Chapter 11 Properties of Solutions Solution Basics Solution

Chapter 11 Properties of Solutions

Solution Basics • Solution – a homogeneous mixture (can contain gases, liquids, or solids) • Solute – the substance being dissolved • Solvent – the substance doing the dissolving

Enthalpy of Solution (DHsoln) Dissolving a solute in a solvent can be viewed in 3 discreet steps. DHsoln = DH 1 + DH 2 + DH 3

Step 1: Expanding the solvent Step 2: Expanding the solute Endothermic Step 3: Allowing solute and solvent interactions Exothermic DHsoln can be either endo or exothermic depending upon the values of each step.
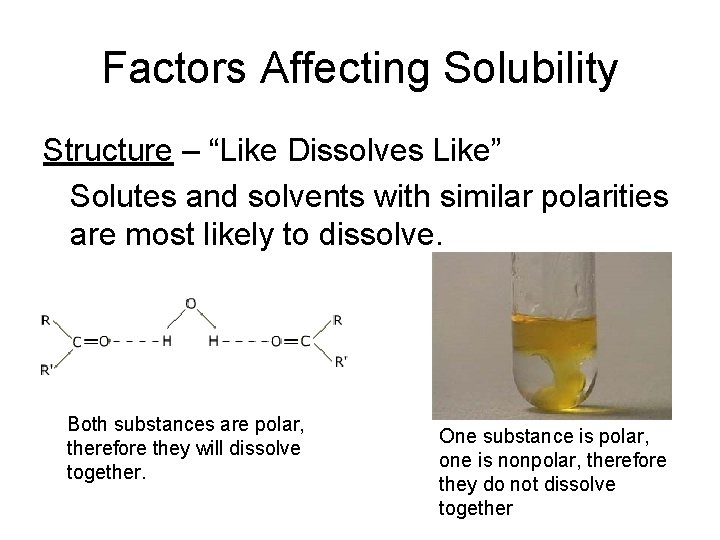
Factors Affecting Solubility Structure – “Like Dissolves Like” Solutes and solvents with similar polarities are most likely to dissolve. Both substances are polar, therefore they will dissolve together. One substance is polar, one is nonpolar, therefore they do not dissolve together
Factors Affecting Solubility Pressure has little effect on the solubility of solids and liquids. Henry’s Law: The partial pressure of a gaseous solute is directly related to the concentration of dissolved gas C=k. P where: C = concentration of dissolved gas k = a constant P = partial pressure of the gas
