Chapter 11 recombinant DNA and related techniques Fig
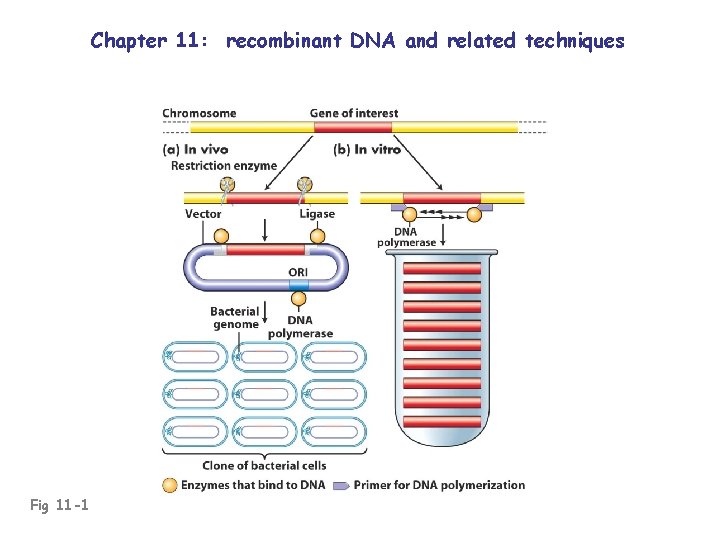
Chapter 11: recombinant DNA and related techniques Fig 11 -1
Recombinant (chimeric) DNA: fused DNA from two different organisms Recombinant clone: vector (bacterial plasmid, virus) + insert (DNA fragment to be cloned) Recombinant (transgenic) organisms: host genome + clone from another organism
c. DNA: “complementary DNA”; DNA complementary to RNA • Usually made against m. RNA • c. DNA is essentially an intron-less copy of a gene, minus 5’ and 3’ flanking regulatory regions of the gene • Prepared using reverse transcriptase (an RNAdependent DNA polymerase enzyme of RNA viruses)
Creating c. DNA (DNA complementary to m. RNA) Fig 11 -2
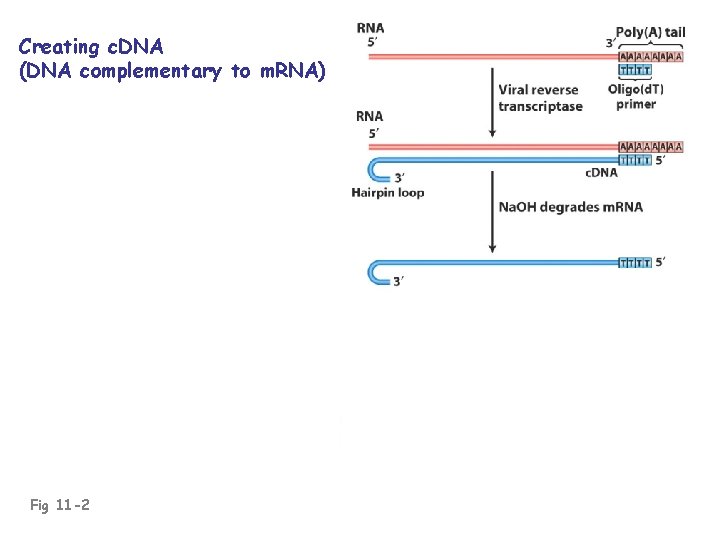
Creating c. DNA (DNA complementary to m. RNA) Fig 11 -2
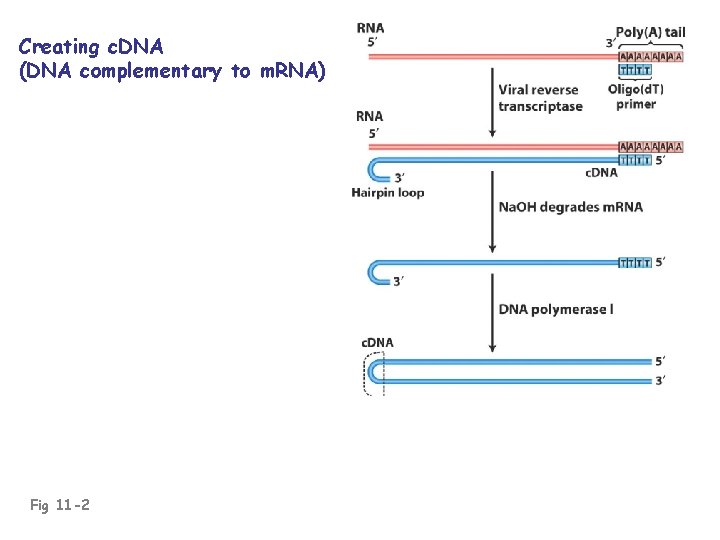
Creating c. DNA (DNA complementary to m. RNA) Fig 11 -2
