Chapter 11 Matter and Atoms Part 1 Substances

Chapter 11 Matter and Atoms Part 1 - Substances and Mixtures

What is matter? Anything that has a mass and takes up space (has a volume)
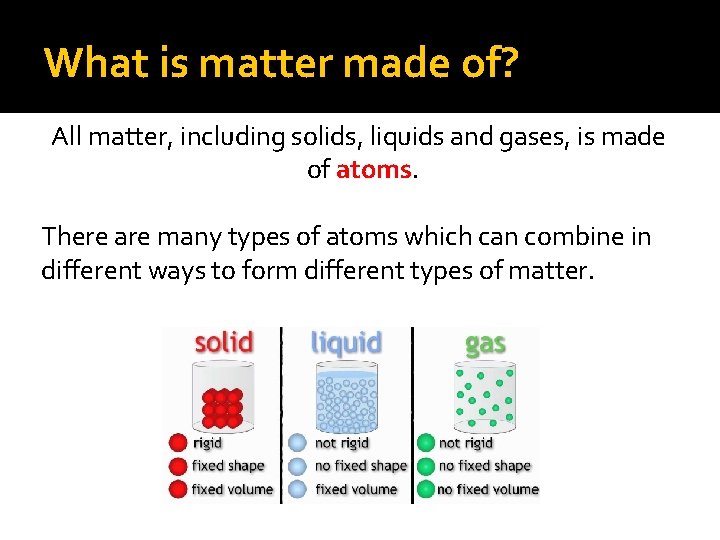
What is matter made of? All matter, including solids, liquids and gases, is made of atoms. There are many types of atoms which can combine in different ways to form different types of matter.

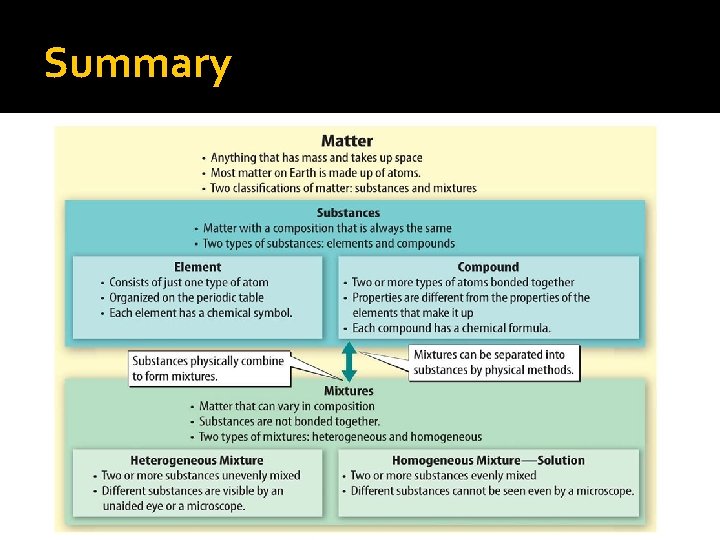
Classifying Matter can be classified by its characteristics and put into one of two groups: q. Substances q. Mixtures How are substances and mixtures different?

