Chapter 11 Lesson 1 2 Part 1 Substances

Chapter 11 Lesson 1. 2 – Part 1 Substances and Mixtures Ms. Diana and Ms. Amanda

What is matter? � Everything � Matter in the world is made out of matter. – anything that has mass and takes up space. � Everything you can see and some things that you cannot see are all made up of matter. ◦ Examples: water, trees, air, balloons, books, tv’s
What is matter made of? � Matter is made up of atoms. � Atoms – small particles that are the building blocks of matter. � Atoms are made up of even smaller particles. � Atoms can combine with each other in many ways to form different types of matter.

Classifying Matter � There are so many different types of atoms. � Matter can be divided into 2 main groups: ◦ Substances – matter with a composition that is always the same. ◦ Mixtures – matter that can vary in composition

What is a substance? � Substance – matter with a composition that is always the same. � Example: ◦ A bar of gold has the same composition of a gold ring because they are both made from GOLD.
What is a Substance? � Some substances are made from one kind of atom. Others are made from more than one kind of atom. � Element – a substance made of only one kind of atom. � All atoms of 1 element are the same. � But atoms of 1 element are different from atoms of other elements. ◦ Example: Gold is made from gold atoms and they are all the same. But gold atoms are different from silver atoms.
Smallest Part of an Element � If you broke an element into its smallest part, you would find an atom. � But some atoms are made up of molecules. � Molecules – two or more atoms that are held together by chemical bonds and act as a unit.
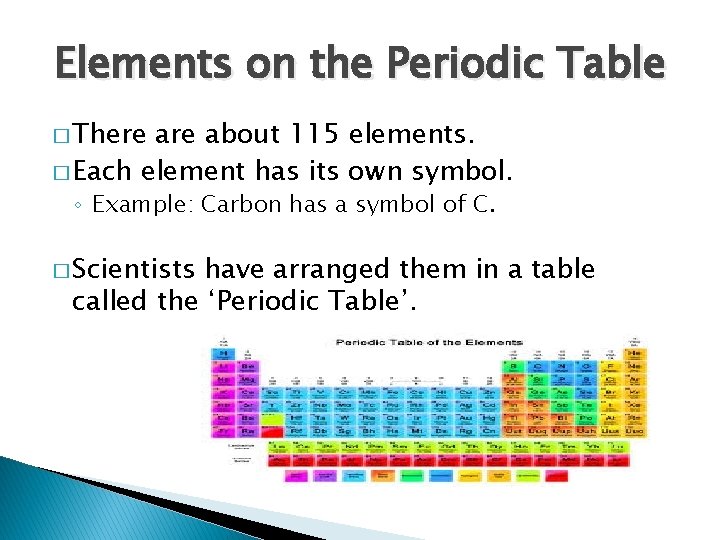
Elements on the Periodic Table � There about 115 elements. � Each element has its own symbol. ◦ Example: Carbon has a symbol of C. � Scientists have arranged them in a table called the ‘Periodic Table’.
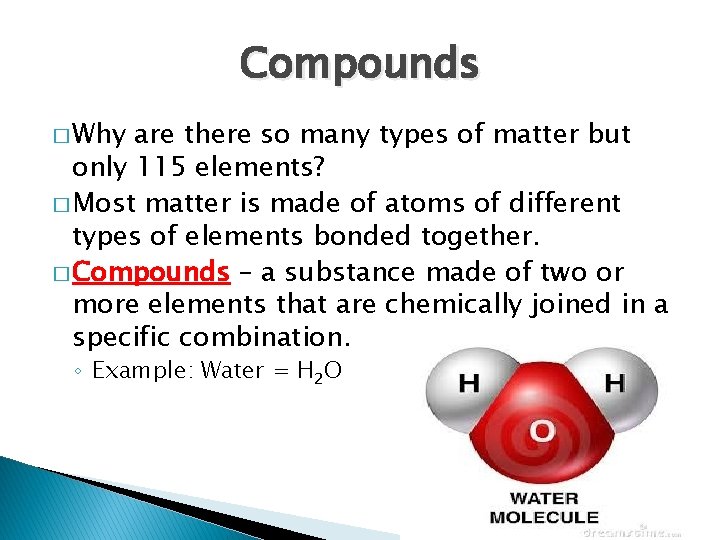
Compounds � Why are there so many types of matter but only 115 elements? � Most matter is made of atoms of different types of elements bonded together. � Compounds – a substance made of two or more elements that are chemically joined in a specific combination. ◦ Example: Water = H 2 O
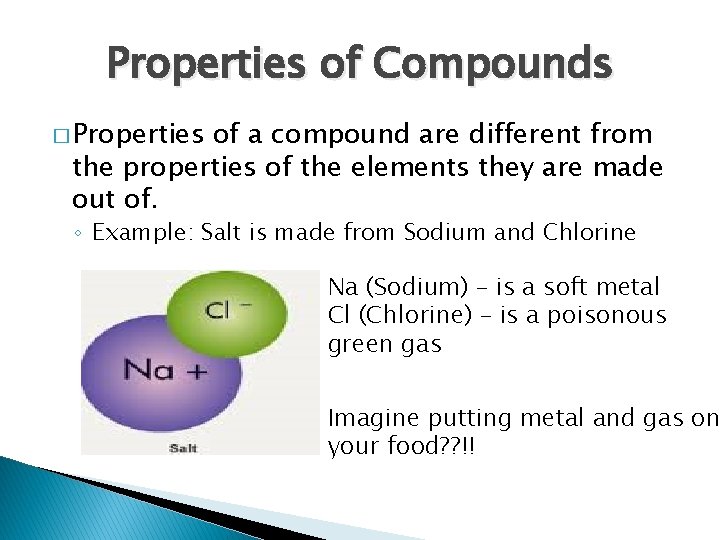
Properties of Compounds � Properties of a compound are different from the properties of the elements they are made out of. ◦ Example: Salt is made from Sodium and Chlorine Na (Sodium) – is a soft metal Cl (Chlorine) – is a poisonous green gas Imagine putting metal and gas on your food? ? !!
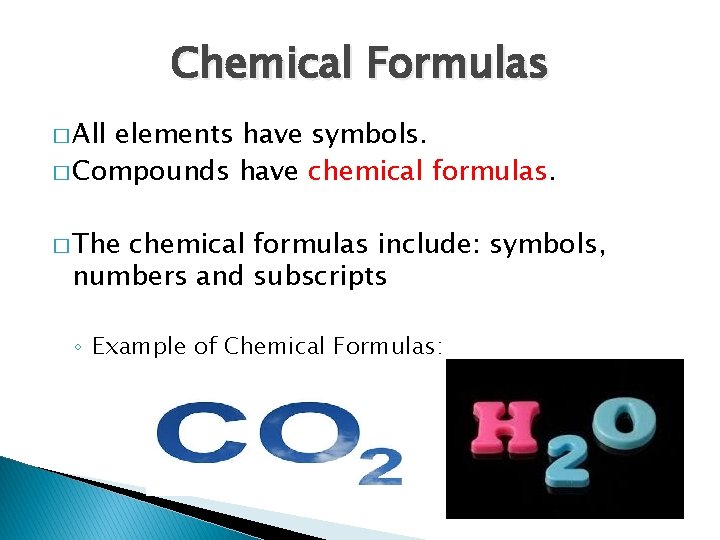
Chemical Formulas � All elements have symbols. � Compounds have chemical formulas. � The chemical formulas include: symbols, numbers and subscripts ◦ Example of Chemical Formulas:

Different Combinations of Atoms � Sometimes one element can combine to form many different compounds. ◦ Example: Nitrogen and Oxygen �N 2 O = Nitrous Oxide = gas used in anesthesia �NO 2 = Nitrogen Dioxide = brown gas air pollutant �N 2 O 3 = Dinitrogen Trioxide = blue liquid
- Slides: 12