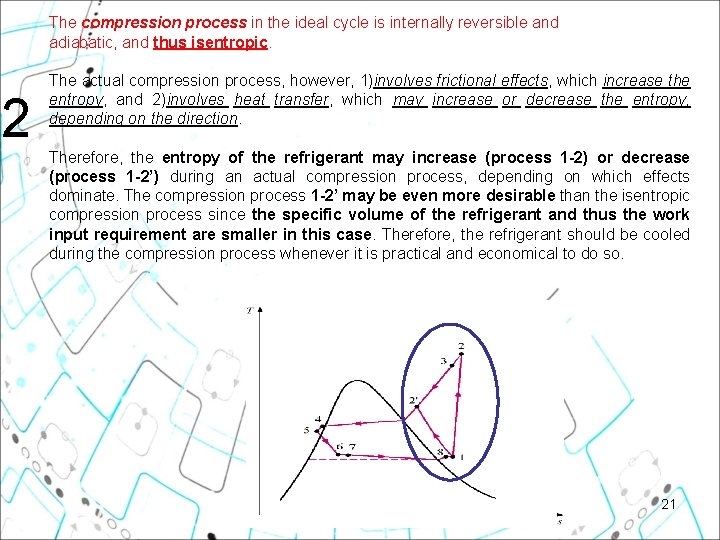
The compression process in the ideal cycle is internally reversible and adiabatic, and thus isentropic. 2 The actual compression process, however, 1)involves frictional effects, which increase the entropy, and 2)involves heat transfer, which may increase or decrease the entropy, depending on the direction. Therefore, the entropy of the refrigerant may increase (process 1 2) or decrease (process 1 2’) during an actual compression process, depending on which effects dominate. The compression process 1 2’ may be even more desirable than the isentropic compression process since the specific volume of the refrigerant and thus the work input requirement are smaller in this case. Therefore, the refrigerant should be cooled during the compression process whenever it is practical and economical to do so. 21

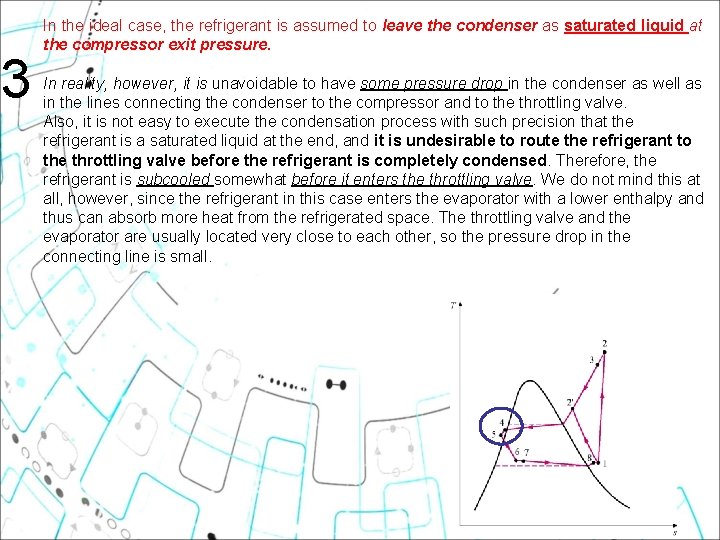
3 In the ideal case, the refrigerant is assumed to leave the condenser as saturated liquid at the compressor exit pressure. In reality, however, it is unavoidable to have some pressure drop in the condenser as well as in the lines connecting the condenser to the compressor and to the throttling valve. Also, it is not easy to execute the condensation process with such precision that the refrigerant is a saturated liquid at the end, and it is undesirable to route the refrigerant to the throttling valve before the refrigerant is completely condensed. Therefore, the refrigerant is subcooled somewhat before it enters the throttling valve. We do not mind this at all, however, since the refrigerant in this case enters the evaporator with a lower enthalpy and thus can absorb more heat from the refrigerated space. The throttling valve and the evaporator are usually located very close to each other, so the pressure drop in the connecting line is small. 22

