Chapter 11 Preparing Sterile Intravenous Products Chapter 11
































































































- Slides: 121


Chapter 11 Preparing Sterile Intravenous Products

Chapter 11 Topics • • Intravenous Preparations Equipment Used in IV Preparation Preparing IVs Calculations for the Hospital Pharmacy Technician

Learning Objectives • Describe the characteristics of intravenous solutions including solubility, osmolality, and p. H • Identify common vehicles for intravenous solutions • Describe the equipment and procedures used in preparing parenterals • Identify the components of an intravenous administration set • Convert from Fahrenheit to Centigrade and vice versa • Calculate the molecular weight and milliequivalents of certain substances used in the pharmacy • Compute the specific gravity of liquids • Calculate intravenous rates and administration

Intravenous Preparations • The IV route of administration is used – – – to reach appropriate drug serum levels to guarantee compliance for drugs with unreliable gastrointestinal (GI) absorption for the patient who can have nothing by mouth for the patient who is unconscious or uncooperative, and for rapid correction of fluid or electrolytes • Most parenterals are introduced directly into the bloodstream – must be free of air bubbles or particulate matter – have many characteristics including solubility, osmolality, and p. H

Characteristics of IV Preparations • Intravenous (IV) preparations are either: – solutions (in which ingredients are dissolved) – suspensions (in which ingredients are suspended) • Most parenteral preparations are made of ingredients in a sterile water medium • Some parenteral preparations may be oleaginous (oily)

Characteristics of IV Preparations • Parenteral IV preparations must have chemical properties that will not – damage vessels or blood cells – alter the chemical properties of the blood serum • With blood, IVs must be – iso-osmotic (having the same number of particles in solution per unit volume) – isotonic (have the same osmotic pressure, meaning the pressure produced by or associated with osmosis)

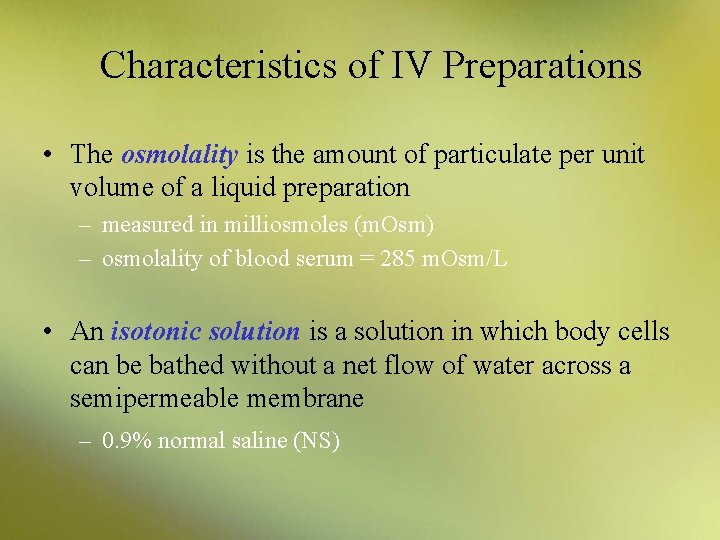
Characteristics of IV Preparations • The osmolality is the amount of particulate per unit volume of a liquid preparation – measured in milliosmoles (m. Osm) – osmolality of blood serum = 285 m. Osm/L • An isotonic solution is a solution in which body cells can be bathed without a net flow of water across a semipermeable membrane – 0. 9% normal saline (NS)

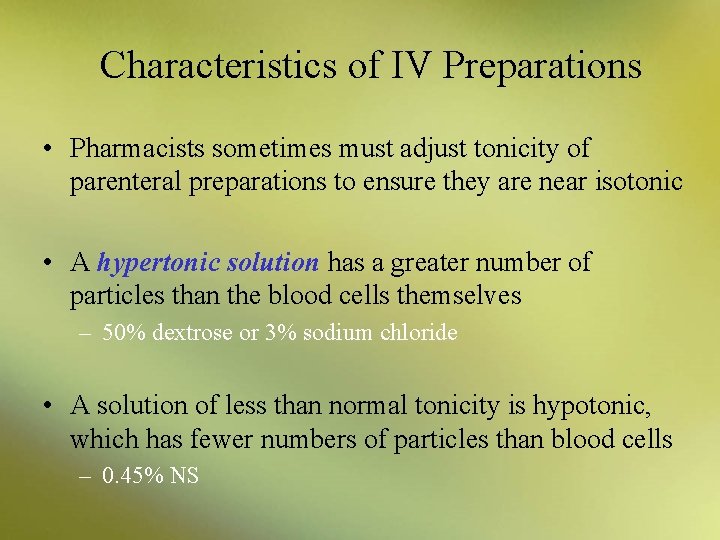
Characteristics of IV Preparations • Pharmacists sometimes must adjust tonicity of parenteral preparations to ensure they are near isotonic • A hypertonic solution has a greater number of particles than the blood cells themselves – 50% dextrose or 3% sodium chloride • A solution of less than normal tonicity is hypotonic, which has fewer numbers of particles than blood cells – 0. 45% NS

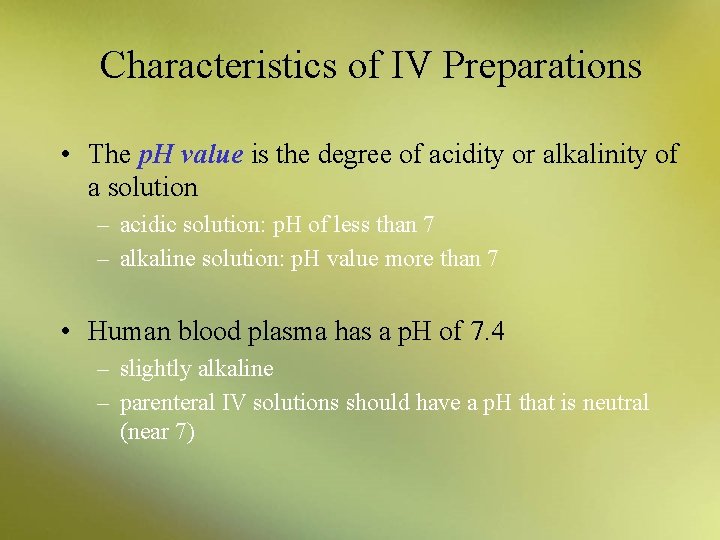
Characteristics of IV Preparations • The p. H value is the degree of acidity or alkalinity of a solution – acidic solution: p. H of less than 7 – alkaline solution: p. H value more than 7 • Human blood plasma has a p. H of 7. 4 – slightly alkaline – parenteral IV solutions should have a p. H that is neutral (near 7)

Methods of Injection • The bolus, or injection, is one of the most common routes of IV administration • The injection is performed using a syringe – prepackaged in the form of filled, disposable plastic syringes – injectable drug must be taken up into the syringe from a single- or multi-dose glass or plastic vial, or from a glass ampule • Sometimes the solid drug in the vial has to be reconstituted by addition of a liquid before use

Methods of Injection • IV infusions deliver: – large amounts of liquid into the bloodstream over prolonged periods of time • IV infusion is used to deliver: – blood – electrolytes – water – other fluids – drugs – nutrients

Discussion What are some characteristics of parenteral preparations, and why are they important?

Discussion What are some characteristics of parenteral preparations, and why are they important? Answer: Tonicity, osmolality, and p. H are characteristics of parenteral preparations. It is important that they be adjusted to be as close as possible to the values for human blood, to prevent damage to blood cells and organs.

