Chapter 11 Lecture Conceptual Integrated Science Second Edition














- Slides: 28

Chapter 11 Lecture Conceptual Integrated Science Second Edition Investigating Matter © 2013 Pearson Education, Inc.

This lecture will help you understand: • • Chemistry: The Central Science The Submicroscopic World The Phases of Matter Physical and Chemical Properties Determining Physical and Chemical Changes Elements to Compounds Naming Compounds © 2013 Pearson Education, Inc.

Chemistry: The Central Science • Chemistry is the study of matter and the transformations it can undergo. © 2013 Pearson Education, Inc.

Chemistry: The Central Science © 2013 Pearson Education, Inc.

Chemistry: The Central Science © 2013 Pearson Education, Inc.

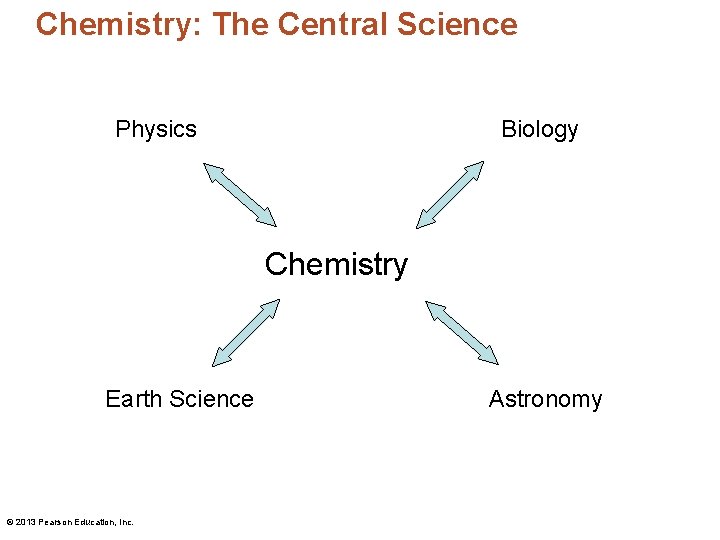
Chemistry: The Central Science • Chemistry is the "central" science. © 2013 Pearson Education, Inc.

Chemistry: The Central Science Physics Biology Chemistry Earth Science © 2013 Pearson Education, Inc. Astronomy

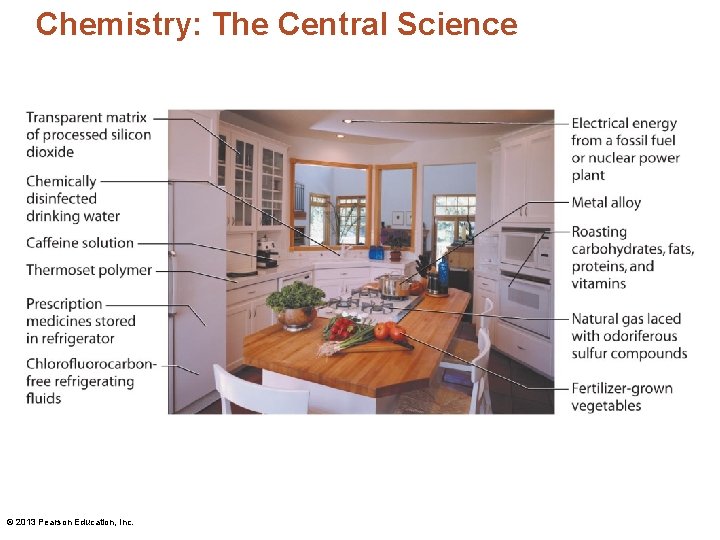
Chemistry: The Central Science • Chemistry is a "materials" science. – Most of the material items in any modern house are shaped by some human-devised chemical process. © 2013 Pearson Education, Inc.

Chemistry: The Central Science © 2013 Pearson Education, Inc.

Chemistry: The Central Science • More than 70% of all legislation placed before the U. S. Congress addresses science-related questions and issues. © 2013 Pearson Education, Inc.

Chemistry: The Central Science A situation to ponder… • Collagen cross-link inhibitors that significantly reverse various aspects of aging have recently been discovered. © 2013 Pearson Education, Inc.

Chemistry: The Central Science CHECK YOUR NEIGHBOR Collagen cross-link inhibitors that are clinically tested to be safe and effective should be A. B. C. D. E. available to the general public. available only to those 21 and older. available only by prescription. prohibited because of their abuse potential. prohibited because growing old should be natural. Explain your answer to your neighbor. © 2013 Pearson Education, Inc.

The Submicroscopic World • A single grain of sand contains about 125 million trillion atoms. • How much is 125 million trillion? ? © 2013 Pearson Education, Inc.

The Submicroscopic World • Roughly 250, 000 dunes of this size contain about 125 million trillion grains of sand. • Yet, that's how many atoms there are in a single grain of sand. (Atoms are small. ) © 2013 Pearson Education, Inc.