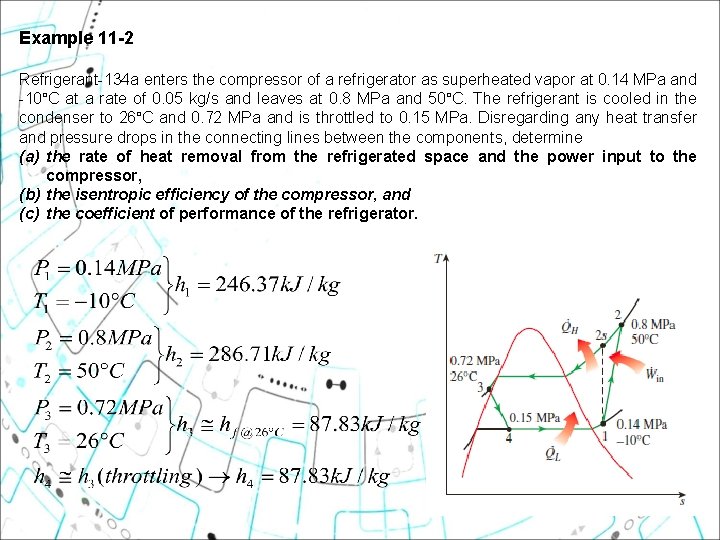
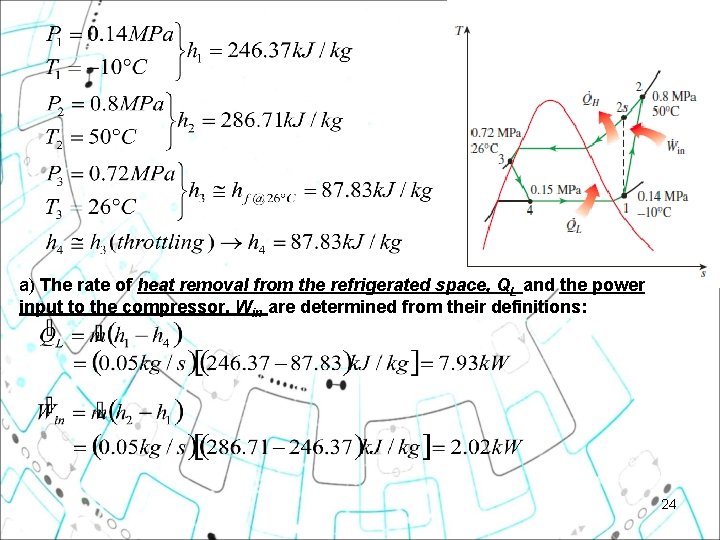
Example 11 2 Refrigerant-134 a enters the compressor of a refrigerator as superheated vapor at 0. 14 MPa and -10°C at a rate of 0. 05 kg/s and leaves at 0. 8 MPa and 50°C. The refrigerant is cooled in the condenser to 26°C and 0. 72 MPa and is throttled to 0. 15 MPa. Disregarding any heat transfer and pressure drops in the connecting lines between the components, determine (a) the rate of heat removal from the refrigerated space and the power input to the compressor, (b) the isentropic efficiency of the compressor, and (c) the coefficient of performance of the refrigerator. 23

a) The rate of heat removal from the refrigerated space, QL and the power input to the compressor, Win are determined from their definitions: 24

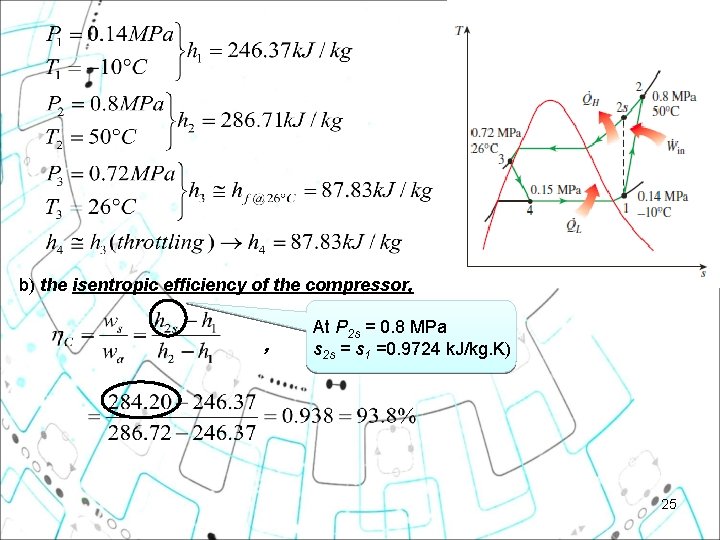
b) the isentropic efficiency of the compressor, , At P 2 s = 0. 8 MPa s 2 s = s 1 =0. 9724 k. J/kg. K) 25