Terms to Remember • • • osmotic pressure osmolality isotonic solution hypertonic solution p. H value

Equipment Used in IV Preparation • Pharmacies use plastic disposable products to – save time and money – provide the patient with an inexpensive sterile product • Often the entire system sent out to the patient floors is composed of plastic – thin, flexible plastic catheters are replacing metal shafts that deliver the medication into the vein – in many cases the only durable, nondisposable product used to deliver IV medication is the IV pump or controller

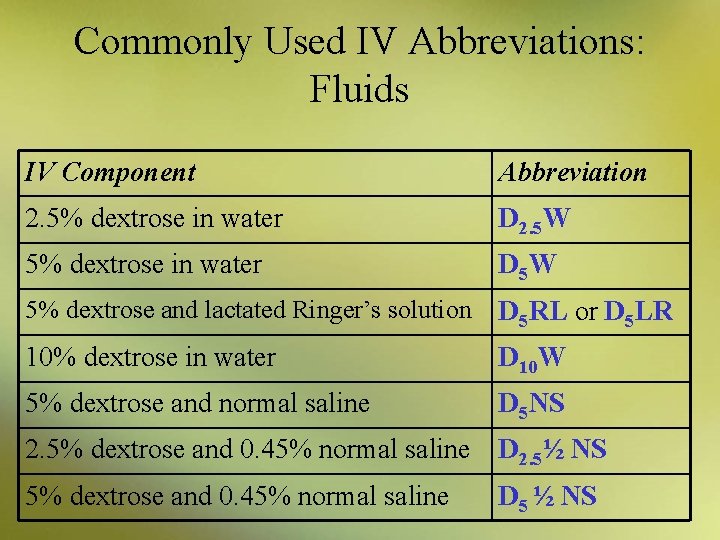
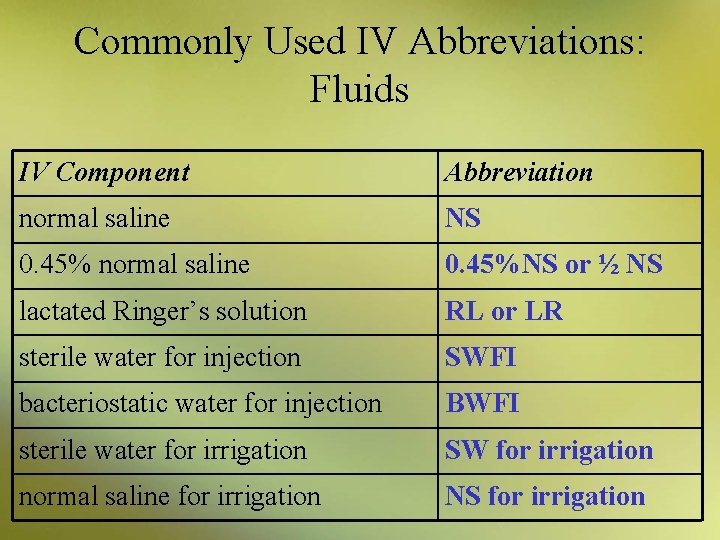
Commonly Used IV Abbreviations: Fluids IV Component Abbreviation 2. 5% dextrose in water D 2. 5 W 5% dextrose in water D 5 W 5% dextrose and lactated Ringer’s solution D 5 RL or D 5 LR 10% dextrose in water D 10 W 5% dextrose and normal saline D 5 NS 2. 5% dextrose and 0. 45% normal saline D 2. 5½ NS 5% dextrose and 0. 45% normal saline D 5 ½ NS

Commonly Used IV Abbreviations: Fluids IV Component Abbreviation normal saline NS 0. 45% normal saline 0. 45%NS or ½ NS lactated Ringer’s solution RL or LR sterile water for injection SWFI bacteriostatic water for injection BWFI sterile water for irrigation SW for irrigation normal saline for irrigation NS for irrigation

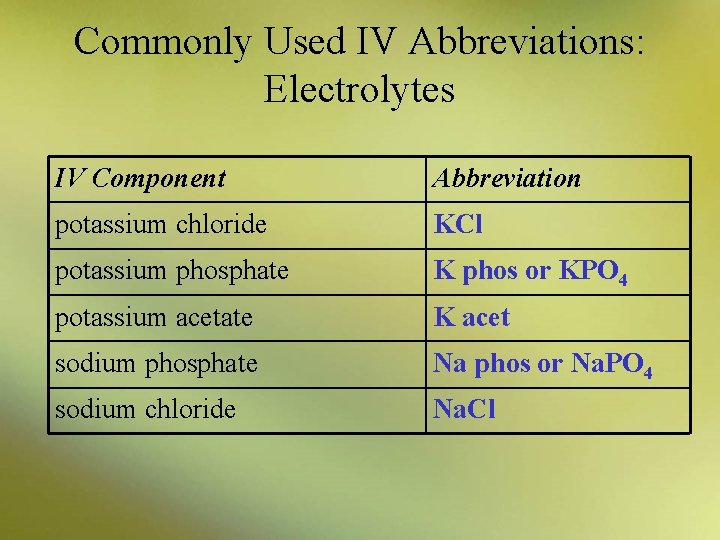
Commonly Used IV Abbreviations: Electrolytes IV Component Abbreviation potassium chloride KCl potassium phosphate K phos or KPO 4 potassium acetate K acet sodium phosphate Na phos or Na. PO 4 sodium chloride Na. Cl

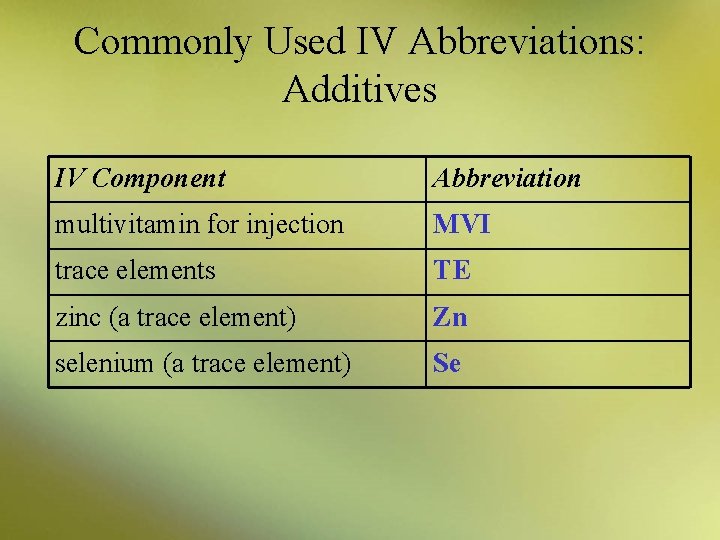
Commonly Used IV Abbreviations: Additives IV Component Abbreviation multivitamin for injection MVI trace elements TE zinc (a trace element) Zn selenium (a trace element) Se

Syringes and Needles • Syringes are used for IV push and in the preparation of infusions, are made of glass or plastic • Glass syringes are more expensive – use limited to medications that are absorbed by plastic • Plastic syringes – are less expensive – are disposable – come from the manufacturer sterile

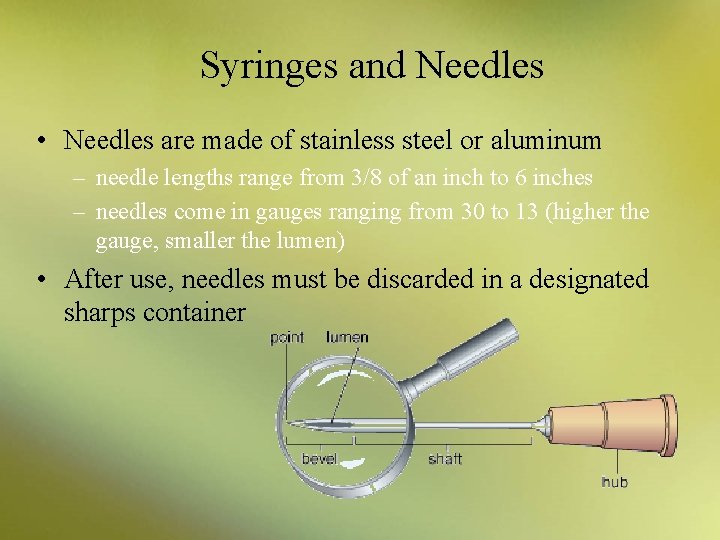
Syringes and Needles • Needles are made of stainless steel or aluminum – needle lengths range from 3/8 of an inch to 6 inches – needles come in gauges ranging from 30 to 13 (higher the gauge, smaller the lumen) • After use, needles must be discarded in a designated sharps container