Chemistry: The Submicroscopic World A situation to ponder… • Are atoms made of molecules, or are molecules made of atoms? © 2013 Pearson Education, Inc.

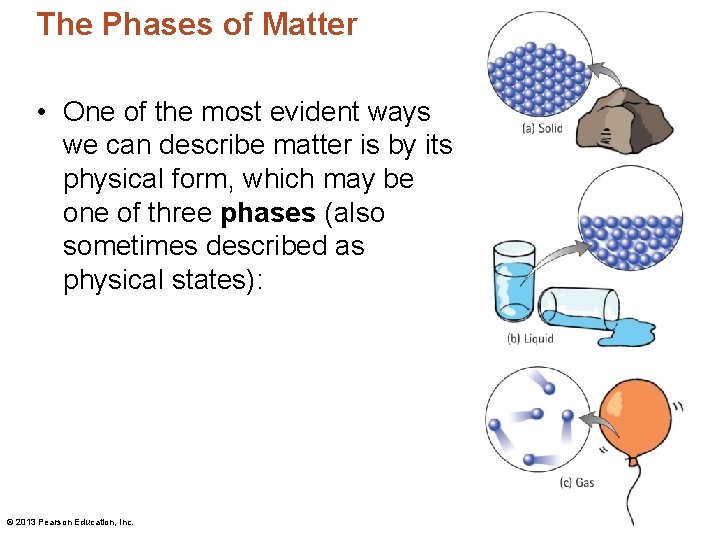
The Phases of Matter • One of the most evident ways we can describe matter is by its physical form, which may be one of three phases (also sometimes described as physical states): © 2013 Pearson Education, Inc.

The Phases of Matter • The gaseous phase of any material occupies significantly more volume than either its solid or liquid phase. • Frozen carbon dioxide, CO 2, "dry ice" © 2013 Pearson Education, Inc.

Phases of Matter © 2013 Pearson Education, Inc.

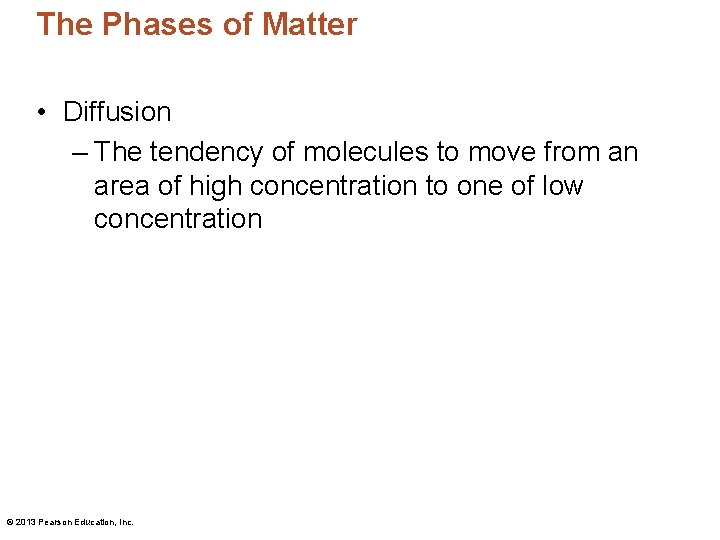
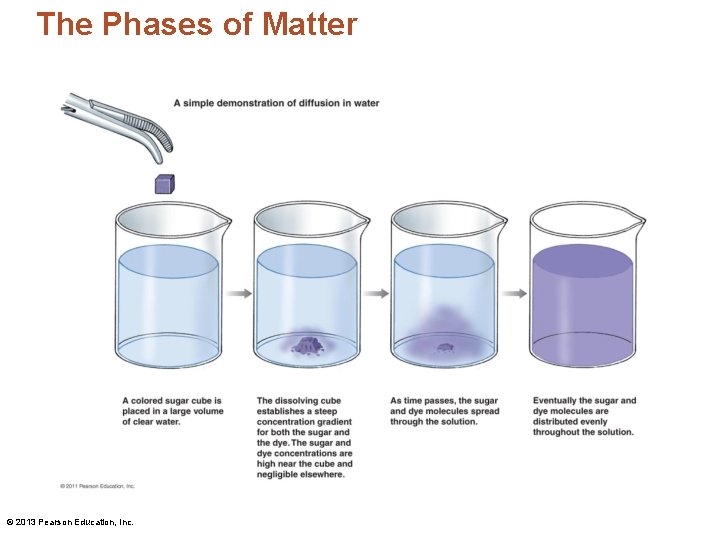
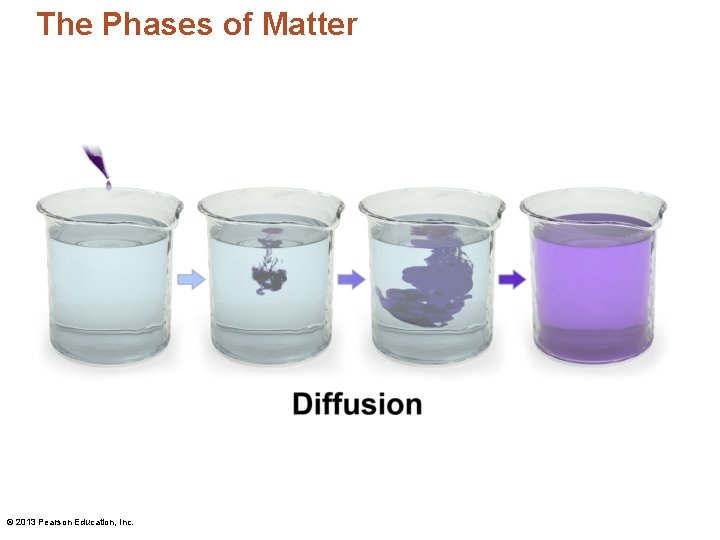
The Phases of Matter • Diffusion – The tendency of molecules to move from an area of high concentration to one of low concentration © 2013 Pearson Education, Inc.

