Chapter 11 Introduction to Organic Chemistry Alkanes 11
- Slides: 11

Chapter 11 Introduction to Organic Chemistry: Alkanes 11. 1 Organic Compounds General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 1

Organic Compounds An organic compound § is a compound made from carbon atoms § has one or more C atoms § has many H atoms § may also contain O, S, N, P, and halogens General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 2

Properties of Organic Compounds Typical organic compounds § contain carbon § have covalent bonds § have low melting points § have low boiling points § are flammable § are soluble in nonpolar solvents § are not soluble in water General, Organic, and Biological Chemistry Oil (organic) and water (inorganic) Copyright © 2010 Pearson Education, Inc. 3

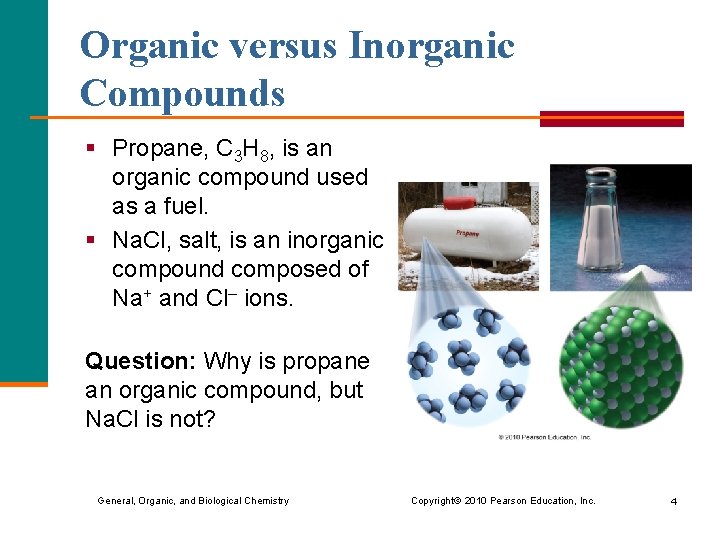
Organic versus Inorganic Compounds § Propane, C 3 H 8, is an organic compound used as a fuel. § Na. Cl, salt, is an inorganic compound composed of Na+ and Cl– ions. Question: Why is propane an organic compound, but Na. Cl is not? General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 4

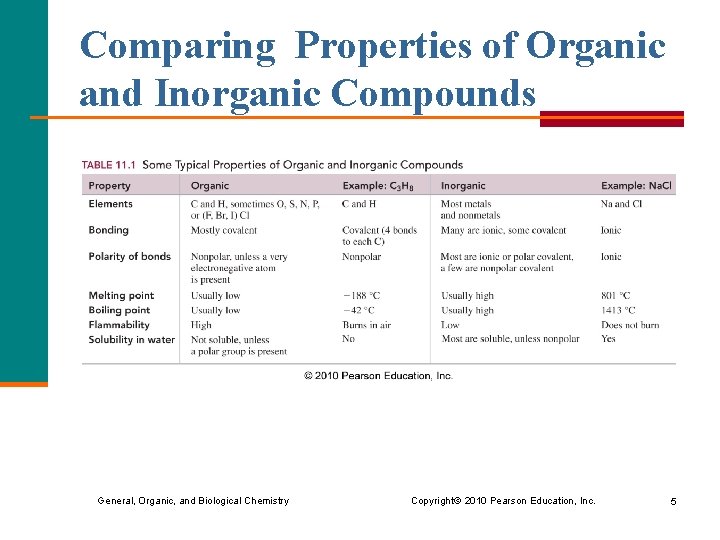
Comparing Properties of Organic and Inorganic Compounds General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 5