Factors Affecting Solubility Temperature For gaseous solutes – the higher the temperature, the lower the solubility. For solid solutes – the rate of dissolving always increases as temperature increases and for MOST solids, the solubility of the solid also increases.
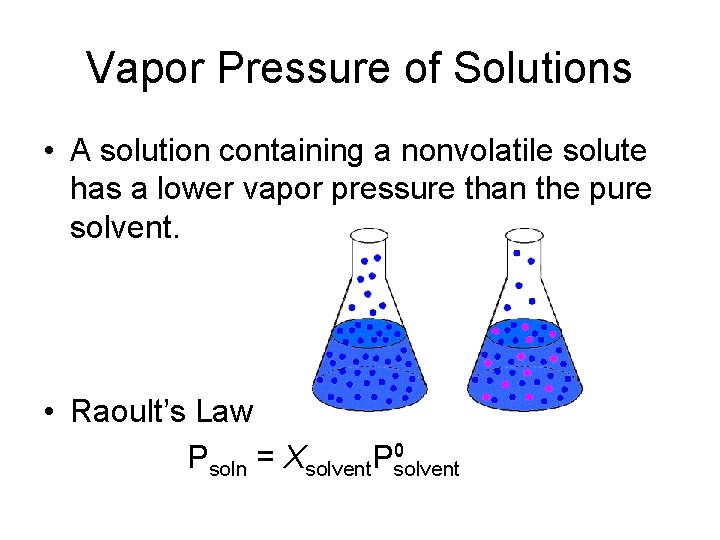
Vapor Pressure of Solutions • A solution containing a nonvolatile solute has a lower vapor pressure than the pure solvent. • Raoult’s Law Psoln = Xsolvent. Psolvent
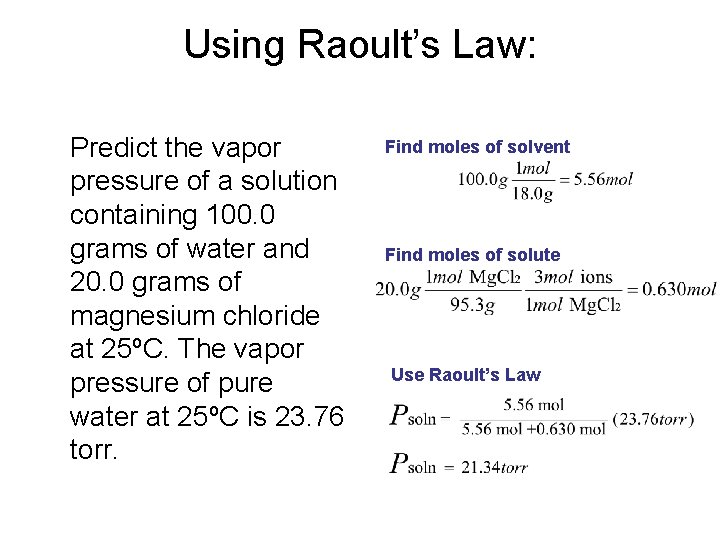
Using Raoult’s Law: Predict the vapor pressure of a solution containing 100. 0 grams of water and 20. 0 grams of magnesium chloride at 25ºC. The vapor pressure of pure water at 25ºC is 23. 76 torr. Find moles of solvent Find moles of solute Use Raoult’s Law
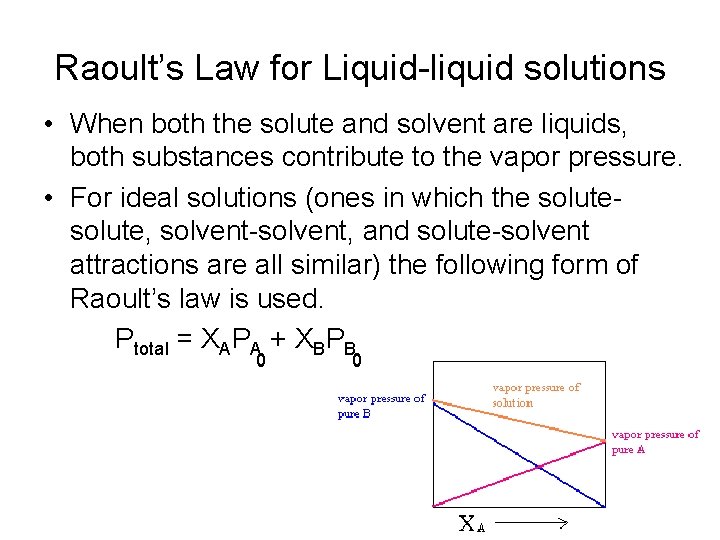
Raoult’s Law for Liquid-liquid solutions • When both the solute and solvent are liquids, both substances contribute to the vapor pressure. • For ideal solutions (ones in which the solute, solvent-solvent, and solute-solvent attractions are all similar) the following form of Raoult’s law is used. Ptotal = XAPA + XBPB
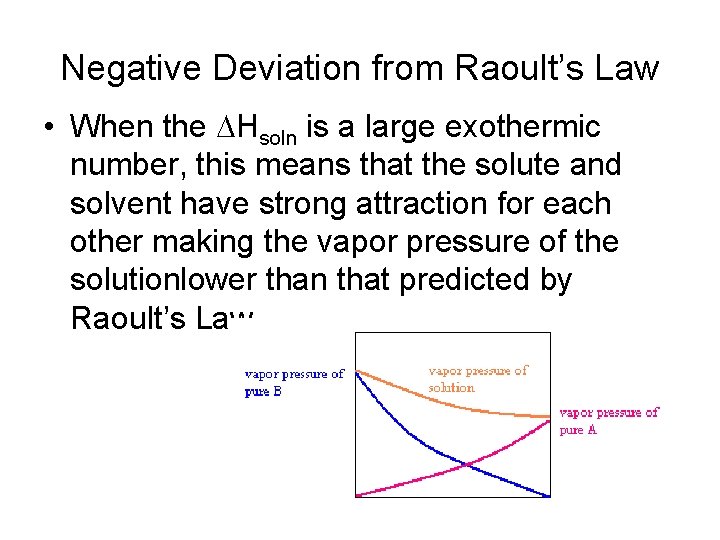
Negative Deviation from Raoult’s Law • When the DHsoln is a large exothermic number, this means that the solute and solvent have strong attraction for each other making the vapor pressure of the solutionlower than that predicted by Raoult’s Law.