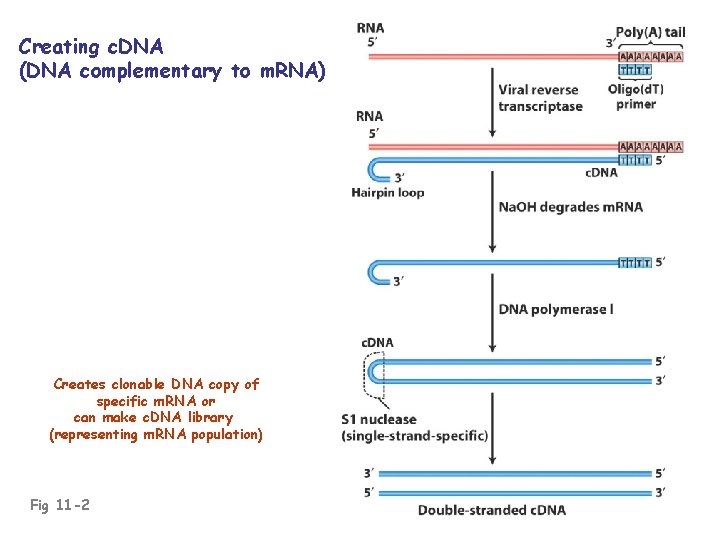
Creating c. DNA (DNA complementary to m. RNA) Creates clonable DNA copy of specific m. RNA or can make c. DNA library (representing m. RNA population) Fig 11 -2
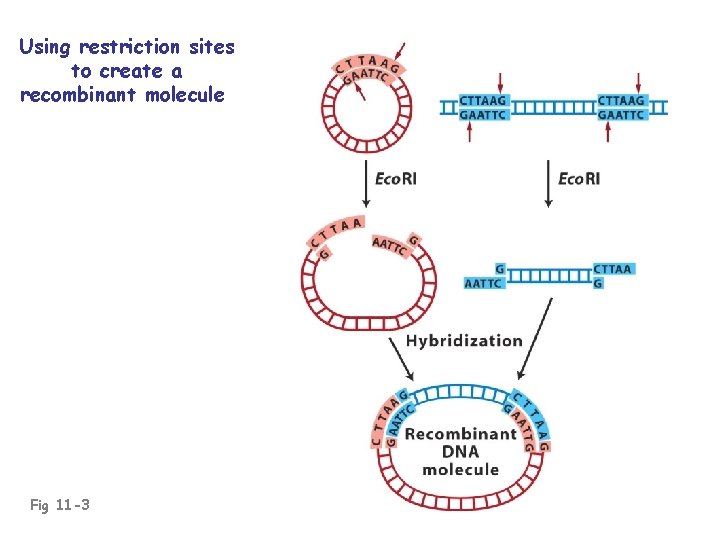
Using restriction sites to create a recombinant molecule Fig 11 -3
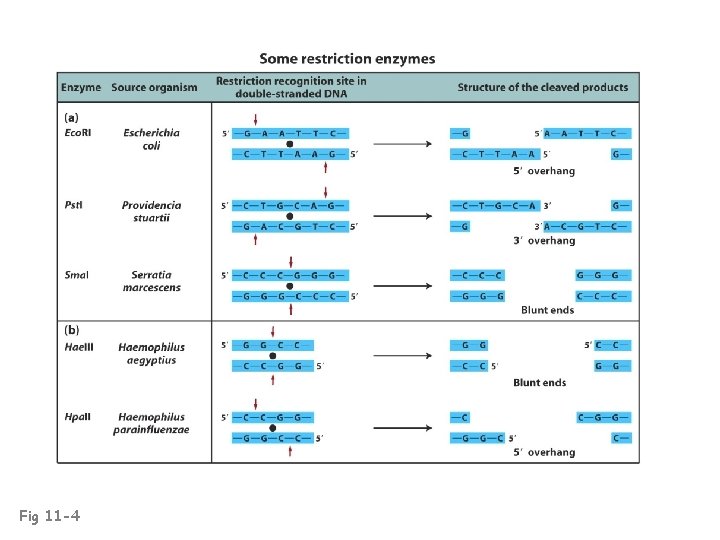
Fig 11 -4
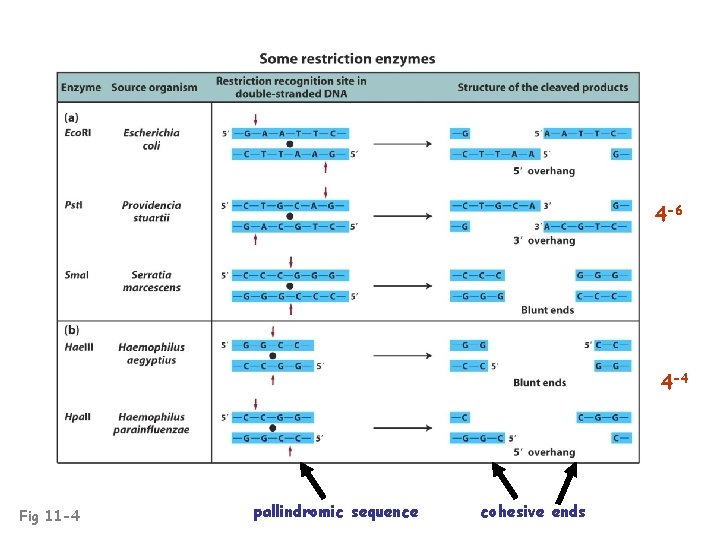
4 -6 4 -4 Fig 11 -4 pallindromic sequence cohesive ends
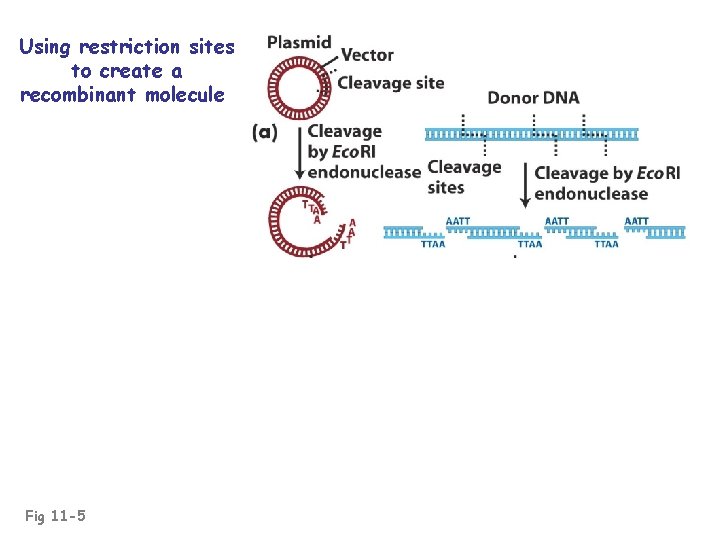
Using restriction sites to create a recombinant molecule Fig 11 -5
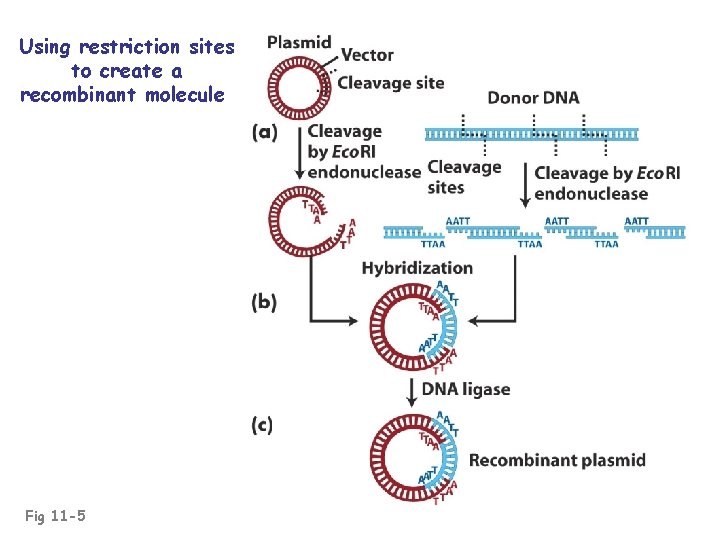
Using restriction sites to create a recombinant molecule Fig 11 -5
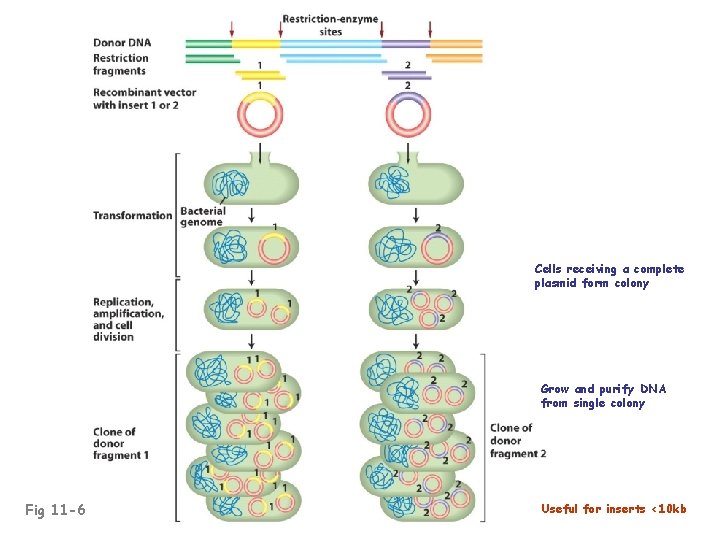
Cells receiving a complete plasmid form colony Grow and purify DNA from single colony Fig 11 -6 Useful for inserts <10 kb
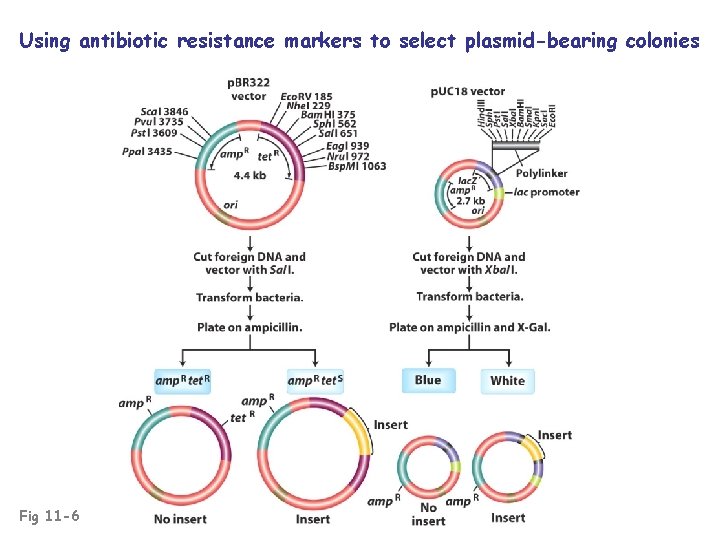
Using antibiotic resistance markers to select plasmid-bearing colonies Fig 11 -6