The Phases of Matter © 2013 Pearson Education, Inc.

The Phases of Matter © 2013 Pearson Education, Inc.

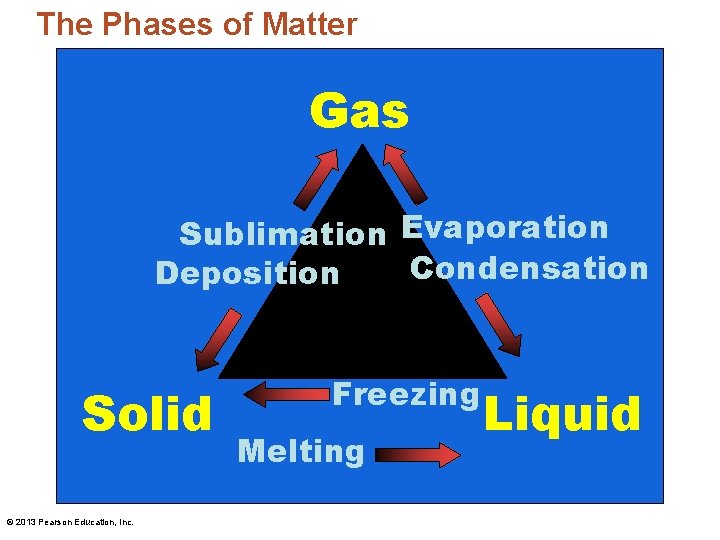
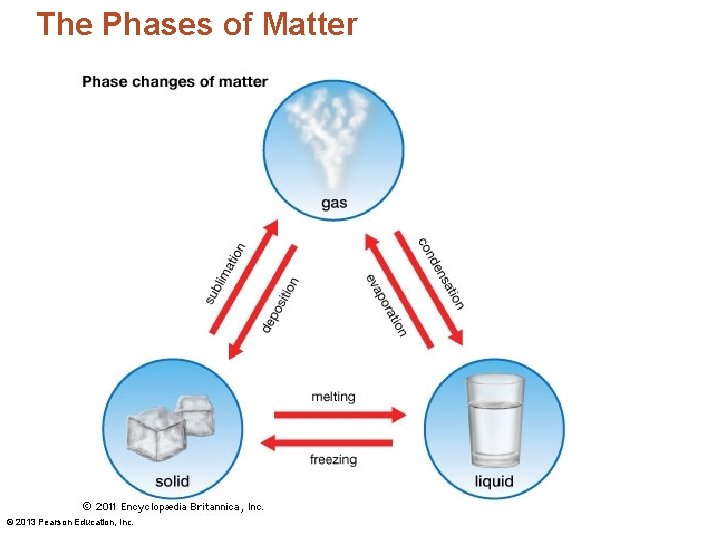
The Phases of Matter Gas Sublimation Evaporation Condensation Deposition Solid © 2013 Pearson Education, Inc. Freezing Melting Liquid

The Phases of Matter • Melting – The process of a solid transforming into a liquid • Freezing – A liquid changing into a solid by the removal of heat © 2013 Pearson Education, Inc.

The Phases of Matter • Evaporation – A liquid that is heated so that it becomes a gas • Boiling – When evaporation occurs beneath the surface of the liquid © 2013 Pearson Education, Inc.

The Phases of Matter • Condensation – The reverse of evaporation • Going from a gas to a liquid • Sublimation – A solid changing to a gas directly without becoming a liquid • Deposition – Opposite of sublimation • A gas transforms directly into a solid © 2013 Pearson Education, Inc.

The Phases of Matter © 2013 Pearson Education, Inc.

The Phases of Matter • Latent Heat – The energy that is absorbed or released in a change of phase © 2013 Pearson Education, Inc.

The Phases of Matter • Heat of Fusion – The energy needed to change any substance from solid to liquid and vice versa – For water it’s 334 J • Heat of vaporization – The energy needed to change any substance from liquid to gas and vice versa – For water it’s 2256 J © 2013 Pearson Education, Inc.