Chapter 11 Introduction to Organic Chemistry Alkanes 11









- Slides: 22

Chapter 11 Introduction to Organic Chemistry: Alkanes 11. 4 Properties of Alkanes Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings 1

Some Properties of Alkanes The properties of alkanes include being § Nonpolar. § Insoluble in water. § Less dense than water § Flammable in air. Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings 2

Alkanes with 1 -4 Carbon Atoms Alkanes with 1 -4 carbon atoms are § Methane, propane, and butane. § Gases at room temperature. § Used as heating fuels. Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings 3

Alkanes with 5 -17 Carbon Atoms Alkanes with 5 -8 carbon atoms are § Liquids at room temperature. § Pentane, hexane, heptane, and octane. § Very volatile. § Used to make gasoline. Alkanes with 9 -17 carbon atoms § Are liquids at room temperature § Have higher boiling points. § Are found in kerosene, diesel, and jet fuels. 4

Alkanes with 18 or more Carbon Atoms Alkanes with 18 or more carbon atoms § Have high molar masses. § Are waxy solids at room temperature. § Used in waxy coatings of fruits and vegetables. Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings 5

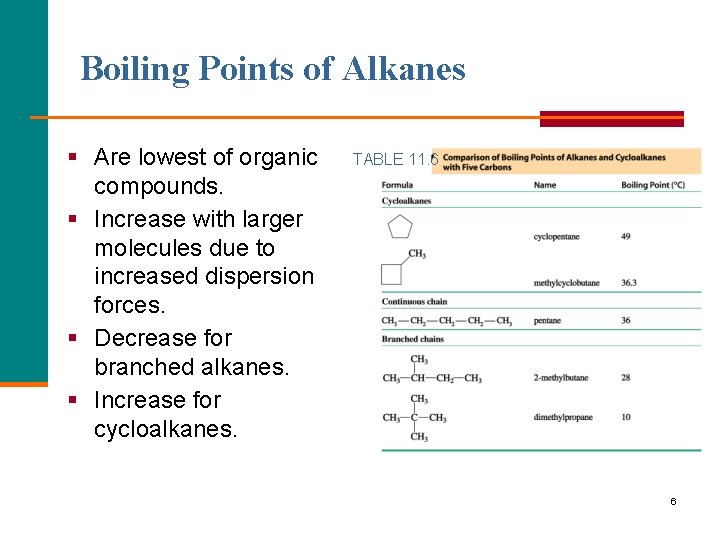
Boiling Points of Alkanes § Are lowest of organic compounds. § Increase with larger molecules due to increased dispersion forces. § Decrease for branched alkanes. § Increase for cycloalkanes. TABLE 11. 6 6

Sample Question – Boiling Point For the following pairs of hydrocarbon, which one has the higher boiling point? Why? 1. butane or octane 2. hexane or 2, 3 -dimethylbutane 7

Solution For the following pairs of hydrocarbon, which one has the higher boiling point? Why? 1. butane or octane has more carbon atoms 2. hexane or 2, 3 -dimethylbutane hexane is not branched 8

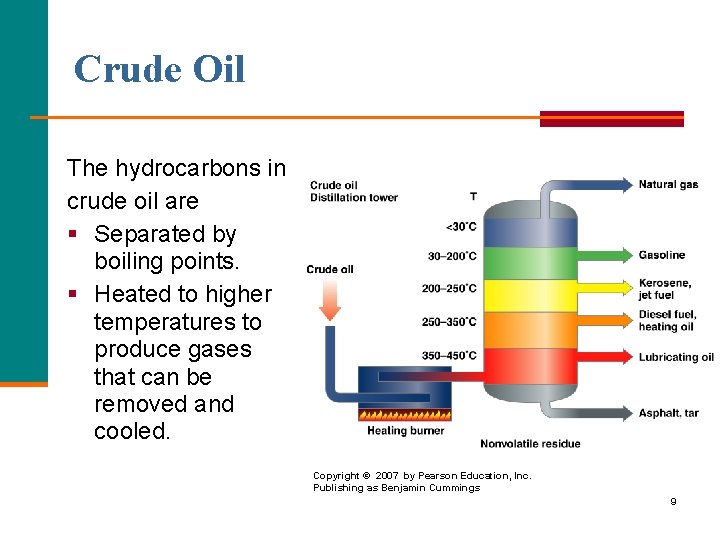
Crude Oil The hydrocarbons in crude oil are § Separated by boiling points. § Heated to higher temperatures to produce gases that can be removed and cooled. Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings 9