What is a substance? A substance is matter with a composition that is always the same. This means they always contain the same atoms bonded in the same way.
Elements Some substances are made of only one kind of atom. The smallest part of an element is an atom. Some elements exist as molecules. 115 elements
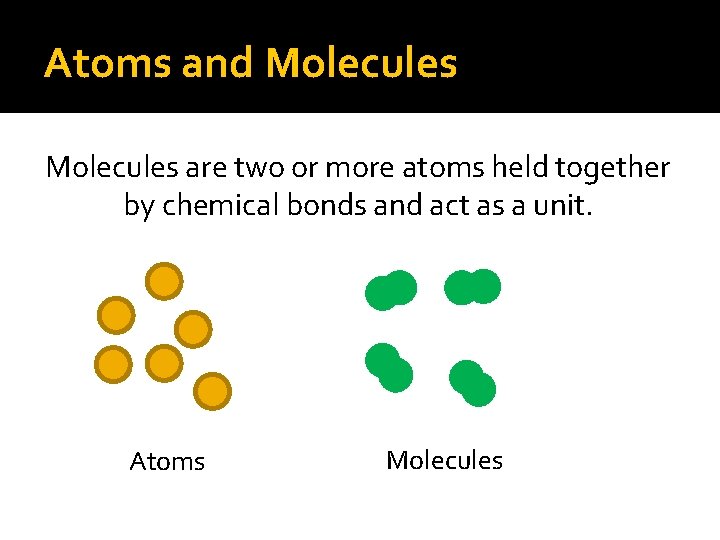
Atoms and Molecules are two or more atoms held together by chemical bonds and act as a unit. Atoms Molecules
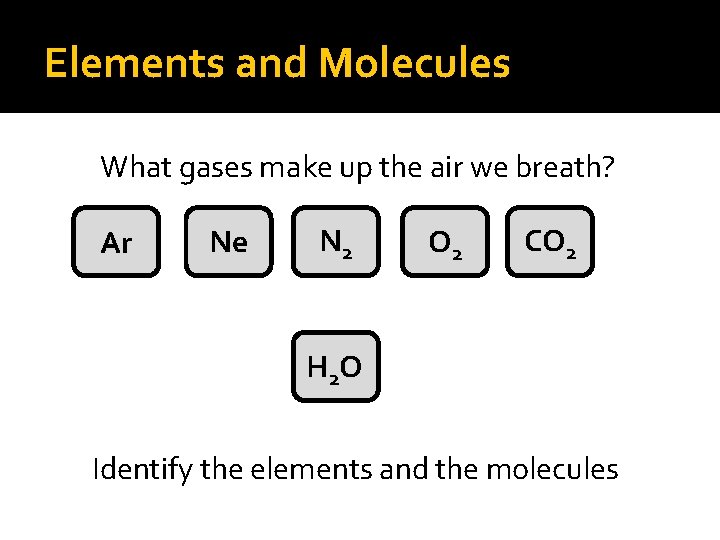
Elements and Molecules What gases make up the air we breath? Ar Ne N 2 O 2 CO 2 H 2 O Identify the elements and the molecules

Compounds q Most matter is made of atoms of different types of elements bonded together. We call these compounds. q Compound: a substance made of two or more elements that are chemically joined in a specific combination.
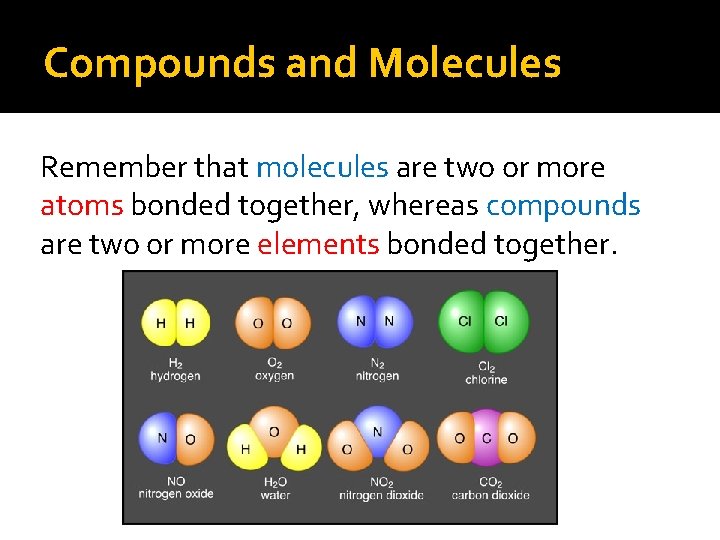
Compounds and Molecules Remember that molecules are two or more atoms bonded together, whereas compounds are two or more elements bonded together.
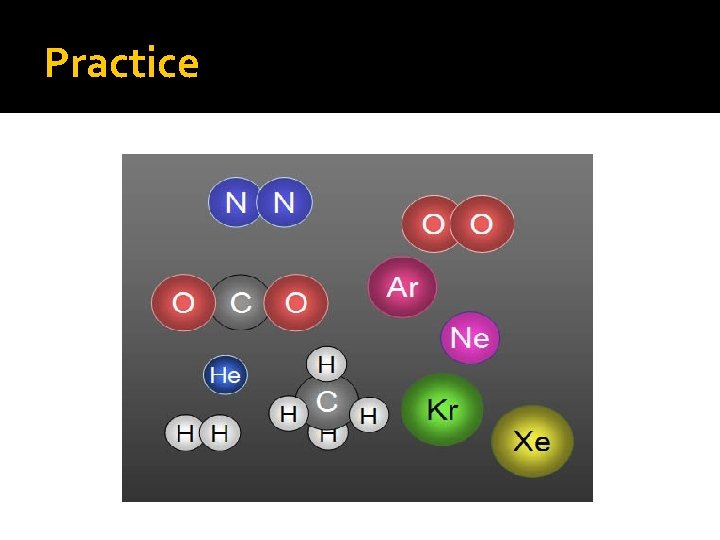
Practice
Mixtures q Matter is either a substance or a mixture. q A substance has a composition that is always the same, a mixture does not. Mixture – matter that can vary in composition. It is made of two or more substances that are blended but not chemically bonded.