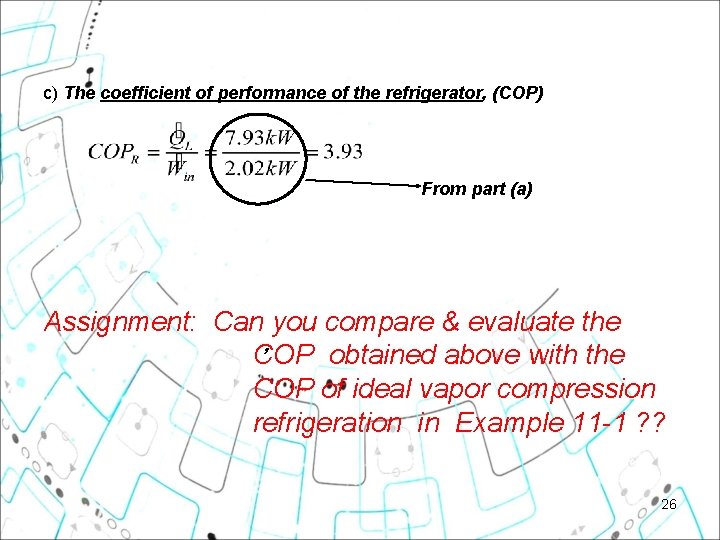
c) The coefficient of performance of the refrigerator, (COP) From part (a) Assignment: Can you compare & evaluate the , COP obtained above with the COP of ideal vapor compression refrigeration in Example 11 -1 ? ? 26

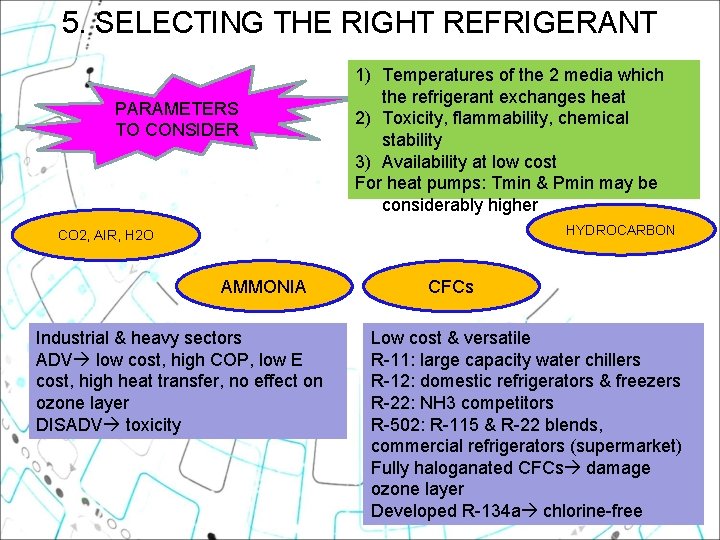
5. SELECTING THE RIGHT REFRIGERANT PARAMETERS TO CONSIDER 1) Temperatures of the 2 media which the refrigerant exchanges heat 2) Toxicity, flammability, chemical stability 3) Availability at low cost For heat pumps: Tmin & Pmin may be considerably higher HYDROCARBON CO 2, AIR, H 2 O AMMONIA Industrial & heavy sectors ADV low cost, high COP, low E cost, high heat transfer, no effect on ozone layer DISADV toxicity CFCs Low cost & versatile R-11: large capacity water chillers R-12: domestic refrigerators & freezers R-22: NH 3 competitors R-502: R-115 & R-22 blends, commercial refrigerators (supermarket) Fully haloganated CFCs damage ozone layer Developed R-134 a chlorine-free 27