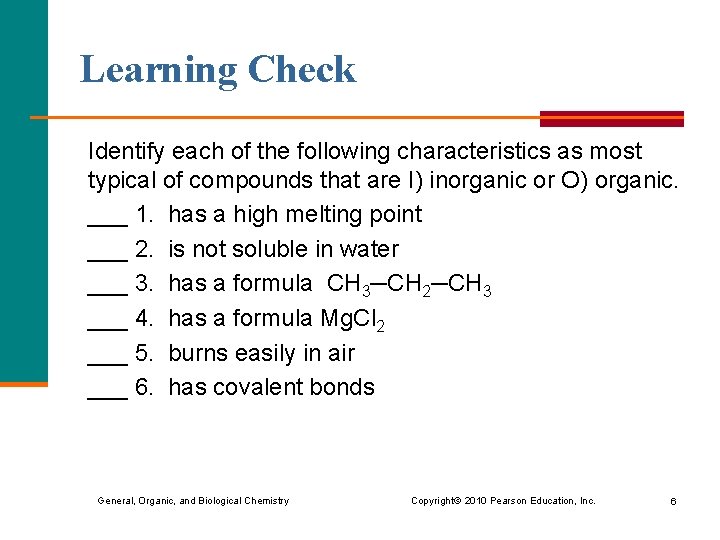
Learning Check Identify each of the following characteristics as most typical of compounds that are I) inorganic or O) organic. ___ 1. has a high melting point ___ 2. is not soluble in water ___ 3. has a formula CH 3─CH 2─CH 3 ___ 4. has a formula Mg. Cl 2 ___ 5. burns easily in air ___ 6. has covalent bonds General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 6

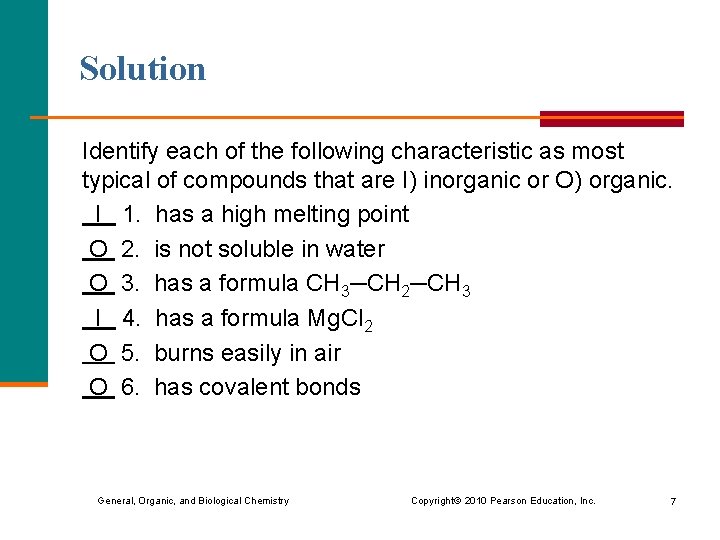
Solution Identify each of the following characteristic as most typical of compounds that are I) inorganic or O) organic. I 1. has a high melting point O 2. is not soluble in water O 3. has a formula CH 3─CH 2─CH 3 I 4. has a formula Mg. Cl 2 O 5. burns easily in air O 6. has covalent bonds General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 7

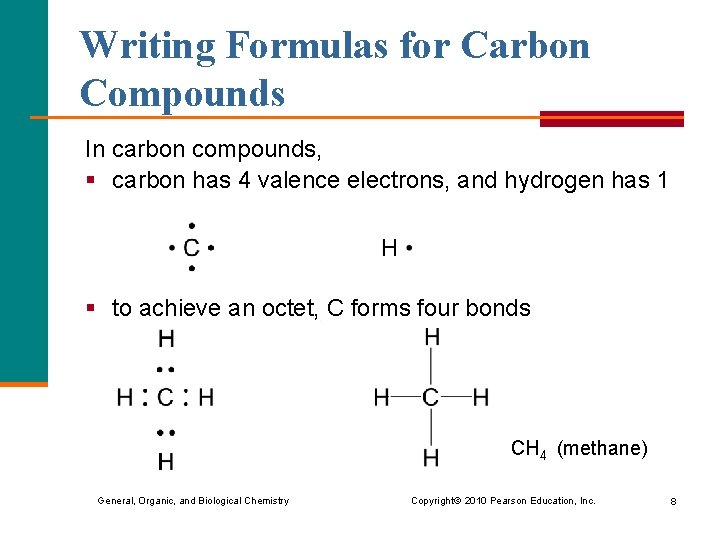
Writing Formulas for Carbon Compounds In carbon compounds, § carbon has 4 valence electrons, and hydrogen has 1 § to achieve an octet, C forms four bonds CH 4 (methane) General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 8