IV Sets • An IV administration set is a sterile, pyrogen-free disposable device used to deliver IV fluids to patients • The set may – be sterilized before use by means of radiation or ethylene oxide – come in sterile packaging and a sealed plastic wrap • Sets do not carry expiration dates • Sets carry the following legend: – “Federal law restricts this device to sale by or on the order of a physician. ”

IV Sets • Nurses generally have the responsibility for – attaching IV tubing to the fluid container – establishing and maintaining flow rate – overall regulation of the system • Pharmacy personnel must assess aspects of IV systems, including infusion sets • A complete understanding of IV sets and their operation is important to pharmacists and pharmacy personnel

IV Sets • IV sets are sterile and nonpyrogenic • Each unit is supplied in packaging that ensures the maintenance of sterility • Flanges and other rigid parts of an IV set are molded from tough plastic • Most of the length of the tubing is molded from a pliable polyvinyl chloride (PVC) • PVC sets should not be used for – nitroglycerin, which is absorbed by the tubing – IV fat emulsions, which may leach out of the tubing

IV Sets A damaged package cannot ensure sterility, even if all protectors are in place. It is best to discard sets that are found in unoriginal, opened, or damaged packages.

IV Sets Do not use PVC IV sets for nitroglycerin or fat emulsions. Special types of plastic sets are required for such infusions.

IV Sets • The length of sets varies from 6 -inch extensions up to 110 - to 120 -inch sets used in surgery – the priming of tubing depends on the length of the set • Standard sets have a lumen diameter of 0. 28 cm – varying the size of the lumen diameter achieves different flow rates – regulation of flow rates is critical in neonates and infants

IV Sets • The tubing’s interior lumen may contain particles that flush out when fluid is run through the set • Use of final filtration has reduced the need for flushing the line with the IV fluid before attaching the set to the patient

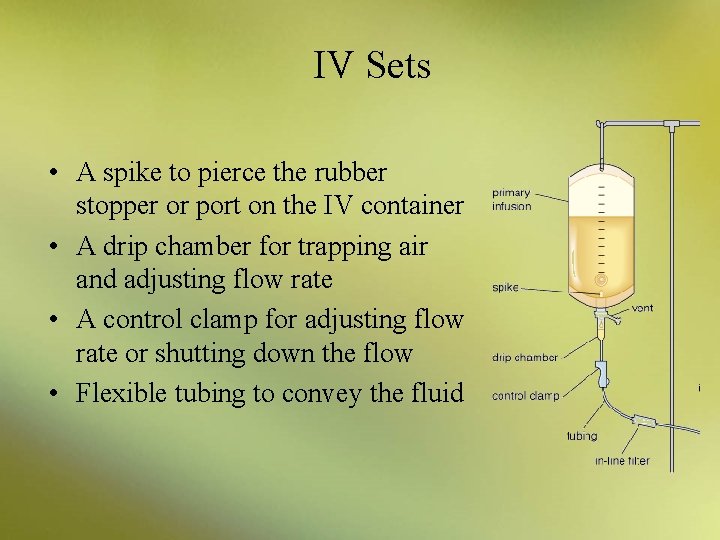
IV Sets • A spike to pierce the rubber stopper or port on the IV container • A drip chamber for trapping air and adjusting flow rate • A control clamp for adjusting flow rate or shutting down the flow • Flexible tubing to convey the fluid

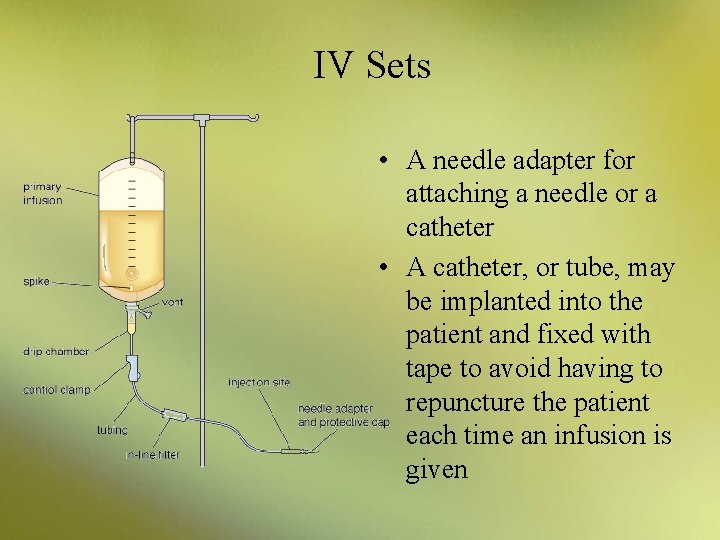
IV Sets • A needle adapter for attaching a needle or a catheter • A catheter, or tube, may be implanted into the patient and fixed with tape to avoid having to repuncture the patient each time an infusion is given

IV Sets • Most IV sets contain a Y-site, or injection port – a rigid piece of plastic with one arm terminating in a resealable port – used for adding medication to the IV • Some IV sets also contain resealable in-line filters – protection for the patient against particulates, including bacteria and emboli Go to www. baxter. com to see IV tubing products from a major manufacturer

IV Sets • The spike is a rigid, sharpened plastic piece used proximal to the IV fluid container – covered with a protective unit to maintain sterility – generally has a rigid area to grip while it is inserted into the IV container • If an air vent is present on a set, it is located below the spike – the air vent points downward and has a bacterial filter covering – the vent allows air to enter the bottle as fluid flows out of it – necessary for glass bottles without an air tube

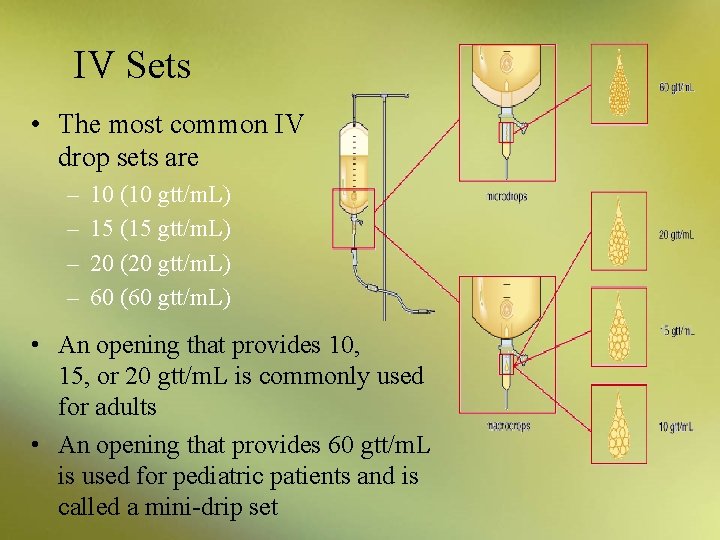
IV Sets • The drip chamber is a transparent, hollow chamber located below the set’s spike – drops of fluid fall into the chamber from an opening at the uppermost end, closest to the spike – number of drops it takes to make 1 m. L identifies an IV set