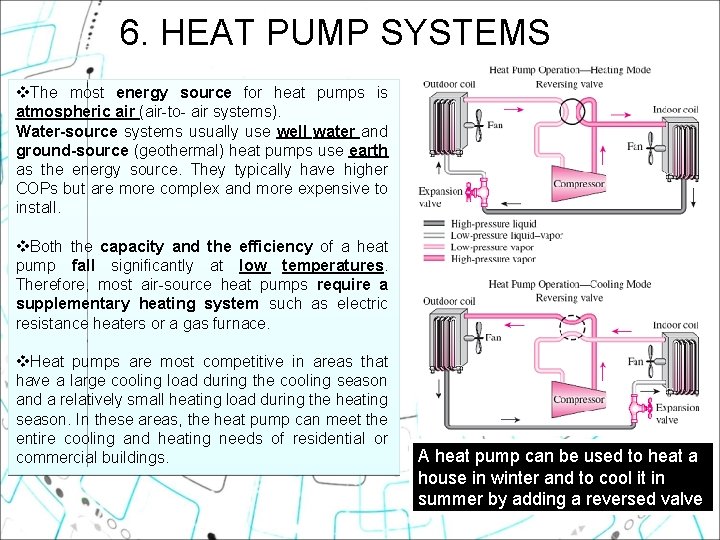
6. HEAT PUMP SYSTEMS v. The most energy source for heat pumps is atmospheric air (air-to- air systems). Water source systems usually use well water and ground source (geothermal) heat pumps use earth as the energy source. They typically have higher COPs but are more complex and more expensive to install. v. Both the capacity and the efficiency of a heat pump fall significantly at low temperatures. Therefore, most air-source heat pumps require a supplementary heating system such as electric resistance heaters or a gas furnace. v. Heat pumps are most competitive in areas that have a large cooling load during the cooling season and a relatively small heating load during the heating season. In these areas, the heat pump can meet the entire cooling and heating needs of residential or commercial buildings. A heat pump can be used to heat a house in winter and to cool it in summer by adding a reversed valve 28

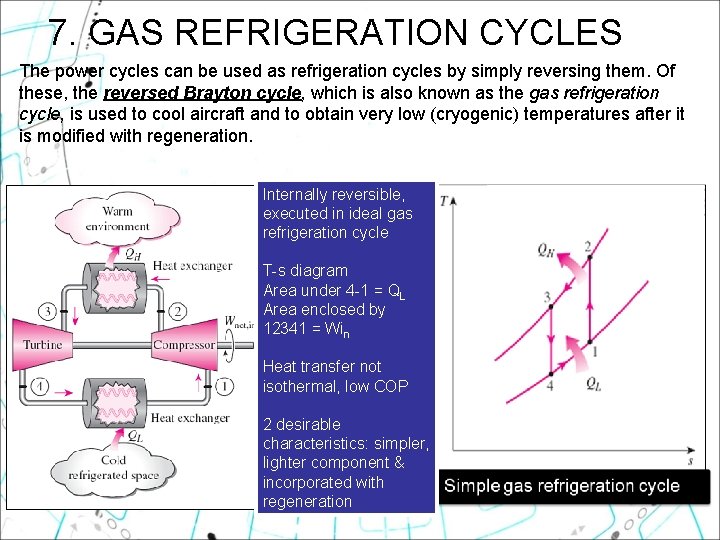
7. GAS REFRIGERATION CYCLES The power cycles can be used as refrigeration cycles by simply reversing them. Of these, the reversed Brayton cycle, which is also known as the gas refrigeration cycle, is used to cool aircraft and to obtain very low (cryogenic) temperatures after it is modified with regeneration. Internally reversible, executed in ideal gas refrigeration cycle T-s diagram Area under 4 -1 = QL Area enclosed by 12341 = Win Heat transfer not isothermal, low COP 2 desirable characteristics: simpler, lighter component & incorporated with regeneration

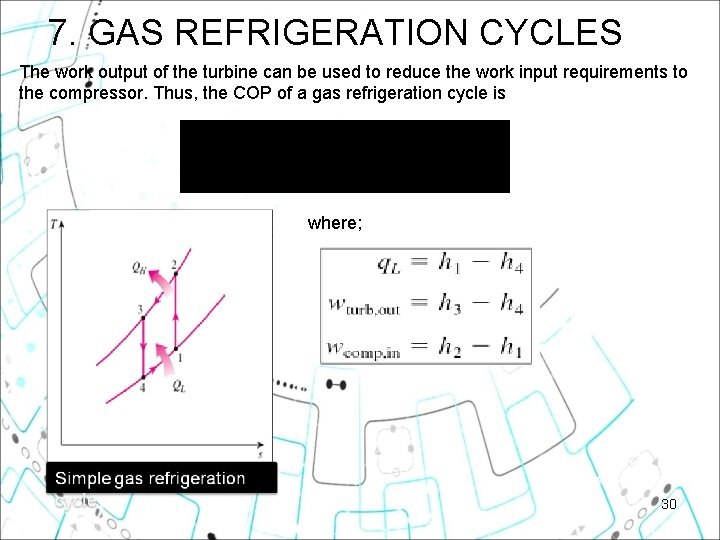
7. GAS REFRIGERATION CYCLES The work output of the turbine can be used to reduce the work input requirements to the compressor. Thus, the COP of a gas refrigeration cycle is where; 30