Mixtures q Most matter exists as a mixture. Pure Water Ocean Water Tap Water q There are two types of mixtures q Heterogeneous q Homogeneous

Heterogeneous Mixtures A mixture in which the substances are not evenly mixed.

Homogeneous Mixtures A mixture in which two or more substances are evenly mixed, but not bonded together.

Homogeneous Mixtures Another name for a homogeneous mixture is a solution. A solution is made up of two parts – a solvent and one or more solutes. Solute Solvent
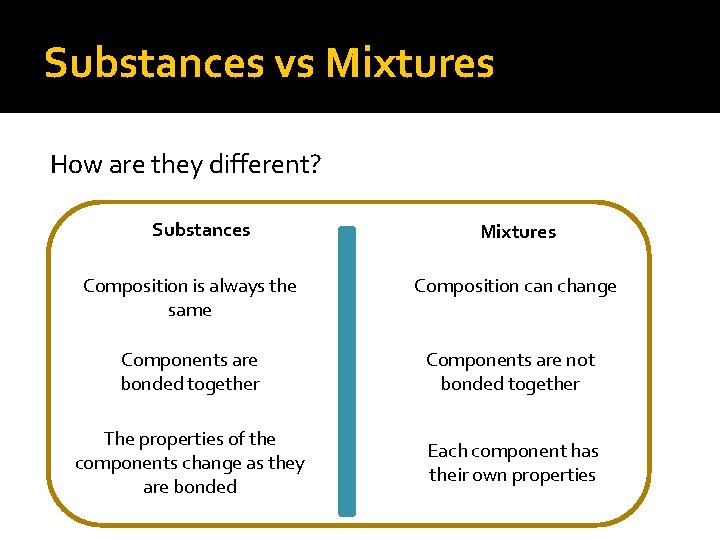
Substances vs Mixtures How are they different? Substances Mixtures Composition is always the same Composition can change Components are bonded together Components are not bonded together The properties of the components change as they are bonded Each component has their own properties

Separating Mixtures �Because the parts of a mixture are not chemically bonded they can be separated by physical processes.