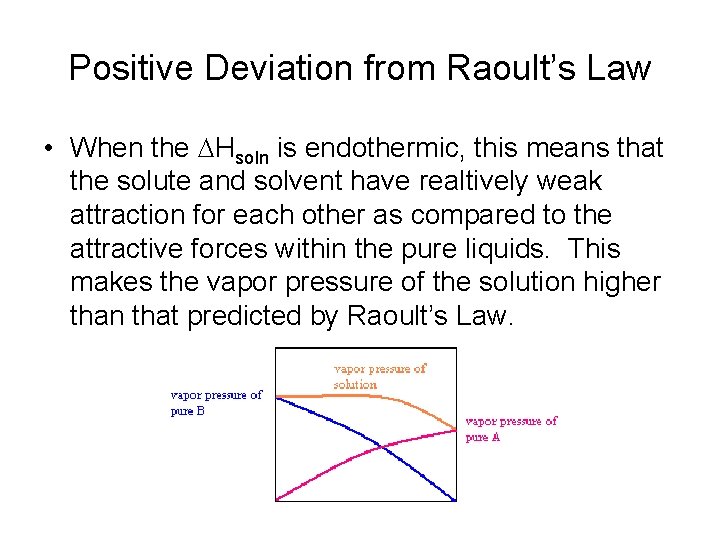
Positive Deviation from Raoult’s Law • When the DHsoln is endothermic, this means that the solute and solvent have realtively weak attraction for each other as compared to the attractive forces within the pure liquids. This makes the vapor pressure of the solution higher than that predicted by Raoult’s Law.
Colligative Properties • Colligative properties depend upon the concentration of solute particles, NOT their identity. • Examples include – Boiling point elevation – Freezing point depression – Osmotic pressure
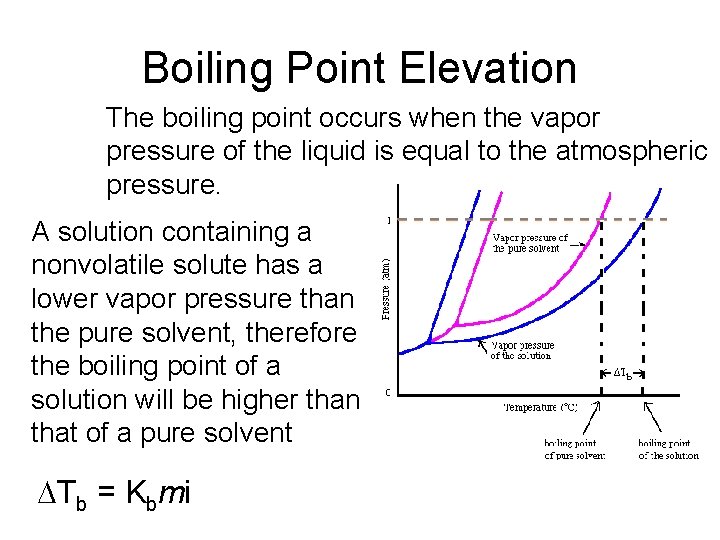
Boiling Point Elevation The boiling point occurs when the vapor pressure of the liquid is equal to the atmospheric pressure. A solution containing a nonvolatile solute has a lower vapor pressure than the pure solvent, therefore the boiling point of a solution will be higher than that of a pure solvent DTb = Kbmi