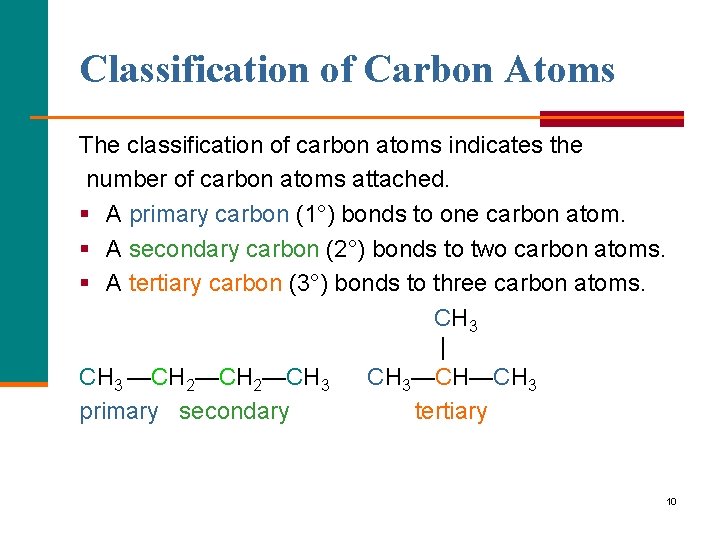
Classification of Carbon Atoms The classification of carbon atoms indicates the number of carbon atoms attached. § A primary carbon (1°) bonds to one carbon atom. § A secondary carbon (2°) bonds to two carbon atoms. § A tertiary carbon (3°) bonds to three carbon atoms. CH 3 | CH 3 —CH 2—CH 3—CH—CH 3 primary secondary tertiary 10

Combustion of Alkanes § Undergo combustion by reacting with oxygen to produce carbon dioxide, water, and energy. § Are typically not very reactive due to strong C -C single bonds. Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings alkane + O 2 CO 2 + H 2 O + energy 11

Balancing a Combustion Reaction Propane is used to provide heat for cooking or warming a room. Write a balanced equation for the complete combustion of propane. Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings 12

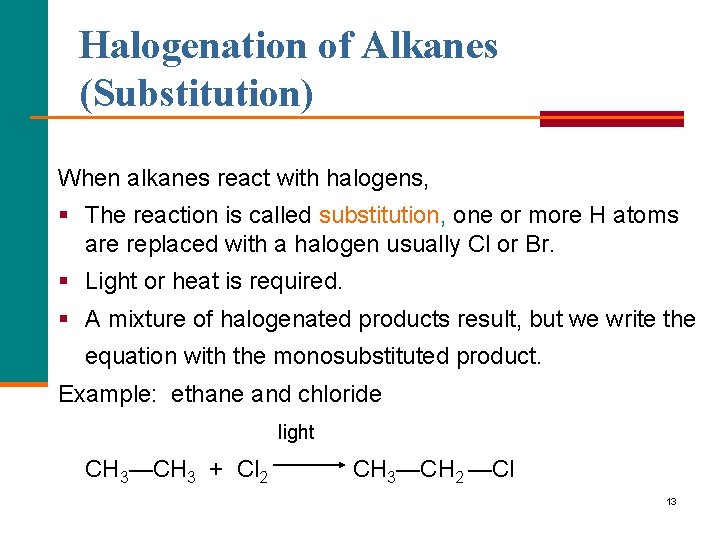
Halogenation of Alkanes (Substitution) When alkanes react with halogens, § The reaction is called substitution, one or more H atoms are replaced with a halogen usually Cl or Br. § Light or heat is required. § A mixture of halogenated products result, but we write the equation with the monosubstituted product. Example: ethane and chloride light CH 3—CH 3 + Cl 2 CH 3—CH 2 —Cl 13