IV Sets • The most common IV drop sets are – – 10 (10 gtt/m. L) 15 (15 gtt/m. L) 20 (20 gtt/m. L) 60 (60 gtt/m. L) • An opening that provides 10, 15, or 20 gtt/m. L is commonly used for adults • An opening that provides 60 gtt/m. L is used for pediatric patients and is called a mini-drip set

IV Sets • The person administering the fluid starts the flow by filling the chamber with fluid from an attached inverted IV container – the chamber sides are squeezed and released – fluid flows into the chamber – procedure is repeated until an indicated level is reached or approximately half the chamber is filled – entering drops are counted for 15 seconds – adjustments made until approximate number of drops desired is obtained – rate should be checked five times, at 30 -second intervals, and again for a last count of 1 minute

IV Sets • Clamps allow for adjusting the rate of the flow and for shutting down the flow • Clamps may be located at any position along the flexible tubing • Usually a clamp moves freely, allowing its location to be changed to one that is convenient for the health professional administering the medication

IV Sets • Types of clamps used for IV solutions include: – slide clamp: has an increasingly narrow channel that constricts IV tubing as it is pressed further into the narrowed area – screw clamp: consists of a thumbscrew that is tightened or loosened to speed or slow the flow – roller clamp: a small roller that is pushed along an incline • moving down the incline, constricts tubing and reduces fluid flow • moving up the incline, increases the flow

IV Sets • Clamp accuracy can be affected by: – creep: tendency of some clamps to return to a more open position with increased fluid flow – cold flow: tendency of PVC tubing to return to its previous position • The needle adapter – usually located at the distal end of the IV set, close to the patient – has a standard taper to fit all needles or catheters – is covered by a sterile cover before removal for connection

IV Sets • A set may have a built-in or in-line filter • Final filtration should protect the patient against particulate matter, bacteria, air emboli, and phlebitis – a 0. 22 -micron filter is optimal – a 5 -micron filter removes particles that block pulmonary microcirculation but will not ensure sterility • A Y-site is an injection port found on most sets – the Y is a rigid plastic piece with one arm terminating in a resealable port – the port, once disinfected with alcohol, is ready for the insertion of a needle and the injection of medication

Filters • Filters are devices used to remove contaminants such as glass, paint, fibers, and rubber cores – will not remove virus particles or toxins – will occasionally become clogged, thus slowing expected flow rates • Filter sizes include: – 5. 0 microns – random path membrane (RPM) filter, removes large particulate matter – 0. 45 microns – in-line filter for IV suspension drug – 0. 22 microns – removes bacteria and produces a sterile solution

Catheters • IV administration for fluids and drug therapy can be accomplished through needle-like devices called catheters • Catheters are devices inserted into veins for direct access to the blood vascular system and are used in two primary ways: – peripheral venous catheters, which are inserted into a vein close to the surface of the skin – central venous catheters, which are inserted deeper in the body

Catheters • A peripheral venous catheter is inserted into veins close to the surface of the skin and used for up to 72 hours – unit is inserted into a vein – needle portion is withdrawn – flexible, Teflon catheter is left in place • Peripheral catheters are easy to insert, and most nurses can do this at a patient’s bedside – usually inserted in sites on the arms or hands – can be inserted in the feet and scalp if the nurse or physician cannot locate “good” veins in the arms or hands

Catheters • Peripheral venous catheters will likely cause problems 20 to 50% of patients – pain – irritation – infiltration • Infiltration is a breakdown or collapse of a vein that allows the drug to leak into tissues surrounding the catheter site, causing edema and/or tissue damage

Catheters • A central venous catheter is one placed deep into the body – more complicated to place – inserted by a physician to minimize the risk of infection • Central catheters are commonly used for therapy of 1 to 2 weeks or even longer • A central venous catheter is used to administer: – hypertonic solutions such as total parenteral nutrition (TPN) solutions – potentially toxic drugs such as cancer chemotherapeutic drugs

Catheters • The most common sites of insertion are – subclavian vein, lying below the clavicle and joining the jugular vein – jugular vein, in the neck – femoral vein, in the groin area • Placed deep in the vein so that the end enters the superior vena cava close to the heart where the blood flow is the greatest Visit the Web site of the American Society of Enteral and Parenteral Nutrition, a good source for information and networking

Catheters • Problems with subclavian catheters are: – possibility of subclavian vein laceration (i. e. , missing the vein and puncturing a lung) – greater risk of infection because the procedure is more invasive • A larger blood flow in the subclavian vein will dilute a more concentrated solution such as TPN – TPN solutions provide • needed calories in the form of amino acids and dextrose • vitamins, minerals, trace elements, and electrolytes

Catheters • A multiple-lumen catheter is used to separately administer potentially physically incompatible drugs – comes with one, two, three, or four lumens • Each lumen exits the catheter at a different location – no opportunity exists for the drugs to mix before being diluted in the bloodstream

Catheters • Midline catheters are longer peripheral catheters that go from insertion site into a deep vein – designed to stay in place 1 week or longer • The peripherally inserted central (PIC) line is a very fine line that is threaded through the peripheral vein into the subclavian vein – has the same characteristics as a central line – can be inserted by a skilled nurse at the bedside

Catheters • Some patients may be on TPN therapy for months or even years in the hospital or at home • Infusion devices are surgically implanted to: – provide long-term therapy – reduce the risk of infection

Catheters • Implantable infusion devices include the Hickman and Broviac external catheters • Surgeon inserts the catheter below the breast and tunnels it under the skin into the subclavian vein – catheter has a cuff to which the body’s connective tissue heals, sealing off bacterial entry – lower point of body insertion makes the catheter easier for the patient to see and clean

Catheters • Another form of implantable device is the internal port, such as the Port-A-Cath, Life Port – when implanted the only evidence is a bump in the skin – drugs, especially cancer chemotherapeutic drugs, are administered by a small needle through the skin in an injection port in the device

Pumps and Controllers • Fluids and drugs are often delivered to catheters by some form of device, including electronic devices, to control the infusion rate • The first system to deliver a drug IV was the syringe system • Care must be taken when administering drugs that have to be diluted or given very slowly • The syringe system is very nurse labor-intensive and pharmacy labor-intensive

Pumps and Controllers • The Buretrol or Soluset were in use before infusion pumps and replaced the syringe system and has a builtin graduated cylinder – fluid is run into the cylinder – nurse can add a drug in the top of the cylinder injection port for dilution and mixing before it is infused • Safer than the syringe system because the drug is being diluted in the cylinder and it can be infused over a long period of time

Pumps and Controllers The Buretrol or the Soluset have the following problems: • labor intensive • potential drug incompatibility • controllers are low-pressure devices (2 to 3 psi) – pressure of controller is generated by gravity – flow rate is controlled by rate of fluid drops falling through a counting chamber • alarms sound with a kink in a line or even interruption of blood flow when patient bends an elbow