Separating Mixtures It is more difficult to separate a homogeneous mixture as the components are evenly mixed. One way to do this is by boiling or evaporation.
Summary

Matter and Atoms Part 2 – The Structure of Atoms
The parts of an Atom �Recall that all elements are made of different atoms and that there about 115 elements. �Atoms are the building blocks of matter; they can combine in many different ways. �Atoms are made of tiny particles. The number of these particles is what makes atoms different.
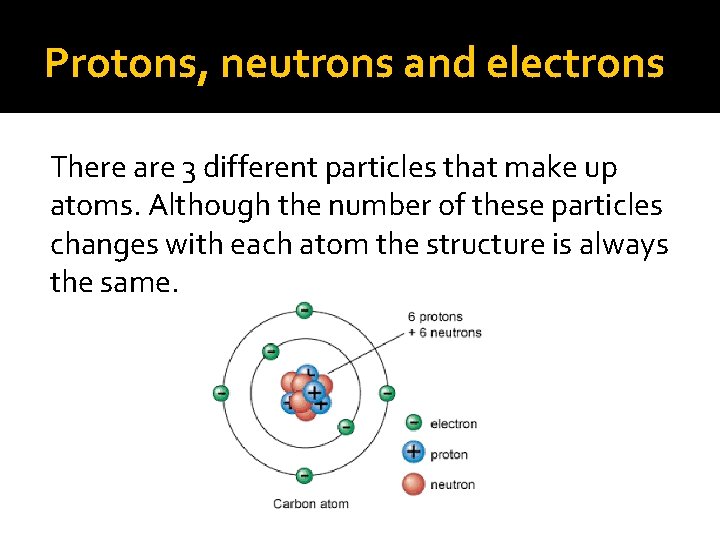
Protons, neutrons and electrons There are 3 different particles that make up atoms. Although the number of these particles changes with each atom the structure is always the same.
The Nucleus The nucleus is at the centre of an atom and contains the protons and neutrons. q Protons – positively charged particles q Neutrons – uncharged particles The nucleus contains most of an atoms mass