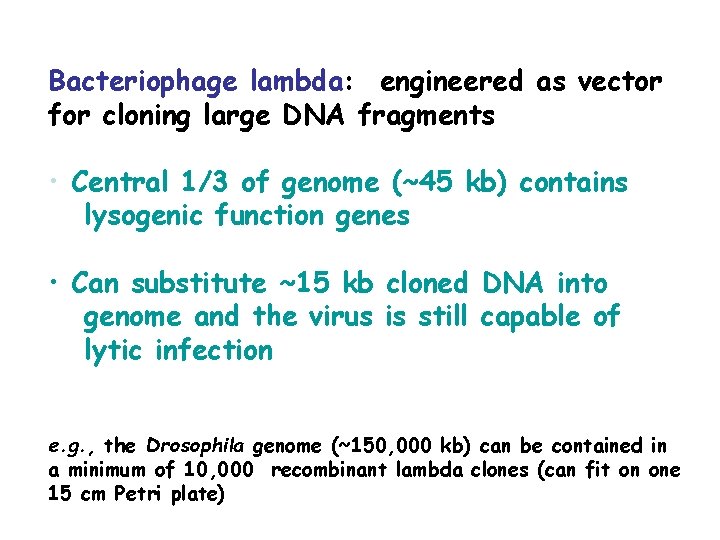
Bacteriophage lambda: engineered as vector for cloning large DNA fragments • Central 1/3 of genome (~45 kb) contains lysogenic function genes • Can substitute ~15 kb cloned DNA into genome and the virus is still capable of lytic infection e. g. , the Drosophila genome (~150, 000 kb) can be contained in a minimum of 10, 000 recombinant lambda clones (can fit on one 15 cm Petri plate)

Creating a genomic library in bacteriophage lambda Fig 11 -7
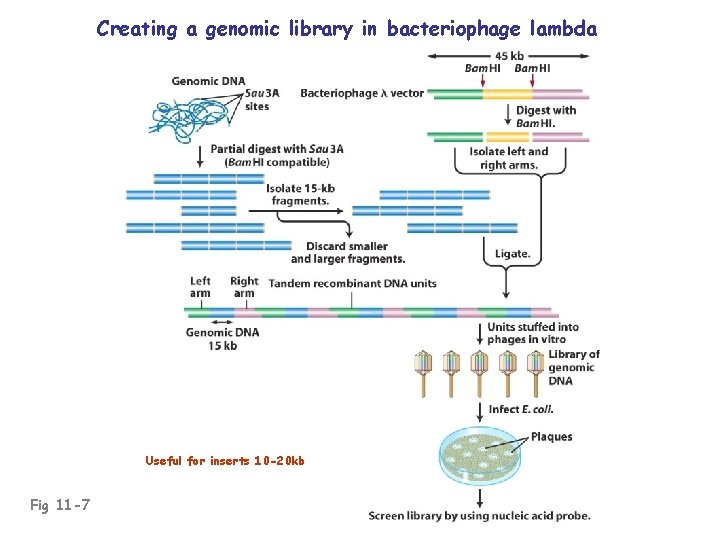
Creating a genomic library in bacteriophage lambda Useful for inserts 10 -20 kb Fig 11 -7
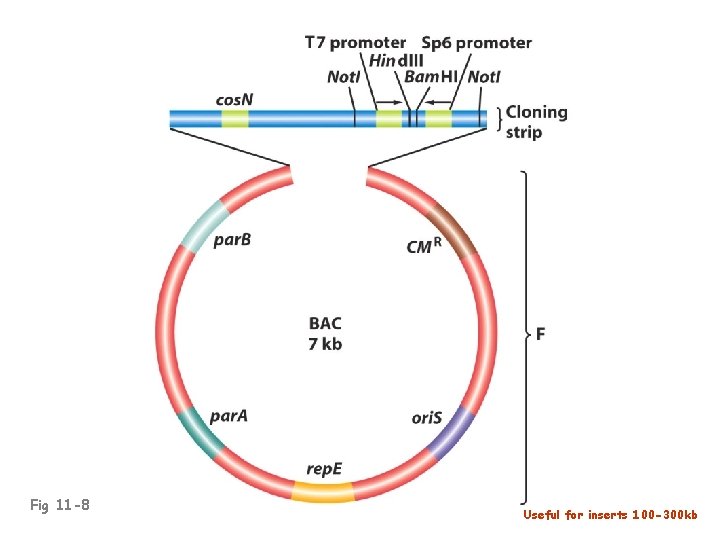
Fig 11 -8 Useful for inserts 100 -300 kb
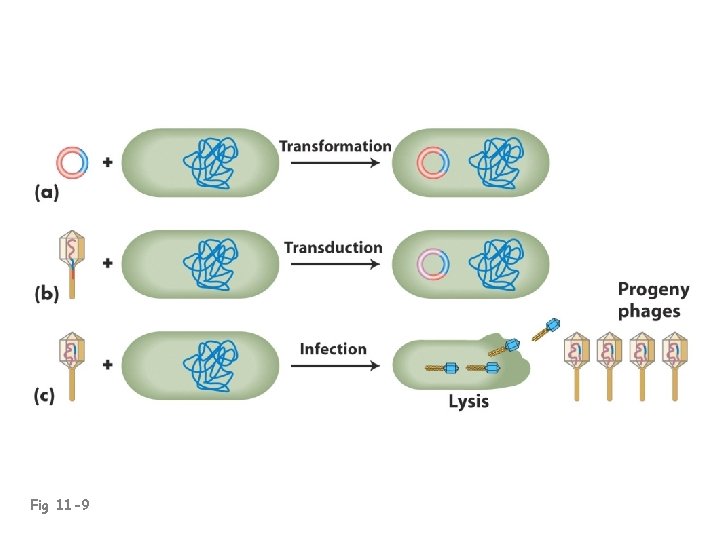
Fig 11 -9

Identifying a desired clone/gene in a library: • Use a probe (previously cloned DNA, oligonucleotide, or antibody)
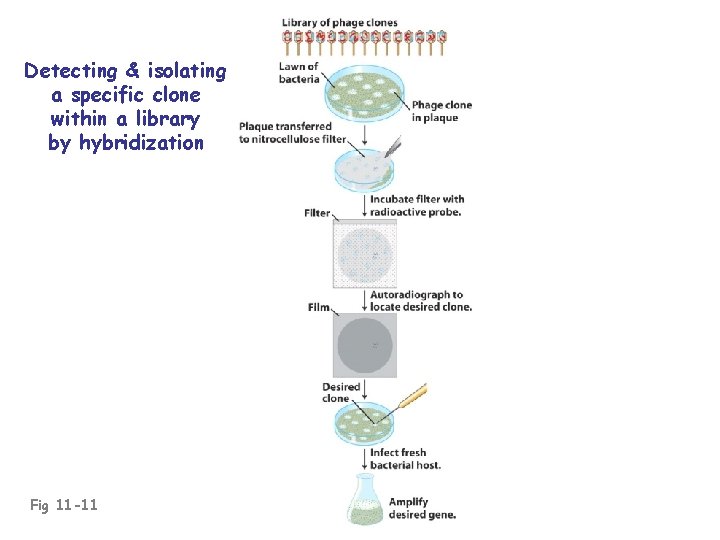