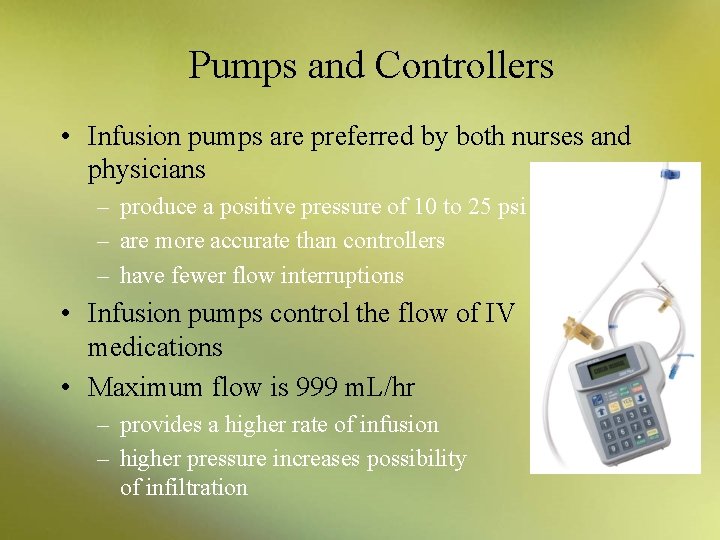
Pumps and Controllers • Infusion pumps are preferred by both nurses and physicians – produce a positive pressure of 10 to 25 psi – are more accurate than controllers – have fewer flow interruptions • Infusion pumps control the flow of IV medications • Maximum flow is 999 m. L/hr – provides a higher rate of infusion – higher pressure increases possibility of infiltration

Pumps and Controllers • A patient-controlled analgesia (PCA) device is a type of medication delivery that uses a parenteral route and allows the patient to administer analgesics by pressing a button – controls the medication so the patient cannot overdose or give the medication too soon after the previous dose • Often, after surgery or severe injuries, a physician will order a PCA for the patient for 24 to 72 hours

Pumps and Controllers PCA pumps should carry the following label: “This PCA pump button should be pushed only by the patient. ”

Discussion What is the most important characteristic that all equipment used in IV preparation and administration have in common?

Discussion What is the most important characteristic that all equipment used in IV preparation and administration have in common? Answer: Sterility is a requirement for all equipment used in IV preparation and administration.

Terms to Remember • • IV administration set filter catheter peripheral venous catheter • infiltration • central venous catheter • subclavian vein • multiple-lumen catheter • peripherally inserted central (PIC) line

Preparing IVs • Pharmacists and technicians prepare drugs and IV solutions in a form ready to be administered to a patient • IV push (i. e. , bolus) and IV infusion dose forms should be prepared in laminar airflow hoods using aseptic techniques – products used during the preparation must always be sterile and handled in such a manner as to prevent contamination

Preparing IVs • Preparation should always be done under the supervision of a licensed pharmacist • Medication that is prepared by the technician must be reviewed and approved by the pharmacist Visit the ASHP Web site for a standard for quality assurance of sterile products

IV Preparation Guidelines • Begin any IV preparation by washing your hands thoroughly using a germicidal agent such as chlorhexidine gluconate or povidone-iodine • All jewelry should be removed from the hands and wrists before scrubbing and while making a sterile product • Wear gloves during procedures • Laminar airflow hoods are normally kept running • Eating, drinking, talking, or coughing is prohibited in the laminar airflow hood • Working in the laminar flow hood should be free from interruptions

IV Preparation Guidelines • Before making the product, thoroughly clean all interior working surfaces • Gather all the necessary materials for the operation and make sure they are: – not expired – free from particulate matter such as dust – check for leaks by squeezing plastic solution containers • Only essential objects and materials necessary for product preparation should be placed in the airflow hood

IV Preparation Guidelines • Work in the center of the work area within the laminar airflow hood – at least six inches inside the edge of the hood – make sure nothing obstructs the flow of air from the highefficiency particulate air (HEPA) filter over the preparation area – nothing should pass behind a sterile object and the HEPA filter in a horizontal airflow hood or above a sterile object in a vertical airflow hood

IV Preparation Guidelines • Follow proper procedure for handling sterile devices and medication containers • Remember that the plunger and tip of the syringe are sterile and must not be touched • For greatest accuracy, use the smallest syringe that can hold the desired amount of solution – syringe should not be larger than twice the volume to be measured – syringe is considered accurate to half the smallest measurement mark on its barrel

IV Preparation Guidelines • The volume of solution drawn into a syringe is measured at the point of contact between the rubber piston and the side of the syringe barrel • Additives are commonly added to IV solutions – medications, electrolytes, vitamins and/or minerals • Additives may be packaged in vials or ampules • Proper technique in using vials and ampules is an important skill for the pharmacy technician to learn

Vials • Powders are reconstituted by introducing a diluent (e. g. , sterile water for injection) • Vials are closed systems – the amount of air introduced should be equal to the volume of fluid removed – an exception to this guideline is the withdrawal of cytotoxic drugs from vials

Vials With the exception of cytotoxic drugs, inject an equal amount of air into the vial with the syringe and needle before withdrawing the medication.

Vials Use a Syringe to Draw Liquid from a Vial 1. Choose the smallest gauge needle appropriate for the task, and avoid coring the rubber top of the vial and thus introducing particulate into the liquid within it 2. Attach the needle to the syringe. 3. Draw into the syringe an amount of air equal to the amount of drug to be drawn from the vial.

Vials 4. Swab or spray the top of the vial with alcohol before entering the laminar flow hood; allow the alcohol to dry. Puncture the rubber top of the vial with the needle bevel up. Then bring the syringe and needle straight up, penetrate the stopper, and depress the plunger of the syringe, emptying the air into the vial.

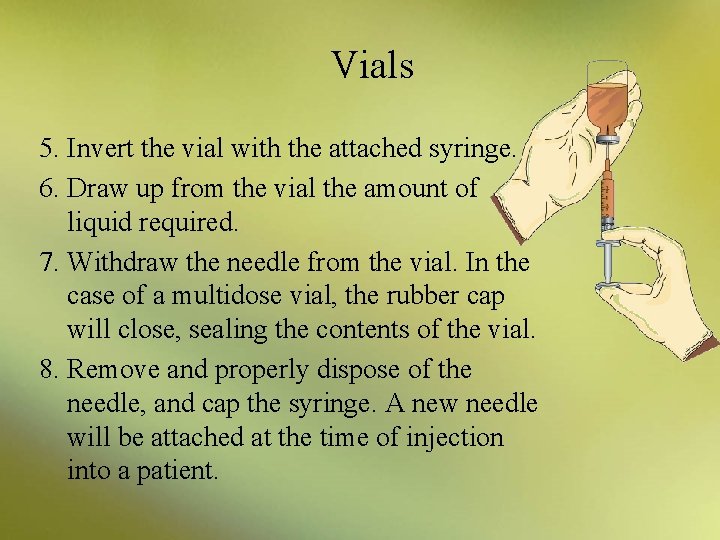
Vials 5. Invert the vial with the attached syringe. 6. Draw up from the vial the amount of liquid required. 7. Withdraw the needle from the vial. In the case of a multidose vial, the rubber cap will close, sealing the contents of the vial. 8. Remove and properly dispose of the needle, and cap the syringe. A new needle will be attached at the time of injection into a patient.

Ampules • An ampule is a single-dose-only drug container • The glass ampule offers another challenge because one must first break the top off the ampule before withdrawing the medication

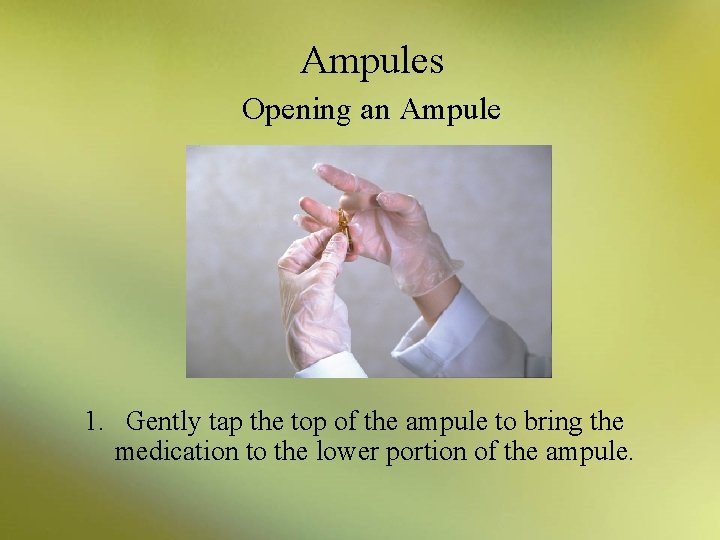
Ampules Opening an Ampule 1. Gently tap the top of the ampule to bring the medication to the lower portion of the ampule.