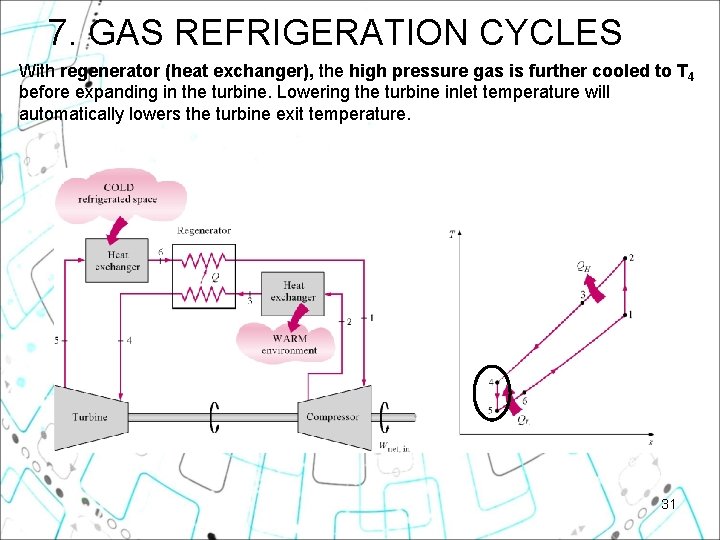
7. GAS REFRIGERATION CYCLES With regenerator (heat exchanger), the high pressure gas is further cooled to T 4 before expanding in the turbine. Lowering the turbine inlet temperature will automatically lowers the turbine exit temperature. 31