Detecting & isolating a specific clone within a library by hybridization Fig 11 -11
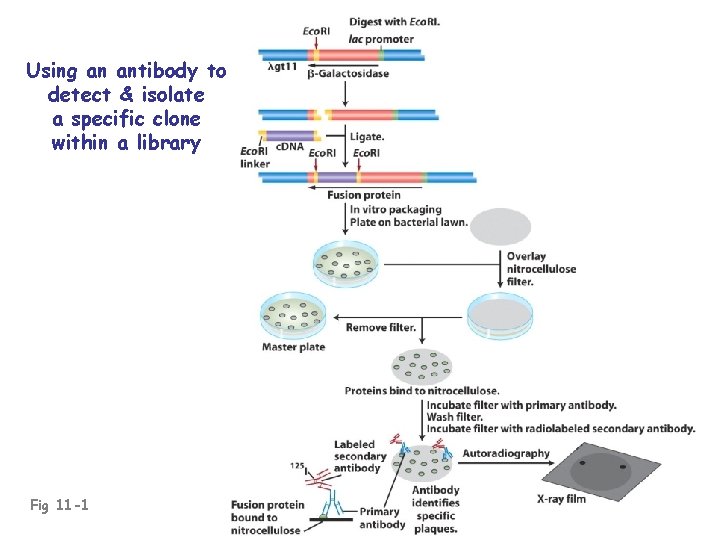
Using an antibody to detect & isolate a specific clone within a library Fig 11 -1

Identifying a desired clone/gene in a library: • Use a probe (previously cloned DNA, oligonucleotide, or antibody) • Functional complementation (useful in organisms with small genomes) • Positional cloning (chromosome “walk” to mutant rearrangement site)
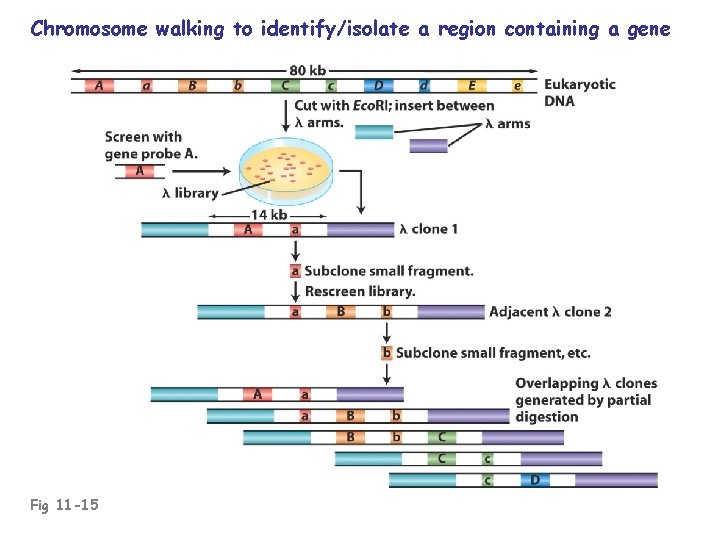
Chromosome walking to identify/isolate a region containing a gene Fig 11 -15
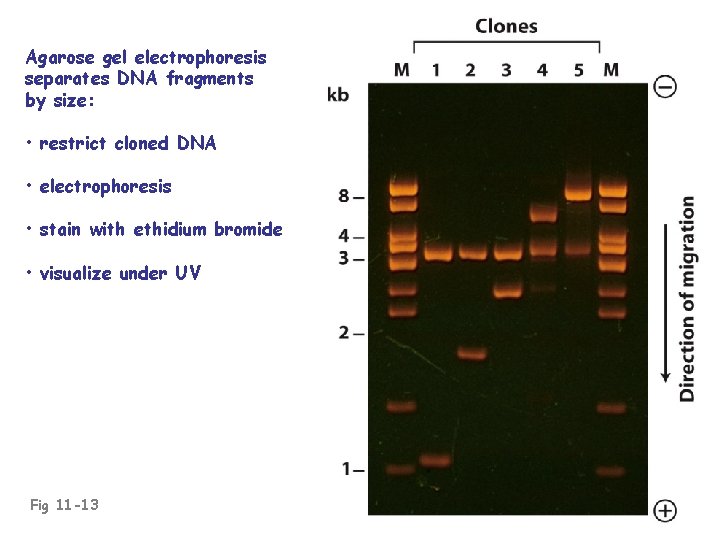
Agarose gel electrophoresis separates DNA fragments by size: • restrict cloned DNA • electrophoresis • stain with ethidium bromide • visualize under UV Fig 11 -13
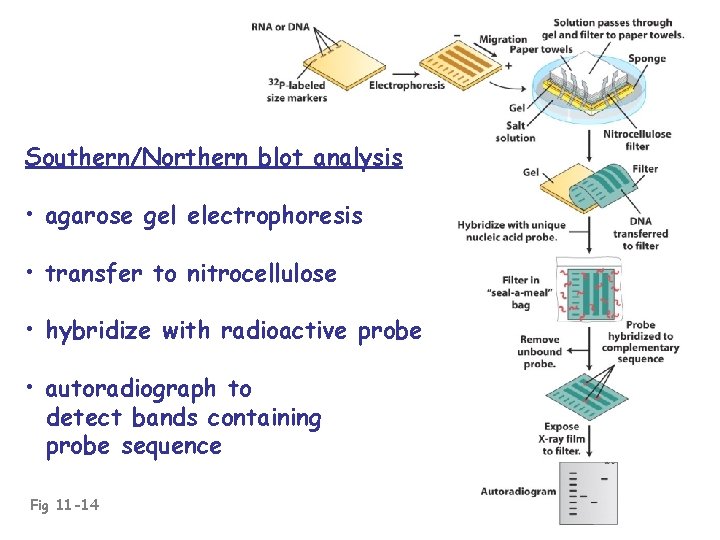
Southern/Northern blot analysis • agarose gel electrophoresis • transfer to nitrocellulose • hybridize with radioactive probe • autoradiograph to detect bands containing probe sequence Fig 11 -14