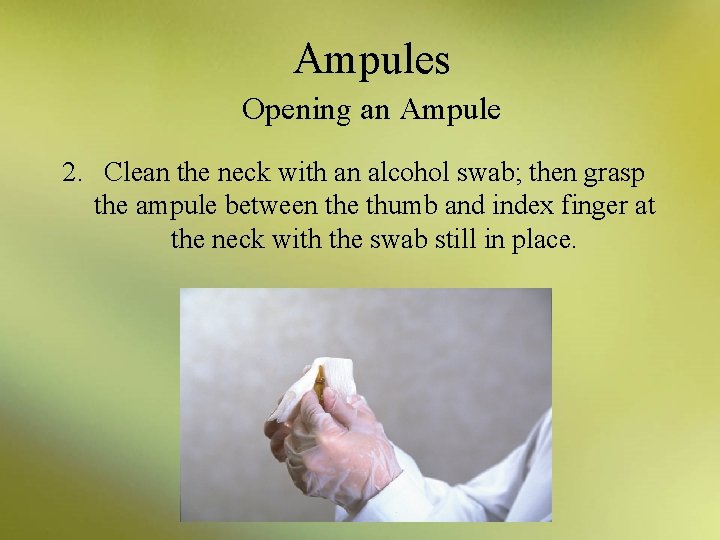
Ampules Opening an Ampule 2. Clean the neck with an alcohol swab; then grasp the ampule between the thumb and index finger at the neck with the swab still in place.

Ampules Opening an Ampule 3. Forcefully snap the neck away from you.

Ampules • To withdraw from an ampule, tilt the ampule, place the needle bevel of a filter needle or tip of a filter straw in the corner near the opening, and withdraw the medication • Use a needle equipped with a filter for filtering out any tiny glass particles, fibers, or paint chips that may have fallen into the ampule

Ampules • Before injecting the contents of a syringe into an IV, the needle must be changed to avoid introducing glass or particles into the admixture • A standard needle could be used to withdraw the drug from the ampule; it is then replaced with a filter device before the drug is pushed out of the syringe • Filter needles are for one directional use only

IV Solutions • Most IV, intrathecal, intra-arterial, and intracardiac injections will be solutions • A diluent is a sterile fluid to be added to a powder to reconstitute or dissolve the medication – check the medication package insert to verify which diluent and what volume should be added to the medication vial to make a sterile solution • Alternative routes of administration may also require special preparation, storage, and needles – it is best to check with the pharmacist if such a medication order is received

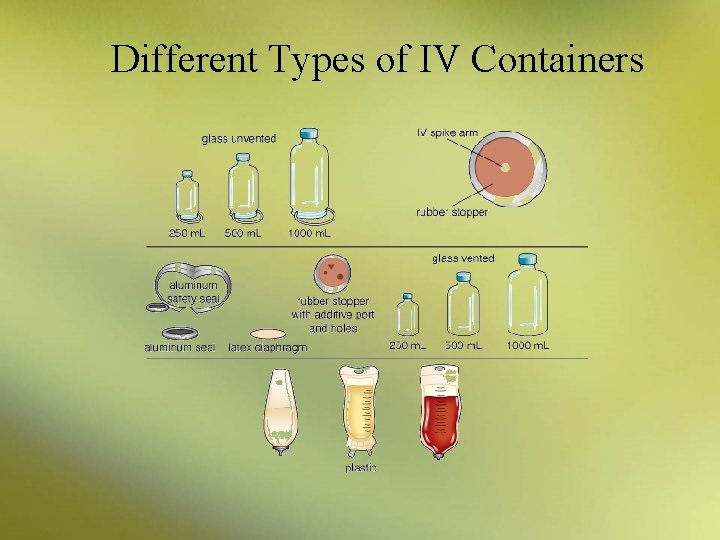
IV Solutions • The vehicles most commonly used for IV infusions are: – dextrose in water – NS solution – dextrose in saline solution • The two main types of IV solutions are: – small-volume parenterals (SVPs) of 50 or 100 m. L – large-volume parenterals (LVPs) of more than 100 m. L

IV Solutions • SVPs are typically used for delivering medications at a controlled infusion rate • Large-volume parenterals (LVPs) are used – to replenish fluids – to provide electrolytes (i. e. , essential minerals) – to provide nutrients such as vitamins and glucose – LVPs are commonly available in 250 m. L, 500 m. L, and 1000 m. L sizes

Different Types of IV Containers

IV Solutions • A piggyback is a small-volume parenteral admixture that is attached to an existing IV line • The piggybacked solution is infused into the tubing of the running IV – usually over a short time, from 30 minutes to 1 hour • Some IV piggybacks are prepared in 250 m. L solution because they contain a medication that is irritating to the veins • In some cases, syringes are used instead of piggyback containers to deliver medication into a running IV

IV Solutions • A LVP usually contains one or more electrolytes – potassium chloride is the most common additive – other salts of potassium, magnesium, or sodium can be added • Additives to IV solutions can also be multivitamins or trace elements

Preparing a Label for an IV Admixture Labels for IV admixtures should bear the following information: – patient’s name and identification number – – – – room number fluid and amount drug name and strength (if appropriate) infusion period flow rate (e. g. , 100 m. L/hr or infuse over 30 min) expiration date and time additional information as required by the institution or by state or federal guidelines

Examples of Pharmacy-prepared Labels • for a minibag • for a large-volume parenteral (LVP)

Discussion What principle guides the techniques and procedures applied to IV solution preparation?

Discussion What principle guides the techniques and procedures applied to IV solution preparation? Answer: IV preparation is based on aseptic technique and all dosage forms and equipment must be handled in a way that prevents contamination.

Terms to Remember • • • ampule diluent small-volume parenterals (SVPs) large-volume parenterals (LVPs) piggyback

Calculations for the Hospital Pharmacy Technician • In preparing sterile IV preparations in the hospital or home healthcare setting, it is important to: – understand math skills somewhat unique to these environments – double- and triple-check calculations and flow rates for IV admixtures or TPN solutions

Calculations for the Hospital Pharmacy Technician • Important math skills are needed in the areas of – reading time – temperature and temperature conversions – electrolyte replacement therapy – specific gravity – IV infusion flow rates

Calculations for the Hospital Pharmacy Technician Always carefully check and double check all calculations.