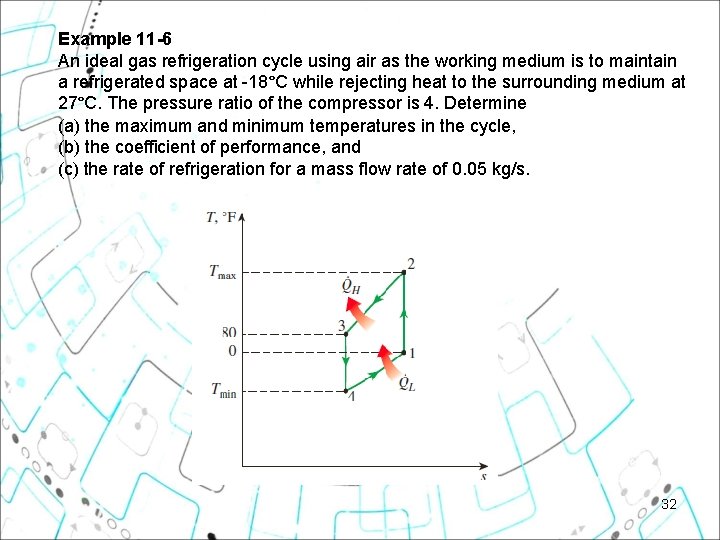
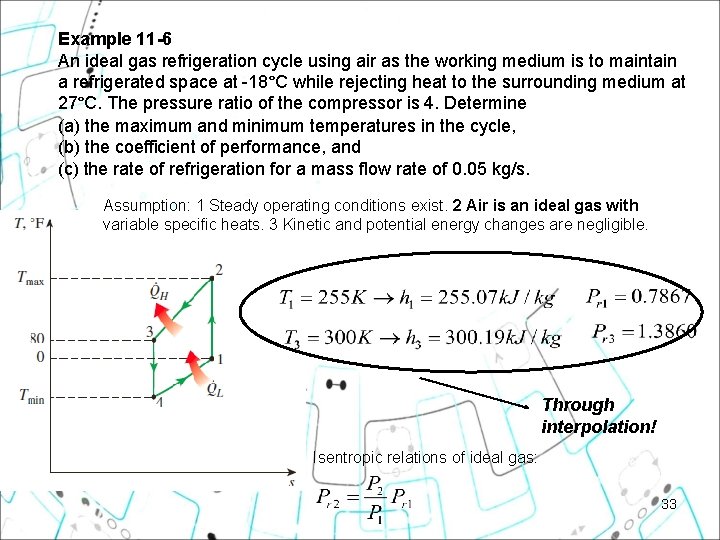
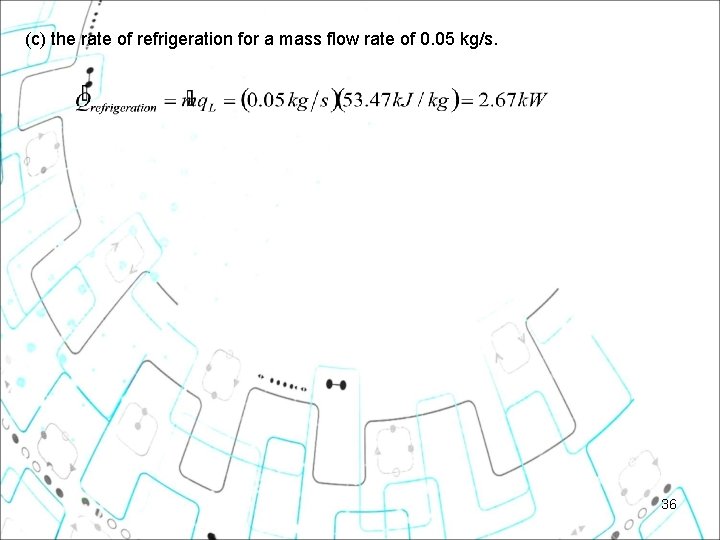
Example 11 6 An ideal gas refrigeration cycle using air as the working medium is to maintain a refrigerated space at -18°C while rejecting heat to the surrounding medium at 27°C. The pressure ratio of the compressor is 4. Determine (a) the maximum and minimum temperatures in the cycle, (b) the coefficient of performance, and (c) the rate of refrigeration for a mass flow rate of 0. 05 kg/s. 32

Example 11 6 An ideal gas refrigeration cycle using air as the working medium is to maintain a refrigerated space at -18°C while rejecting heat to the surrounding medium at 27°C. The pressure ratio of the compressor is 4. Determine (a) the maximum and minimum temperatures in the cycle, (b) the coefficient of performance, and (c) the rate of refrigeration for a mass flow rate of 0. 05 kg/s. Assumption: 1 Steady operating conditions exist. 2 Air is an ideal gas with variable specific heats. 3 Kinetic and potential energy changes are negligible. Through interpolation! Isentropic relations of ideal gas: 33

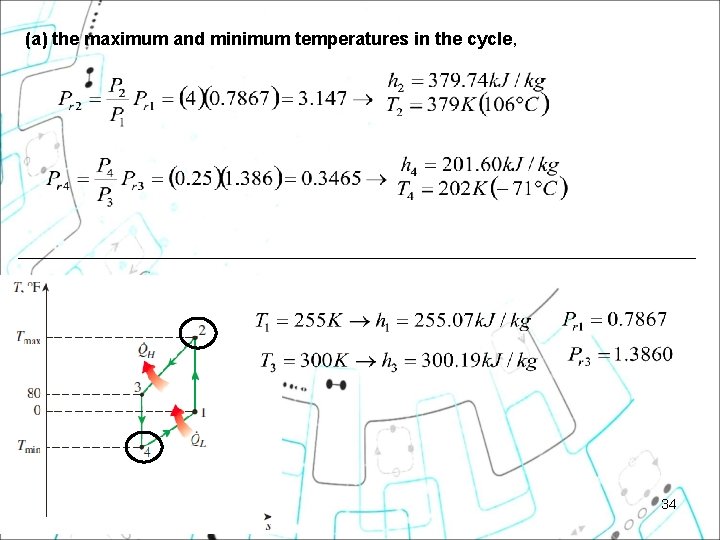
(a) the maximum and minimum temperatures in the cycle, 34