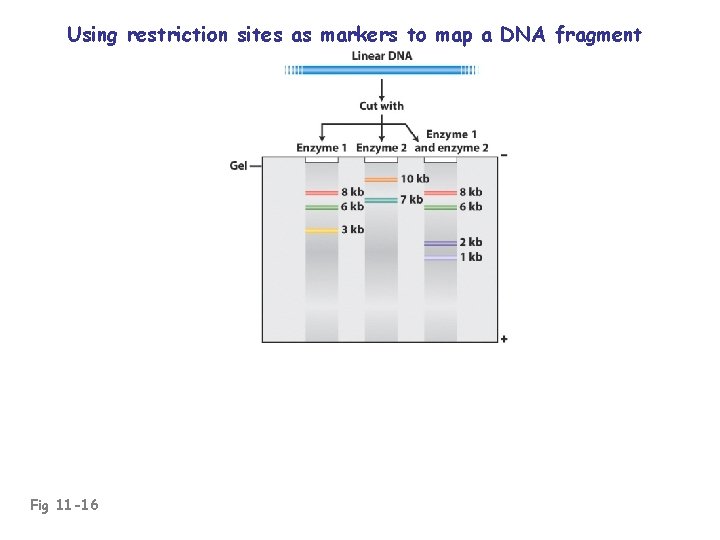
Using restriction sites as markers to map a DNA fragment Fig 11 -16
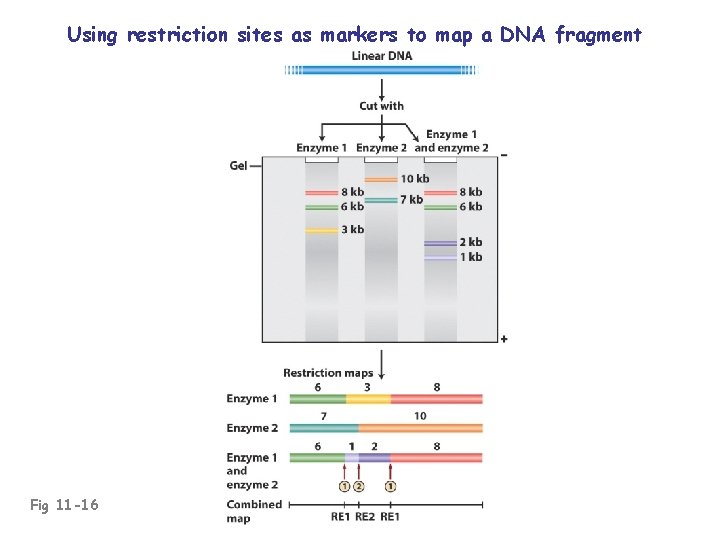
Using restriction sites as markers to map a DNA fragment Fig 11 -16
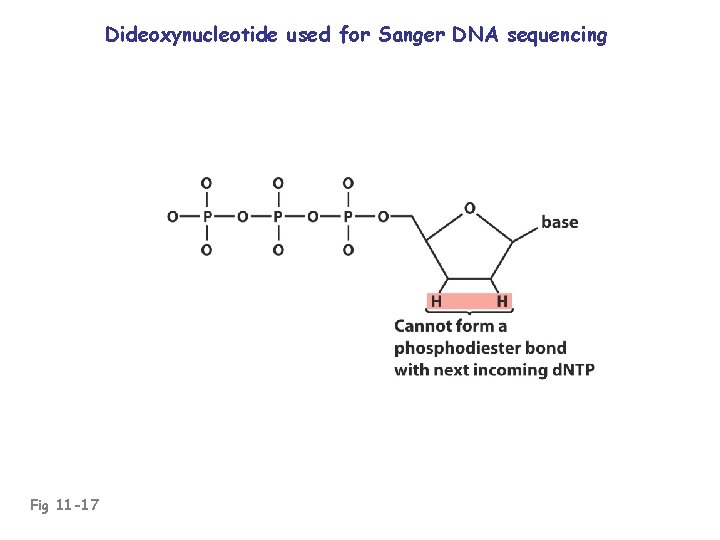
Dideoxynucleotide used for Sanger DNA sequencing Fig 11 -17
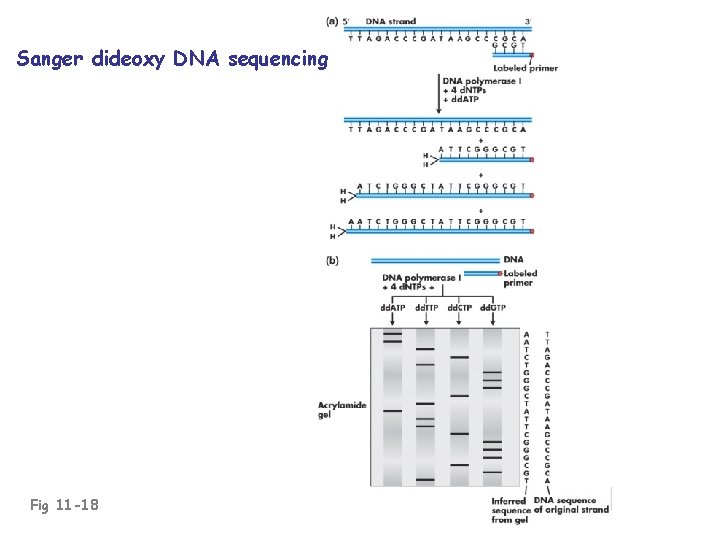
Sanger dideoxy DNA sequencing Fig 11 -18
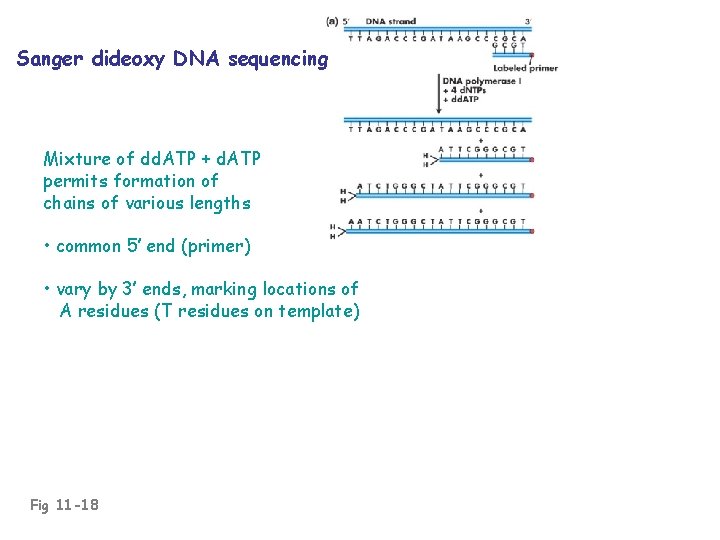
Sanger dideoxy DNA sequencing Mixture of dd. ATP + d. ATP permits formation of chains of various lengths • common 5’ end (primer) • vary by 3’ ends, marking locations of A residues (T residues on template) Fig 11 -18
Sanger dideoxy DNA sequencing Fig 11 -18
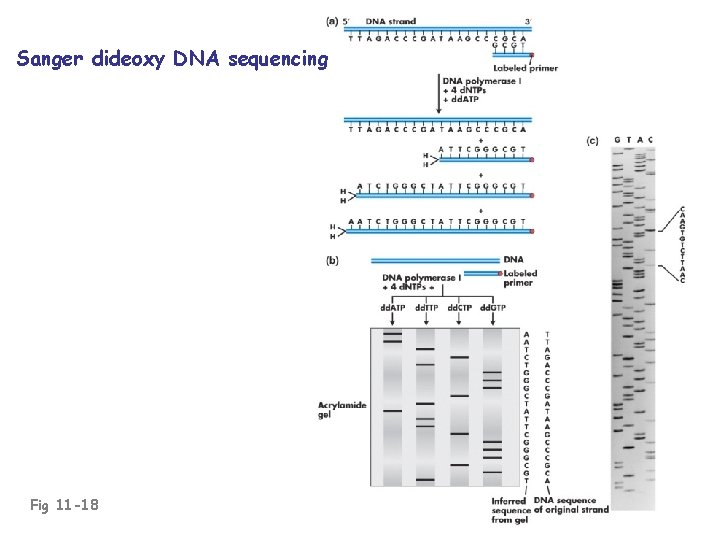
Sanger dideoxy DNA sequencing Fig 11 -18
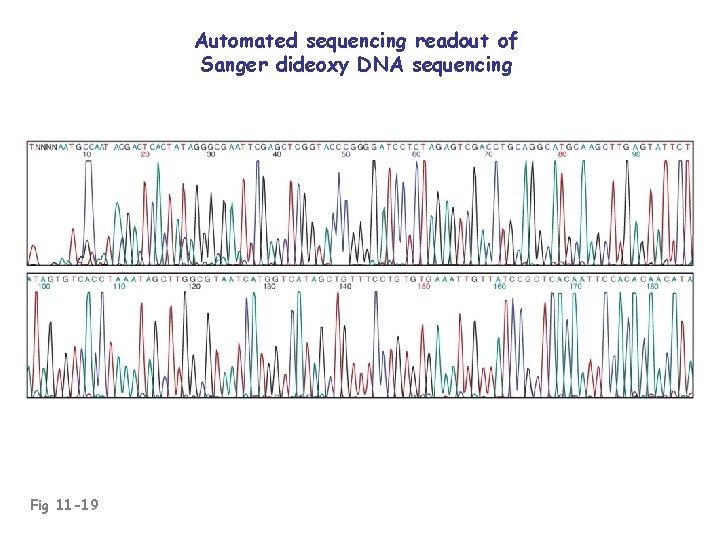