Electrons An electron is a negatively charged particle located outside of the nucleus. Electrons are so small and move so quickly that we cannot tell exactly where they are. Therefore the area outside of the nucleus is known as an electron cloud.
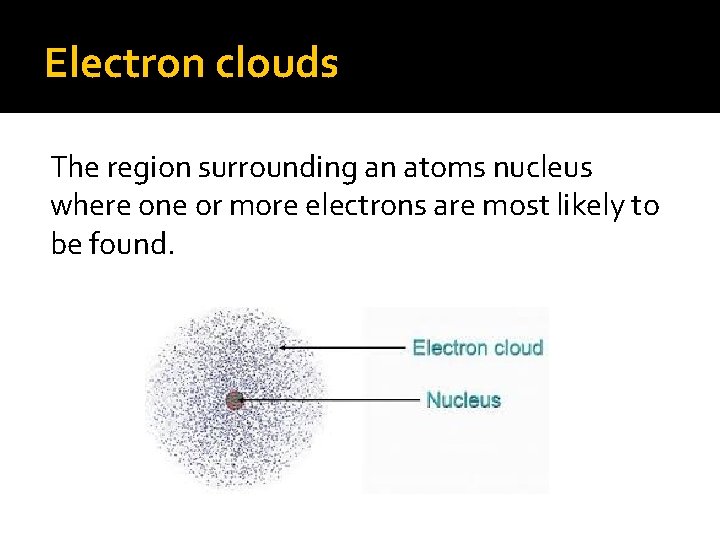
Electron clouds The region surrounding an atoms nucleus where one or more electrons are most likely to be found.
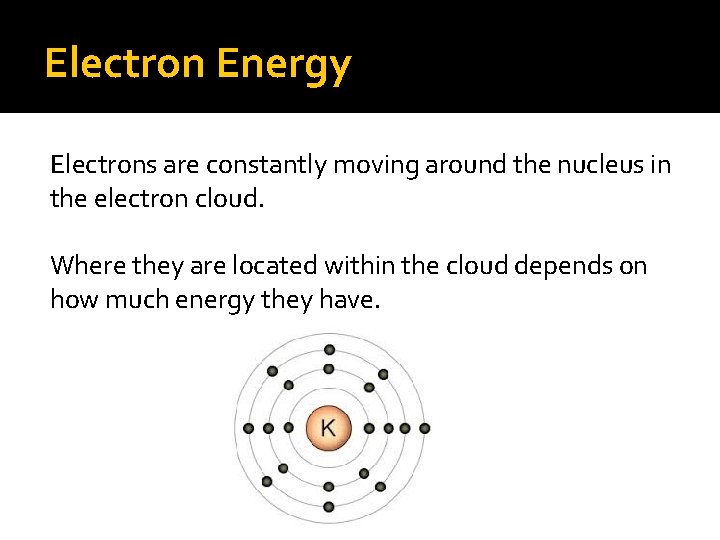
Electron Energy Electrons are constantly moving around the nucleus in the electron cloud. Where they are located within the cloud depends on how much energy they have.
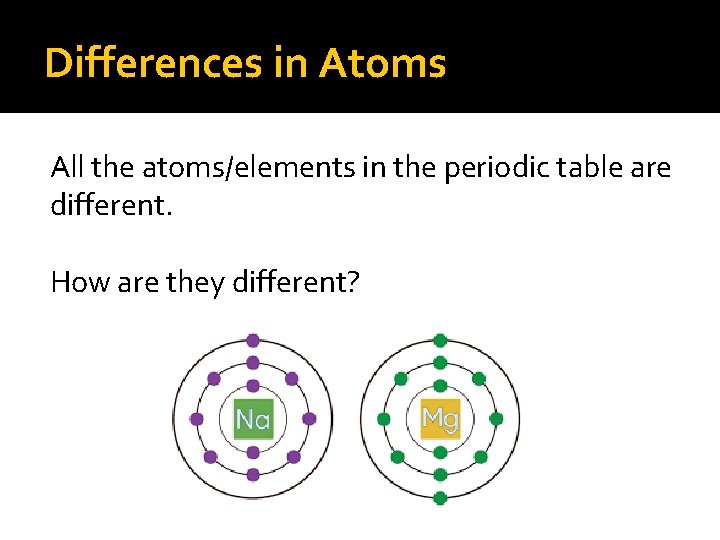
Differences in Atoms All the atoms/elements in the periodic table are different. How are they different?
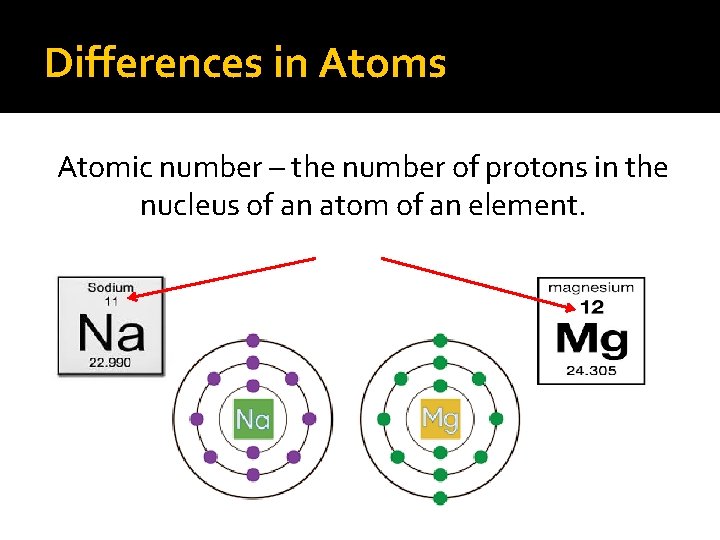
Differences in Atoms Atomic number – the number of protons in the nucleus of an atom of an element.
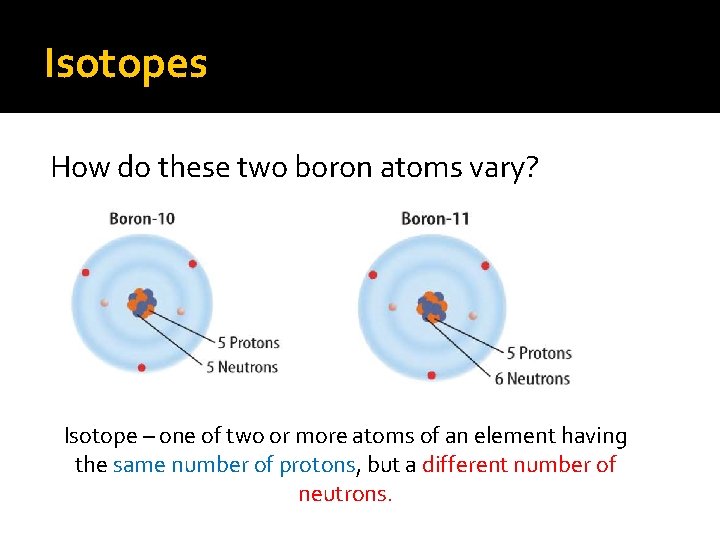
Isotopes How do these two boron atoms vary? Isotope – one of two or more atoms of an element having the same number of protons, but a different number of neutrons.
Ions What charge does an atom have? Neutral What would happen if an atom gained or lost an electron? The charge changes (positive or negative) If the number of electrons changes does the type of atom change? No, because the number of protons and neutrons has not changed What are ions? An atom that has a charge because it has gained or lost electrons
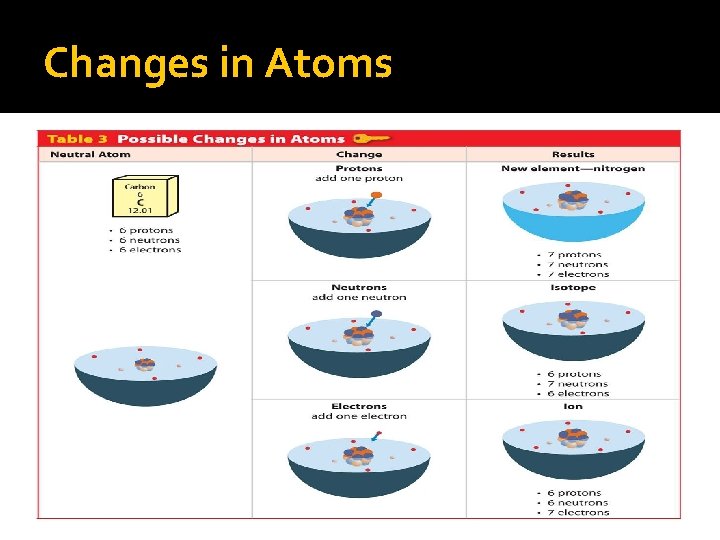
Changes in Atoms
Summary q Matter can be a substance or a mixture q All types of matter are made of atoms q The number of neutrons and electrons can change without changing the element q Changing the number of protons changes the element