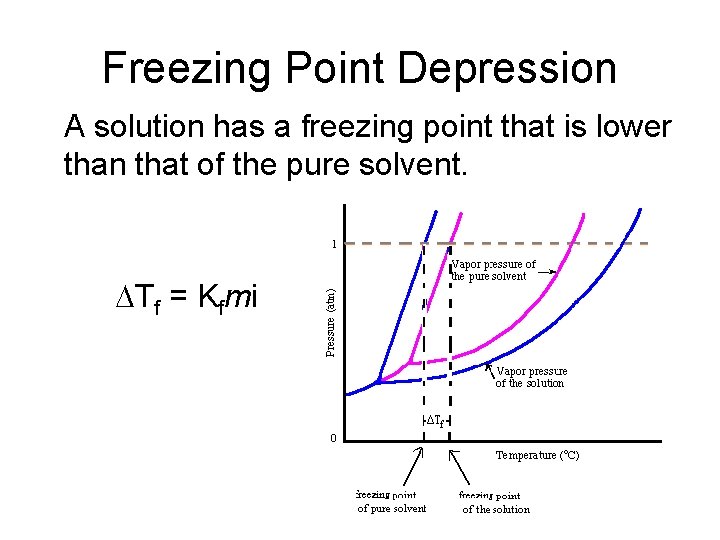
Freezing Point Depression A solution has a freezing point that is lower than that of the pure solvent. DTf = Kfmi
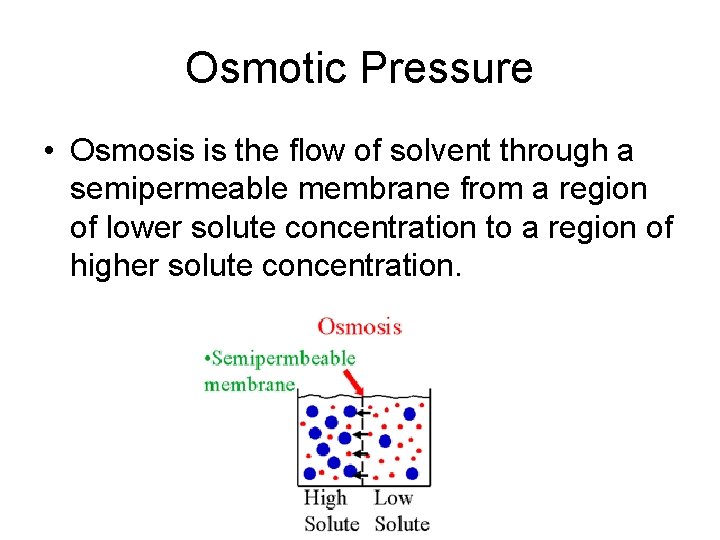
Osmotic Pressure • Osmosis is the flow of solvent through a semipermeable membrane from a region of lower solute concentration to a region of higher solute concentration.
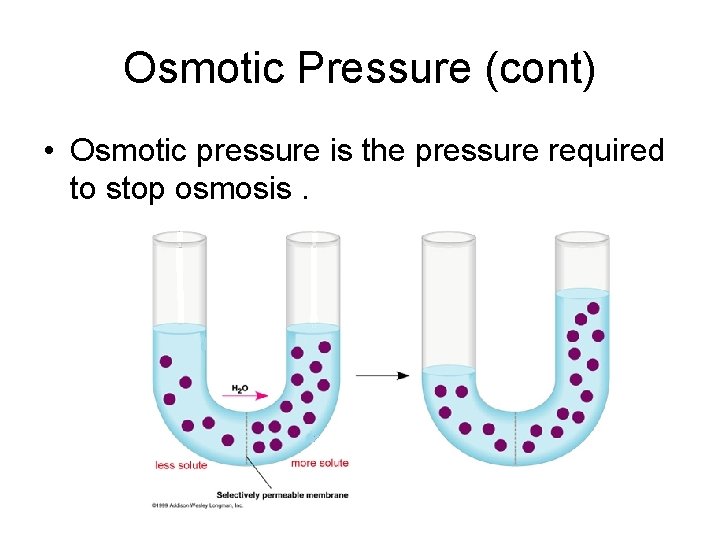
Osmotic Pressure (cont) • Osmotic pressure is the pressure required to stop osmosis.
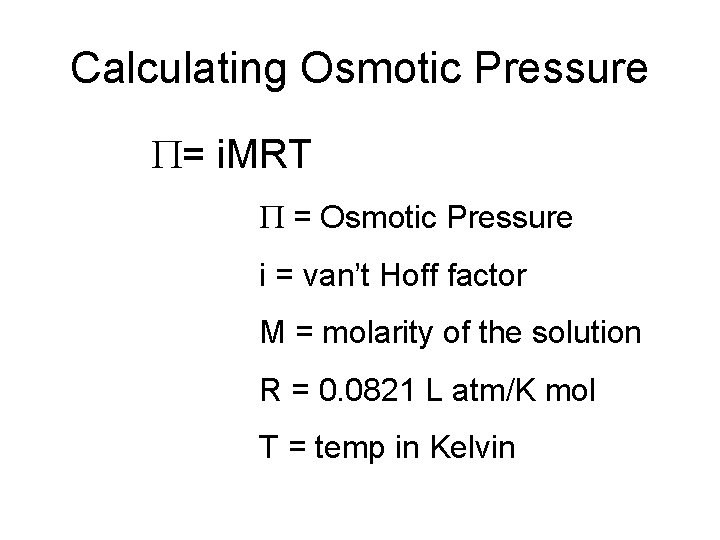
Calculating Osmotic Pressure P= i. MRT P = Osmotic Pressure i = van’t Hoff factor M = molarity of the solution R = 0. 0821 L atm/K mol T = temp in Kelvin
- Slides: 18