Automated sequencing readout of Sanger dideoxy DNA sequencing Fig 11 -19
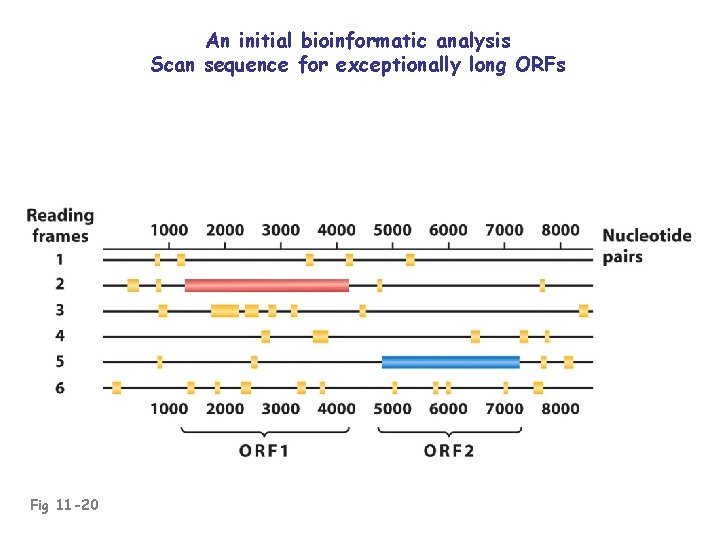
An initial bioinformatic analysis Scan sequence for exceptionally long ORFs Fig 11 -20
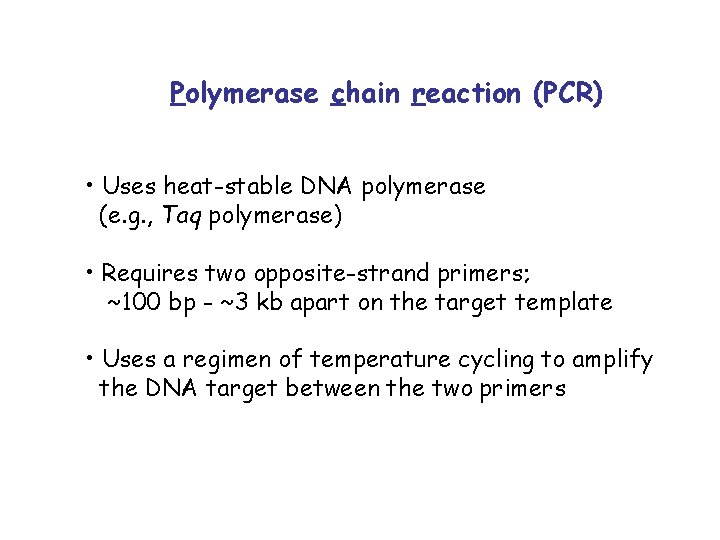
Polymerase chain reaction (PCR) • Uses heat-stable DNA polymerase (e. g. , Taq polymerase) • Requires two opposite-strand primers; ~100 bp - ~3 kb apart on the target template • Uses a regimen of temperature cycling to amplify the DNA target between the two primers
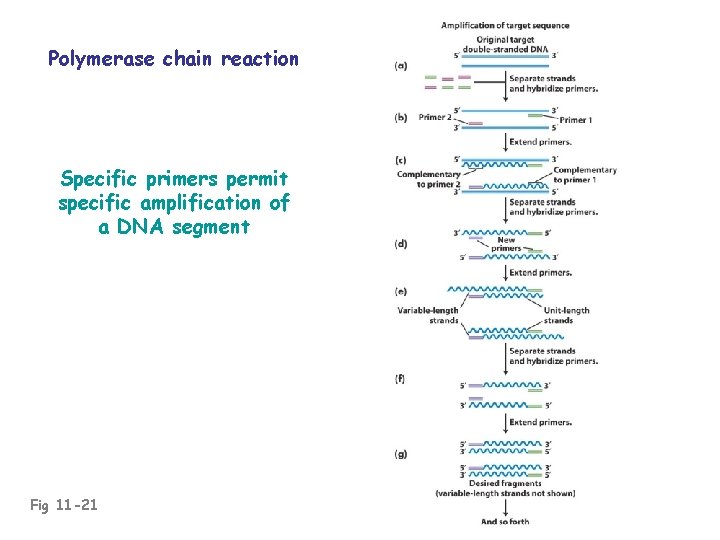
Polymerase chain reaction Specific primers permit specific amplification of a DNA segment Fig 11 -21
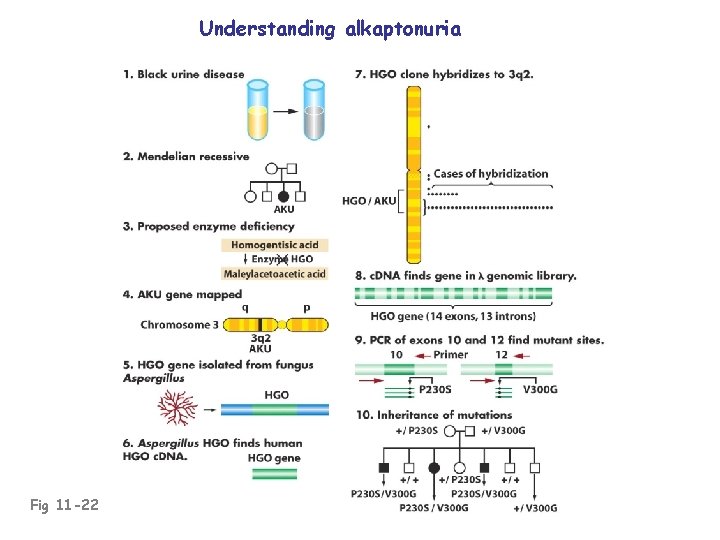
Understanding alkaptonuria Fig 11 -22
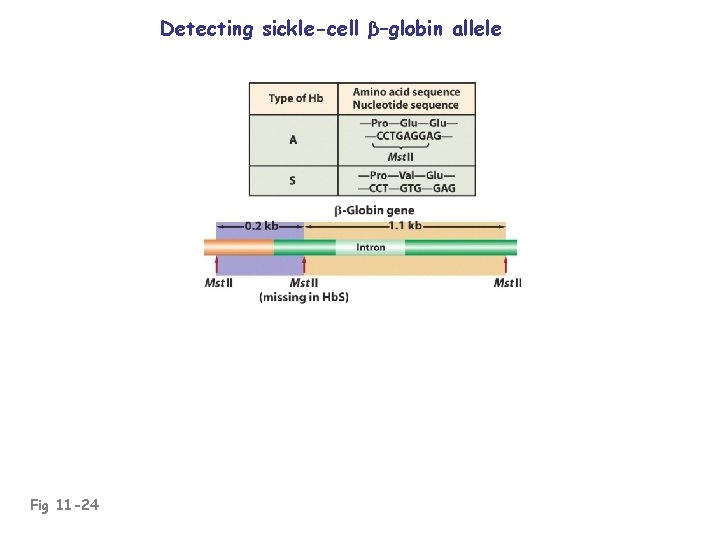
Detecting sickle-cell β–globin allele Fig 11 -24
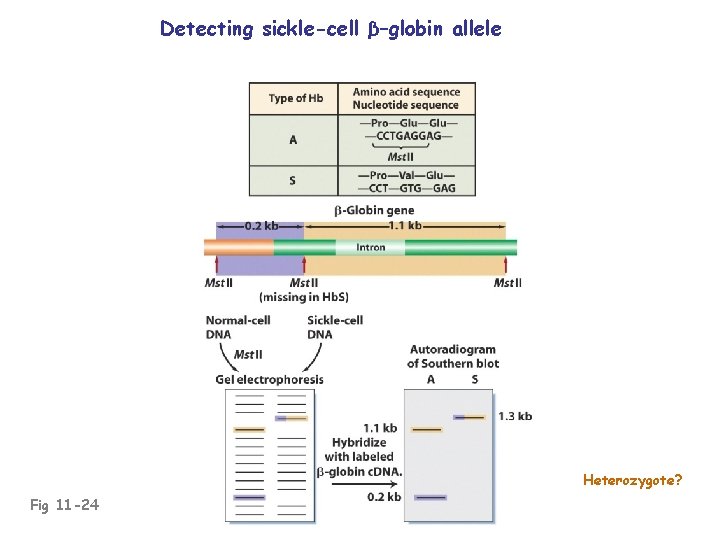
Detecting sickle-cell β–globin allele Heterozygote? Fig 11 -24
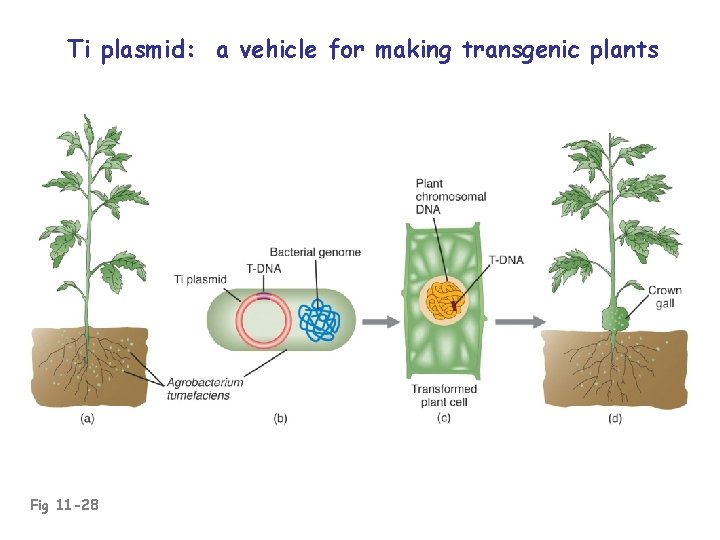