(b) the coefficient of performance, COP 35

(c) the rate of refrigeration for a mass flow rate of 0. 05 kg/s. 36

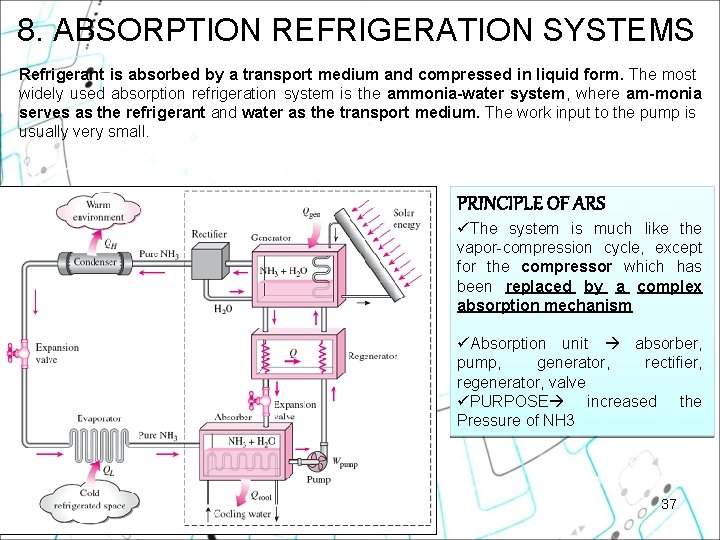
8. ABSORPTION REFRIGERATION SYSTEMS Refrigerant is absorbed by a transport medium and compressed in liquid form. The most widely used absorption refrigeration system is the ammonia water system, where am monia serves as the refrigerant and water as the transport medium. The work input to the pump is usually very small. PRINCIPLE OF ARS üThe system is much like the vapor-compression cycle, except for the compressor which has been replaced by a complex absorption mechanism üAbsorption unit absorber, pump, generator, rectifier, regenerator, valve üPURPOSE increased the Pressure of NH 3 37

8. ABSORPTION REFRIGERATION SYSTEMS 3 4 2 5 6 2 1 MECHANISM: 1. NH 3 vapor leaves evaporator enters the absorber, dissolves & reacts with H 2 O (exo rxn, heat released) 2. The NH 3+H 2 O solution pumped to generator, vaporize some solution by heat transfer to the solution 3. The vapor passes through rectifier to separate the water 4. High P NH 3 vapor enter condenser & the rest of the cycle 5. The hot NH 3+H 2 O solution passes through the regenerator, transfer some heat to rich solution from the pump 38 6. The solution is throttled to the absorber P.

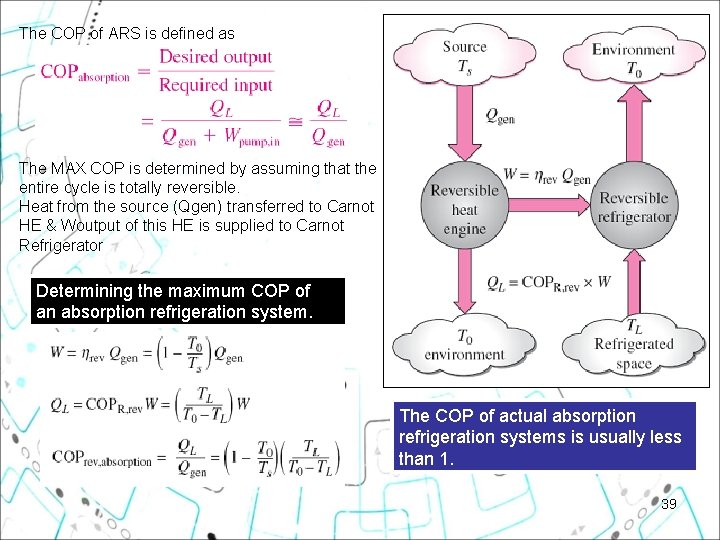
The COP of ARS is defined as The MAX COP is determined by assuming that the entire cycle is totally reversible. Heat from the source (Qgen) transferred to Carnot HE & Woutput of this HE is supplied to Carnot Refrigerator Determining the maximum COP of an absorption refrigeration system. The COP of actual absorption refrigeration systems is usually less than 1. 39

THANK YOU 40