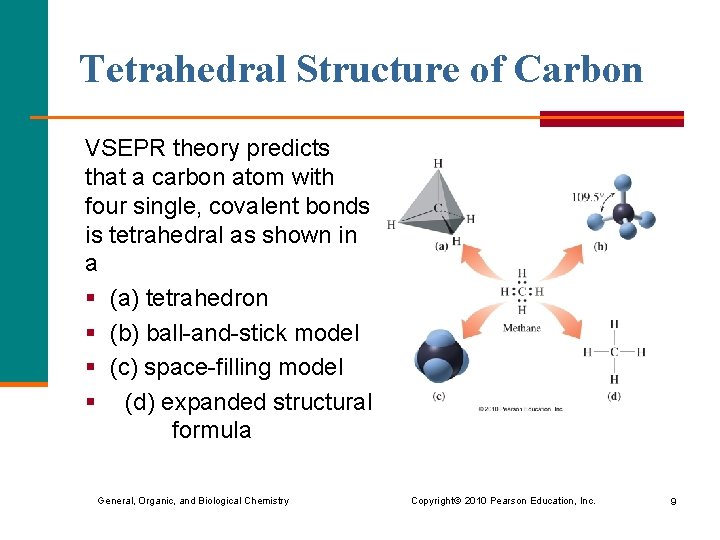
Tetrahedral Structure of Carbon VSEPR theory predicts that a carbon atom with four single, covalent bonds is tetrahedral as shown in a § (a) tetrahedron § (b) ball-and-stick model § (c) space-filling model § (d) expanded structural formula General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 9

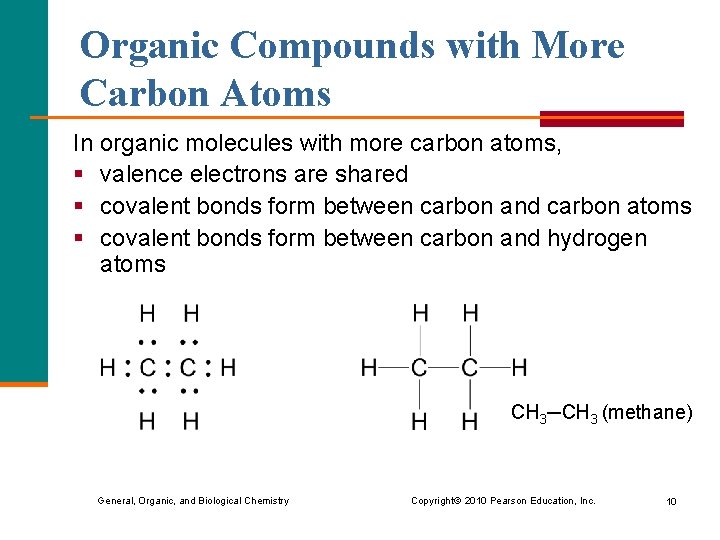
Organic Compounds with More Carbon Atoms In organic molecules with more carbon atoms, § valence electrons are shared § covalent bonds form between carbon and carbon atoms § covalent bonds form between carbon and hydrogen atoms CH 3─CH 3 (methane) General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 10

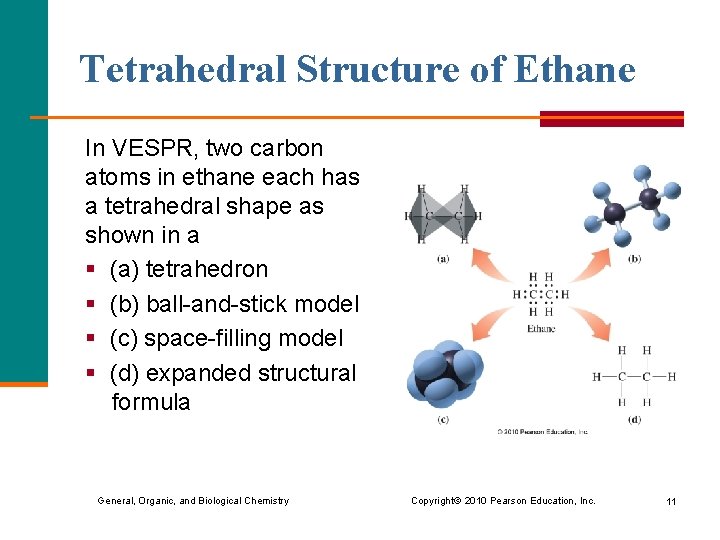
Tetrahedral Structure of Ethane In VESPR, two carbon atoms in ethane each has a tetrahedral shape as shown in a § (a) tetrahedron § (b) ball-and-stick model § (c) space-filling model § (d) expanded structural formula General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 11