Time Conversions • Hospital medication orders are often stamped with international or military time – dose administration schedules for unit dose and IV admixtures also use this method • This time is based on a 24 -hour clock, with midnight being considered time 0000 – time in hours are the first two digits – time in minutes are the last two digits – no A. M. or P. M. are used

Time Conversions Examples: 0000 = Midnight 0600 = 6 AM 1200 = Noon 1800 = 6 PM

Temperature Conversions • The United States is one of the few countries in the world where the Fahrenheit temperature scale is commonly used – The Fahrenheit temperature scale uses 32° F as the temperature when ice freezes and 212° F as the temperature that water boils – the difference between these two extremes is 180° F

Temperature Conversions • The Celsius temperature scale is commonly used in Europe and globally in science, and it is often the scale used in the pharmacy – the Celsius temperature scale uses 0° C as the temperature when ice freezes and 100° C as the temperature that water boils – the difference between these two extremes is 100° C

Temperature Conversions • Storing drugs under the proper refrigeration and maintaining refrigerating equipment at the appropriate temperature are responsibilities of the pharmacy technician – most refrigerators in the pharmacy need to maintain a temperature of 5º C to 10º C • Temperatures in the drug package inserts or in the policy and procedure manual are often in centigrade – pharmacy technicians will need to know how to convert between the Celsius scale and the Fahrenheit scales

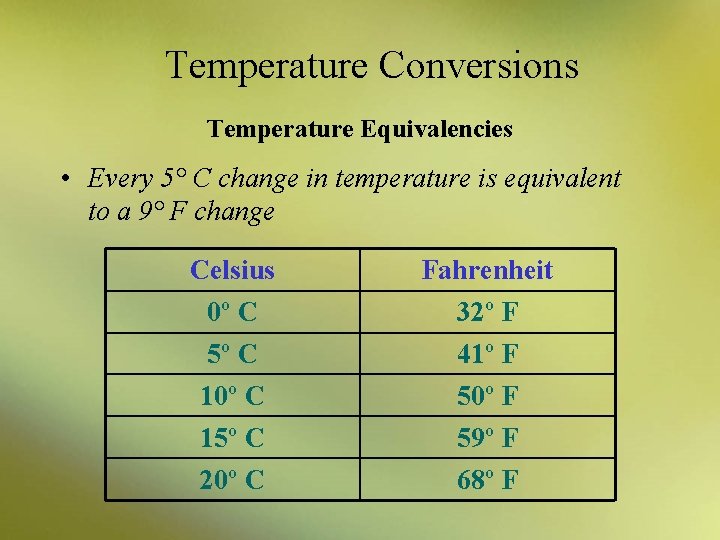
Temperature Conversions Temperature Equivalencies • Every 5° C change in temperature is equivalent to a 9° F change Celsius 0º C 5º C 10º C 15º C 20º C Fahrenheit 32º F 41º F 50º F 59º F 68º F

Temperature Conversions • These equations allow conversions between the two temperature scales: º F = (1. 8 × º C) + 32 º C = (º F – 32) ÷ 1. 8 • An alternative method uses this equation: 5 F = 9 C + 160 • The final temperature is usually rounded up to the closest whole number

Electrolytes • Many IV fluids used in pharmacy practice contain electrolytes: – dissolved mineral salts – so-named because they conduct an electrical charge through the solution when connected to electrodes • Electrolytes are measured in millimoles (m. M) and milliequivalents (m. Eq)

Understanding Millimoles and Milliequivalents • Milliequivalents (m. Eq) are related to molecular weight • Molecular weights are based on the atomic weights of elements – the atomic weight of an element is the weight of a single atom of that element compared with the weight of one atom of hydrogen – the molecular weight of a compound is the sum of the atomic weights of all the atoms in one molecule of the compound

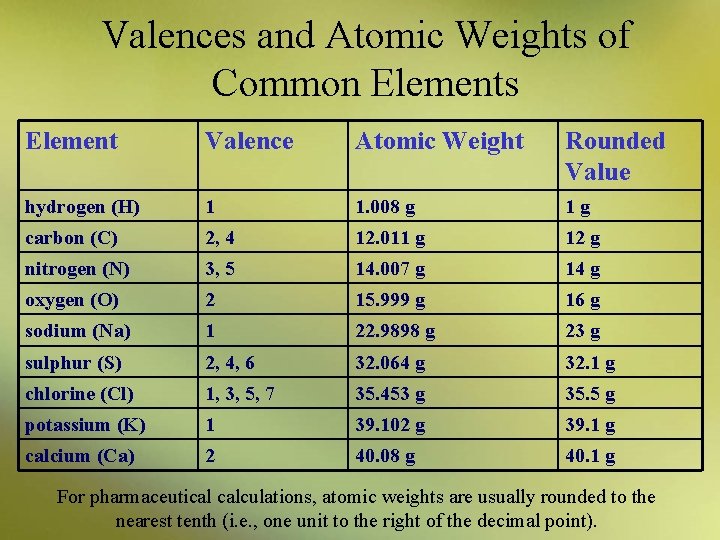
Understanding Millimoles and Milliequivalents • A millimole (m. M) is an amount of a substance weighing its molecular weight in milligrams • The valence of an element is a number that represents its capacity to combine to form a molecule of a stable compound – an element can exist in various forms – valence may vary depending on an elemental form

Valences and Atomic Weights of Common Elements Element Valence Atomic Weight Rounded Value hydrogen (H) 1 1. 008 g 1 g carbon (C) 2, 4 12. 011 g 12 g nitrogen (N) 3, 5 14. 007 g 14 g oxygen (O) 2 15. 999 g 16 g sodium (Na) 1 22. 9898 g 23 g sulphur (S) 2, 4, 6 32. 064 g 32. 1 g chlorine (Cl) 1, 3, 5, 7 35. 453 g 35. 5 g potassium (K) 1 39. 102 g 39. 1 g calcium (Ca) 2 40. 08 g 40. 1 g For pharmaceutical calculations, atomic weights are usually rounded to the nearest tenth (i. e. , one unit to the right of the decimal point).

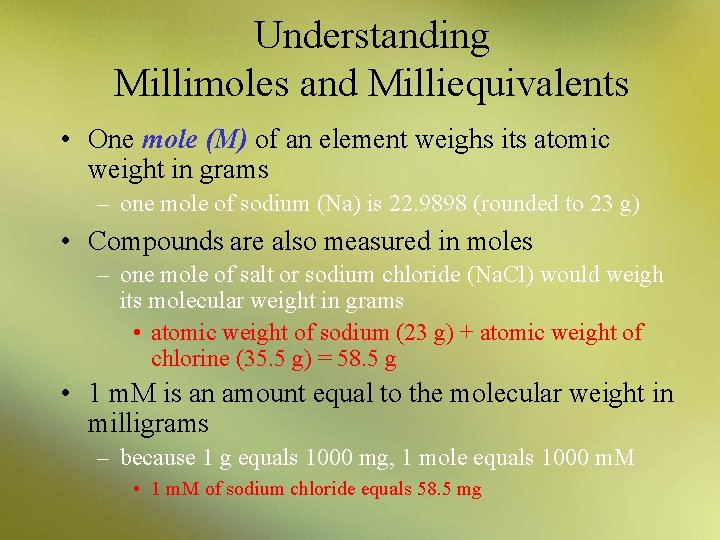
Understanding Millimoles and Milliequivalents • One mole (M) of an element weighs its atomic weight in grams – one mole of sodium (Na) is 22. 9898 (rounded to 23 g) • Compounds are also measured in moles – one mole of salt or sodium chloride (Na. Cl) would weigh its molecular weight in grams • atomic weight of sodium (23 g) + atomic weight of chlorine (35. 5 g) = 58. 5 g • 1 m. M is an amount equal to the molecular weight in milligrams – because 1 g equals 1000 mg, 1 mole equals 1000 m. M • 1 m. M of sodium chloride equals 58. 5 mg

Understanding Millimoles and Milliequivalents • One equivalent (Eq) is equal to one mole divided by its valence Equivalent weight = molecular weight (expressed in milligrams) valence • One milliequivalent (m. Eq) is equal to 1 millimole divided by its valence – thus one equivalent equals 1000 m. Eq, or one thousandth of a gram equivalent

Determining Milliequivalents of Compounds To determine the number of milliequivalents of a compound: (1) identify the formula of the compound (2) separate the formula into atoms (3) determine the moleclular weight of the compound – multiply the weight of each atom by the number of those atoms – add the products together, the sum is the molecular weight m. Eq = molecular weight (expressed in milligrams) valence