Review Questions Which term describes two or more atoms that are held together by chemical bonds and act as a unit? A. B. C. D. Atom Compound Molecule Substance

Review Questions Which term refers to two or more substances that are blended but are not chemically bonded? A. B. C. D. element Compound Molecule Mixture

Review Questions Which term describes the substance in a solution that is present in the largest amount? A. B. C. D. Solute Solvent Element Mixture

Review Questions Which term refers to the region surrounding an atoms nucleus where one or more electrons are most likely to be found? A. B. C. D. Isotope Ion Electron cloud Proton

Review Questions Which term describes one of two or more atoms of an element having the same number of protons but a different number of neutrons? A. B. C. D. Atomic number Ion Molecule Isotope

Review Questions What charge would a neutral atom have if it lost an electron? A. B. C. D. Positive Negative Neutral Atomic

Review Questions Which term refers to matter that can vary in composition? A. B. C. D. Compound Element Mixture Solvent

Review Questions Which type of mixture has two or more substances that are evenly mixed but not chemically bonded together? A. B. C. D. Molecular Homogeneous Heterogeneous Atomic

Review Questions What is the region at the center of an atom that contains most of the mass? A. B. C. D. Proton Nucleus Neutron Electron

Review Questions Which is an atom that has a charge because it has gained or lost electrons? A. B. C. D. Ion Isotope Molecule Compound
- Slides: 43