Ti plasmid: a vehicle for making transgenic plants Fig 11 -28
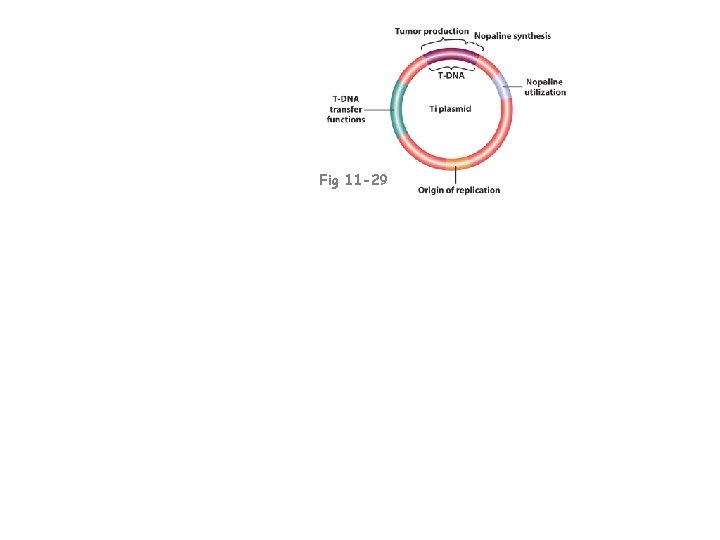
Fig 11 -29
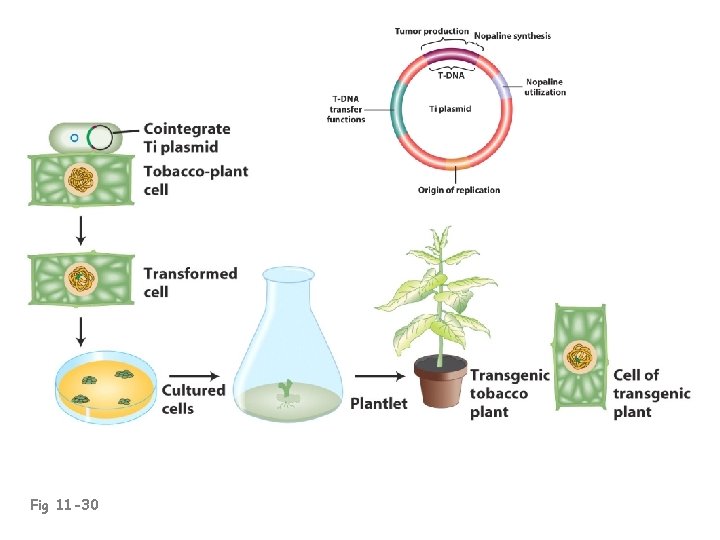
Fig 11 -30
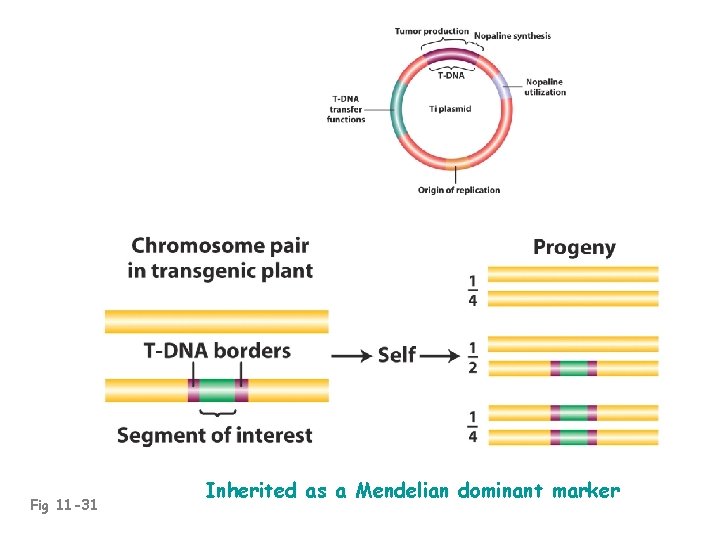
Fig 11 -31 Inherited as a Mendelian dominant marker
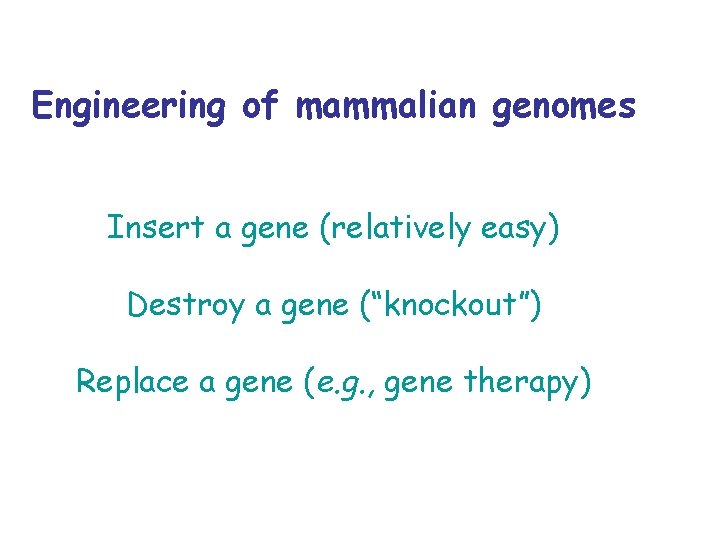
Engineering of mammalian genomes Insert a gene (relatively easy) Destroy a gene (“knockout”) Replace a gene (e. g. , gene therapy)
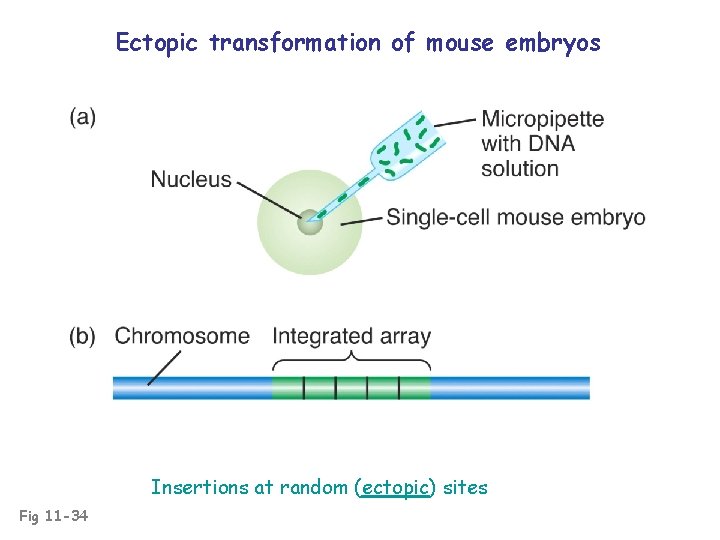
Ectopic transformation of mouse embryos Insertions at random (ectopic) sites Fig 11 -34
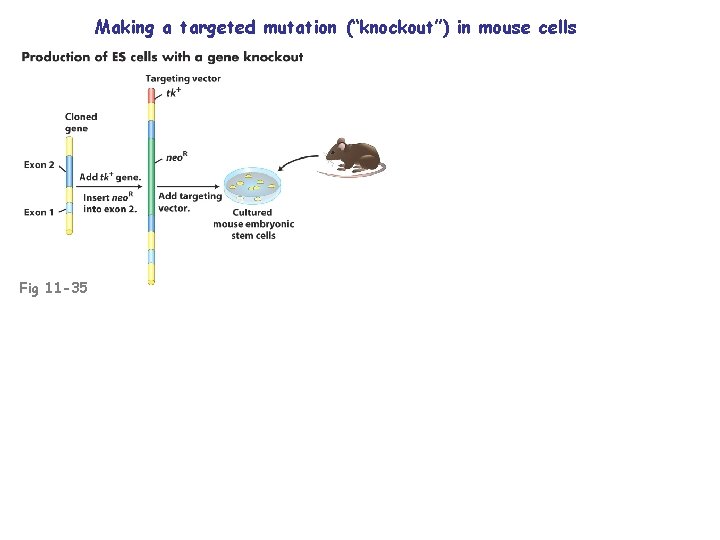
Making a targeted mutation (“knockout”) in mouse cells Fig 11 -35
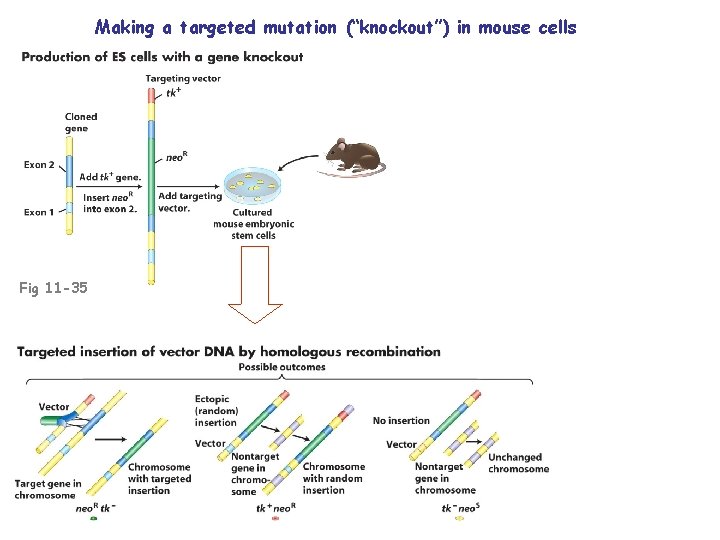
Making a targeted mutation (“knockout”) in mouse cells Fig 11 -35
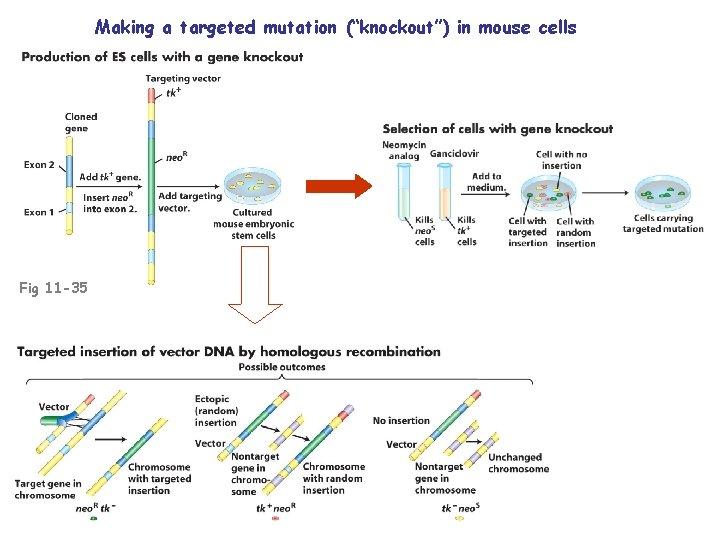
Making a targeted mutation (“knockout”) in mouse cells Fig 11 -35
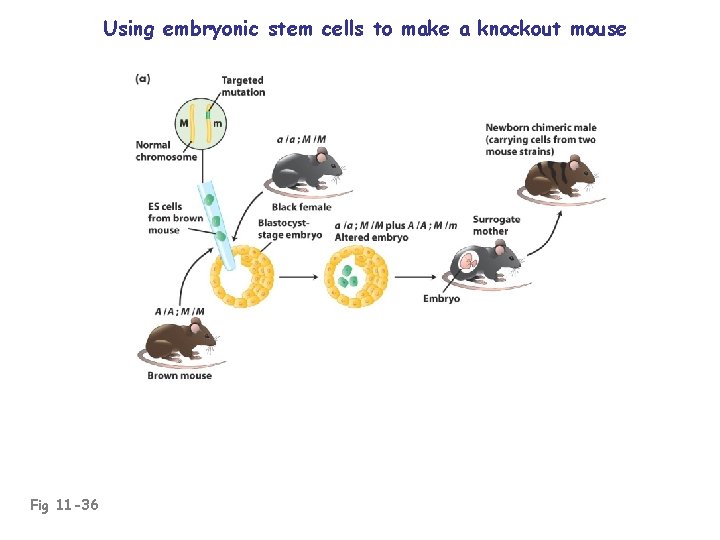