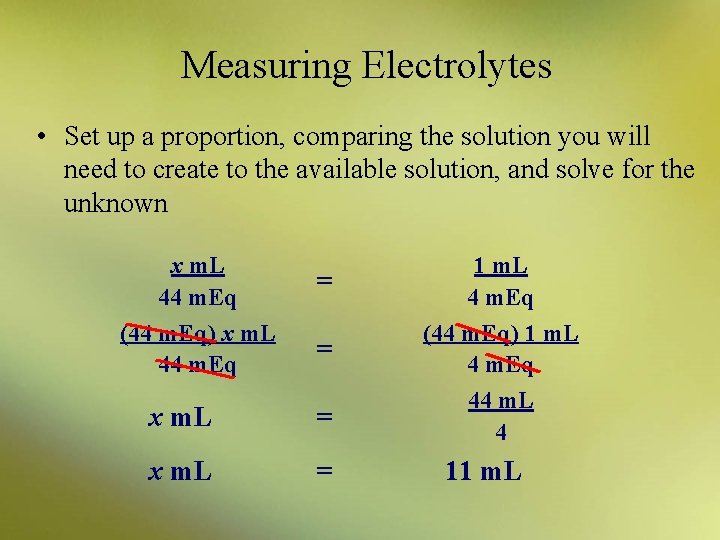
Measuring Electrolytes • Milliequivalents (and sometimes millimoles) are used to measure electrolytes in the bloodstream and/or in an IV preparation • Example: – You must add 44 m. Eq of sodium chloride (Na. Cl) to an IV bag. Sodium chloride is available as a 4 m. Eq/m. L solution. How many milliliters will you add to the bag?

Measuring Electrolytes • You must add 44 m. Eq of sodium chloride (Na. Cl) to an IV bag. Sodium chloride is available as a 4 m. Eq/m. L solution. How many milliliters will you add to the bag? – set up a proportion, comparing the solution you will need to create to the available solution, and solve for the unknown

Measuring Electrolytes • Set up a proportion, comparing the solution you will need to create to the available solution, and solve for the unknown x m. L 44 m. Eq = 1 m. L 4 m. Eq = (44 m. Eq) 1 m. L 4 m. Eq x m. L = 44 m. L 4 x m. L = (44 m. Eq) x m. L 44 m. Eq 11 m. L

Specific Gravity • Specific gravity can be defined as the ratio of the weight of a substance to the weight of an equal volume of water when both have the same temperature • Final weight can be measured in grams because 1 m. L of water weighs 1 g – 1 m. L, volume of water = 1 g, weight of water – specific gravity of water = 1 specific gravity = weight of a substance weight of an equal volume of water

Specific Gravity • When the specific gravity is known, certain assumptions can be made regarding the physical properties of a liquid – liquids that are viscous or have particles floating in them often have a specific gravity higher than 1 – solutions that contain volatile chemicals (or something that is prone to quick evaporation), such as alcohol, often have a specific gravity lower than 1

Specific Gravity Usually numbers are not written without units; however, no units exist for specific gravity. Therefore, you must label specific gravity carefully.

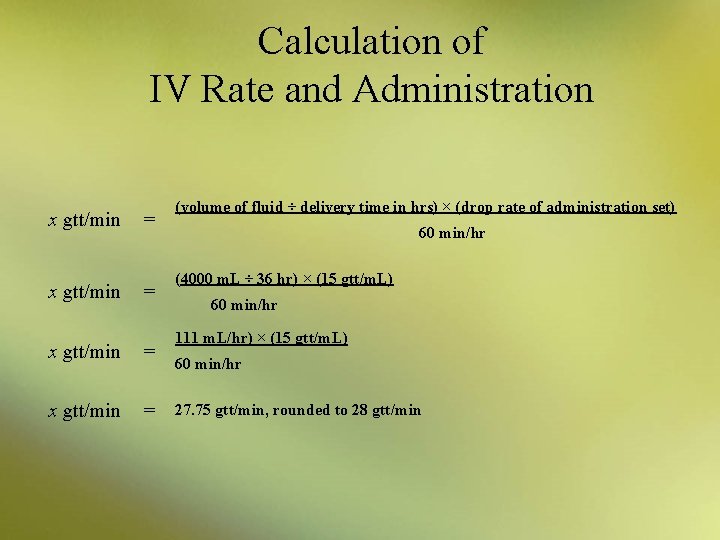
Calculation of IV Rate and Administration • IV flow rates are usually described as milliliters per hour or as drops per minute (expressed as gtt/min) – pharmacy usually uses milliliters per hour – nurses sometimes prefer drops per minute • The formula used to determine the rate in drops per minute is as follows: x gtt/min = (volume of fluid ÷ delivery time in hrs) × (drop rate of administration set) 60 min/hr

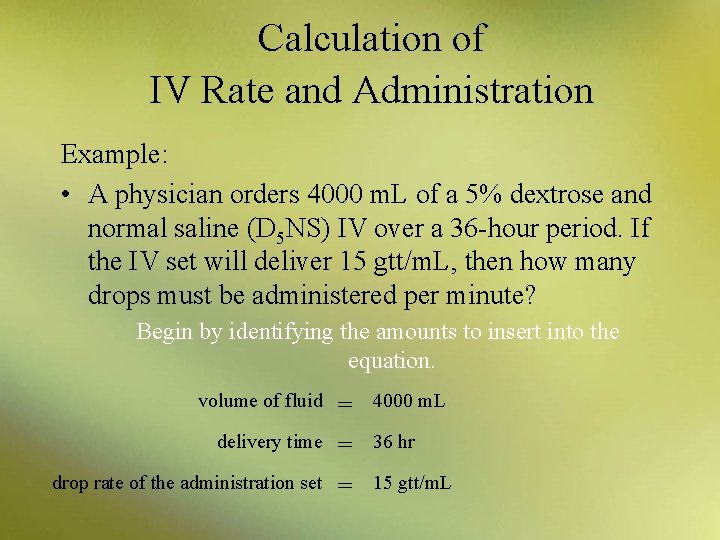
Calculation of IV Rate and Administration Example: • A physician orders 4000 m. L of a 5% dextrose and normal saline (D 5 NS) IV over a 36 -hour period. If the IV set will deliver 15 gtt/m. L, then how many drops must be administered per minute? Begin by identifying the amounts to insert into the equation. volume of fluid delivery time drop rate of the administration set = = = 4000 m. L 36 hr 15 gtt/m. L

Calculation of IV Rate and Administration x gtt/min = = x gtt/min = (volume of fluid ÷ delivery time in hrs) × (drop rate of administration set) 60 min/hr (4000 m. L ÷ 36 hr) × (15 gtt/m. L) 60 min/hr 111 m. L/hr) × (15 gtt/m. L) 60 min/hr 27. 75 gtt/min, rounded to 28 gtt/min

Calculation of IV Rate and Administration • The number of hours that the IV will last can be determined by dividing – the volume of the IV bag (expressed in milliliters) by – the flow rate (expressed in milliliters per hour)

Calculation of IV Rate and Administration Example: • A 1 L IV is running at 125 m. L/hr. How often will a new bag have to be administered? Begin by converting 1 L to 1000 m. L, and then divide the volume by the volume per hour rate. hours the IV will last = 1000 m. L 125 ml/hr = 8 hr

Discussion What are some of the special areas of calculations skills that are important for technicians preparing IV solutions?

Discussion What are some of the special areas of calculations skills that are important for technicians preparing IV solutions? Answer: Skills for accurate conversion between time and temperature systems, electrolyte measurement, specific gravity determinations, and infusion flow rate calculations are vital in IV preparation and labeling.

Terms to Remember • Fahrenheit temperature scale • Celsius temperature scale • electrolytes • atomic weight • molecular weight • • • millimole (m. M) valence mole (M) equivalent (Eq) milliequivalent (m. Eq)