Using embryonic stem cells to make a knockout mouse Fig 11 -36
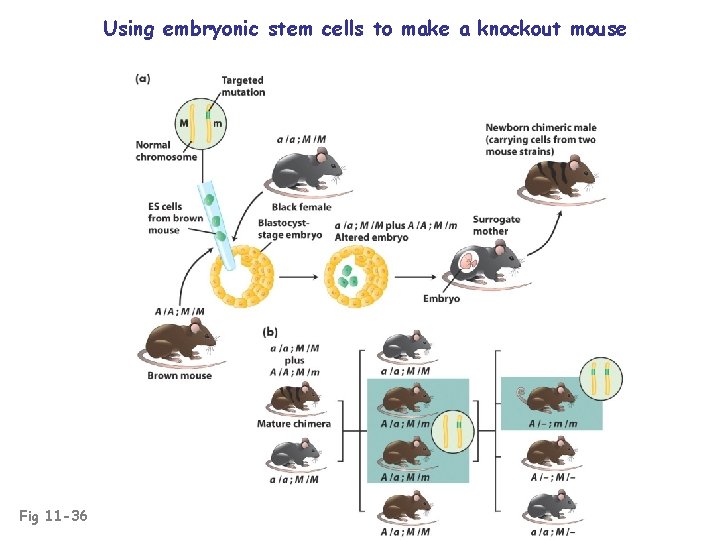
Using embryonic stem cells to make a knockout mouse Fig 11 -36
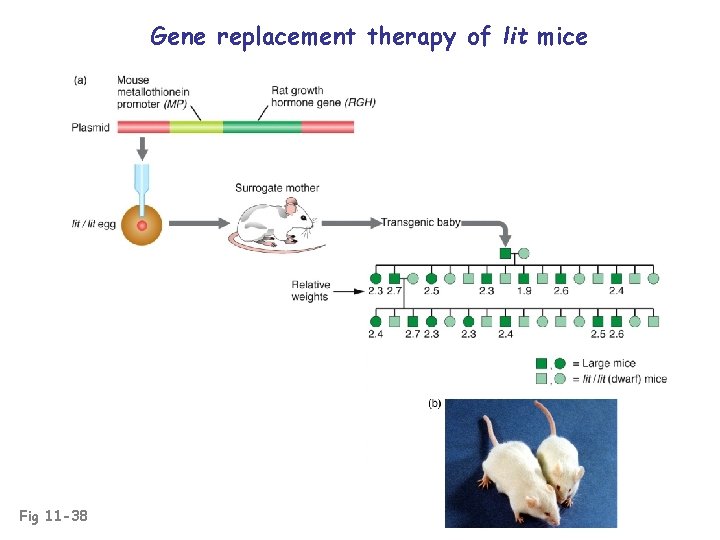
Gene replacement therapy of lit mice Fig 11 -38
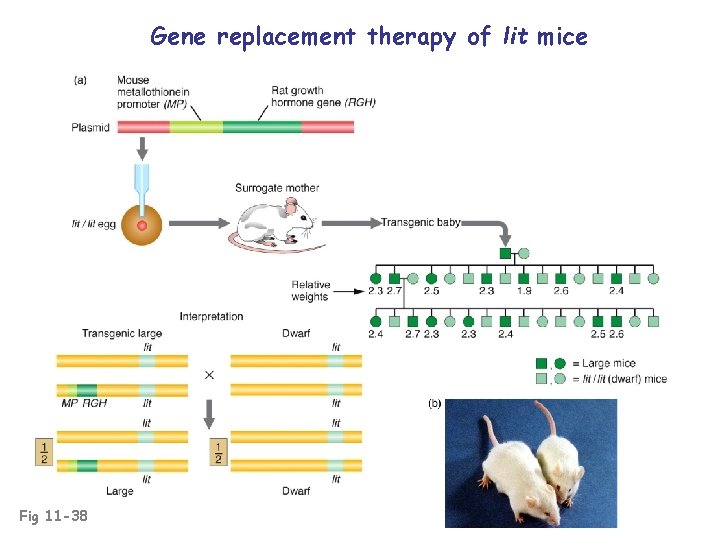
Gene replacement therapy of lit mice Fig 11 -38
Complications arising with germline gene therapy to cure genetic diseases in mammals is that most transgene integration events are random (not targeted) • Transgene does not replace defective gene (just complements it) • Transgene insert might disrupt another gene (creating an undesired mutation) • Transgene will usually segregate independently from the disease-causing gene
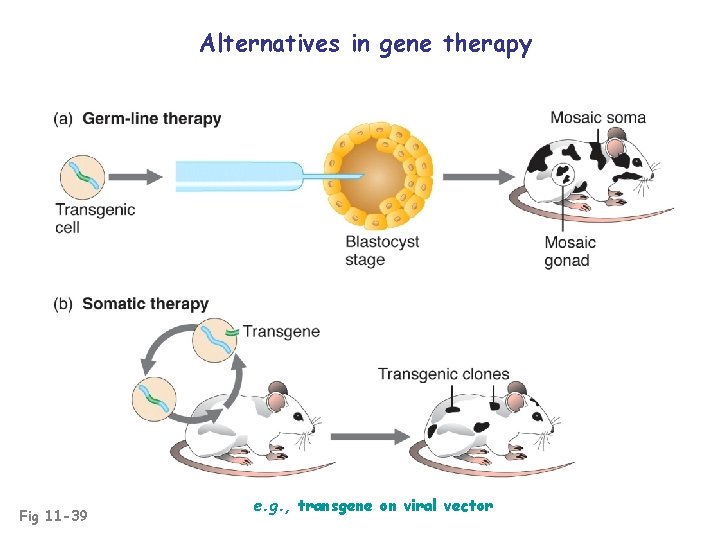
Alternatives in gene therapy Fig 11 -39 e. g. , transgene on viral vector

Fig 11 -

Fig 11 -

Fig 11 -

Fig 11 -

Fig 11 -

Fig 11 -

Fig 11 -

Fig 11 -

Fig 11 -








- Slides: 73