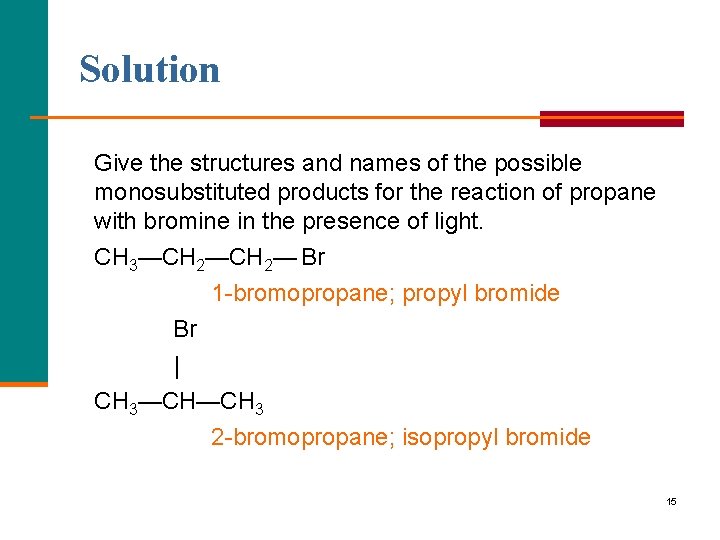
Example of Bromination Give the structures and names of the possible monosubstituted products for the reaction of propane with bromine in the presence of light. 14

Solution Give the structures and names of the possible monosubstituted products for the reaction of propane with bromine in the presence of light. CH 3—CH 2— Br 1 -bromopropane; propyl bromide Br | CH 3—CH—CH 3 2 -bromopropane; isopropyl bromide 15

Functional Groups Functional groups are § A characteristic feature of organic molecules that behave in a predictable way. § Composed of an atom or group of atoms. § Groups that replace an H in the corresponding alkane. § A way to classify families of organic compounds. 16

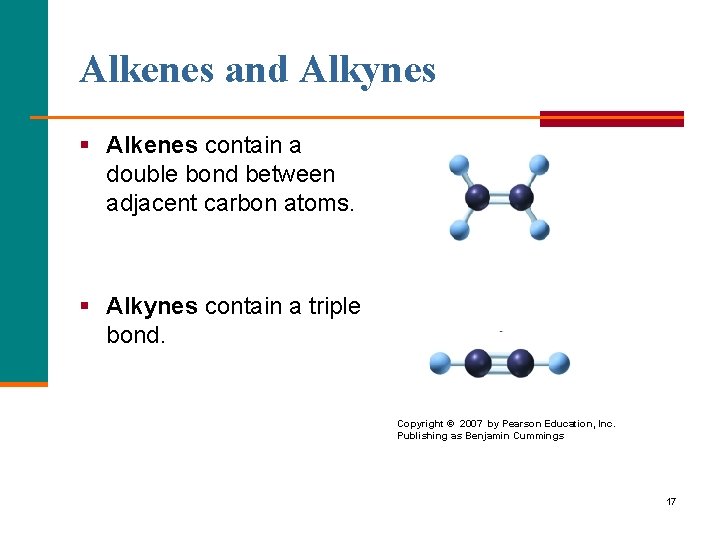
Alkenes and Alkynes § Alkenes contain a double bond between adjacent carbon atoms. § Alkynes contain a triple bond. Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings 17

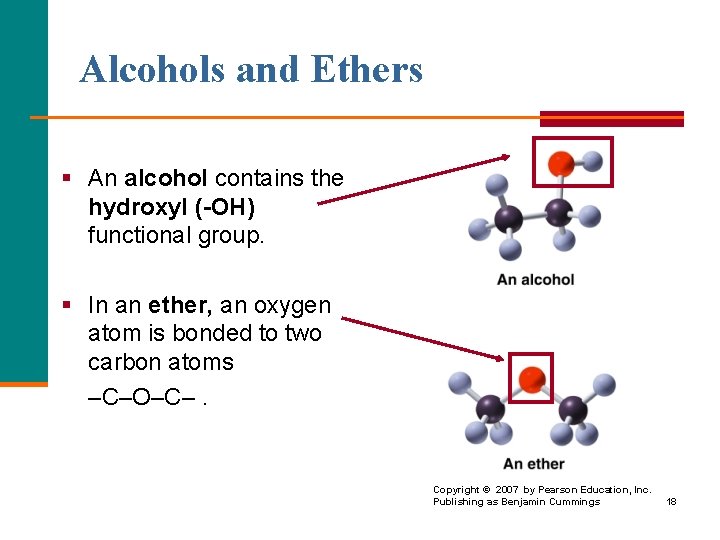
Alcohols and Ethers § An alcohol contains the hydroxyl (-OH) functional group. § In an ether, an oxygen atom is bonded to two carbon atoms –C–O–C–. Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings 18

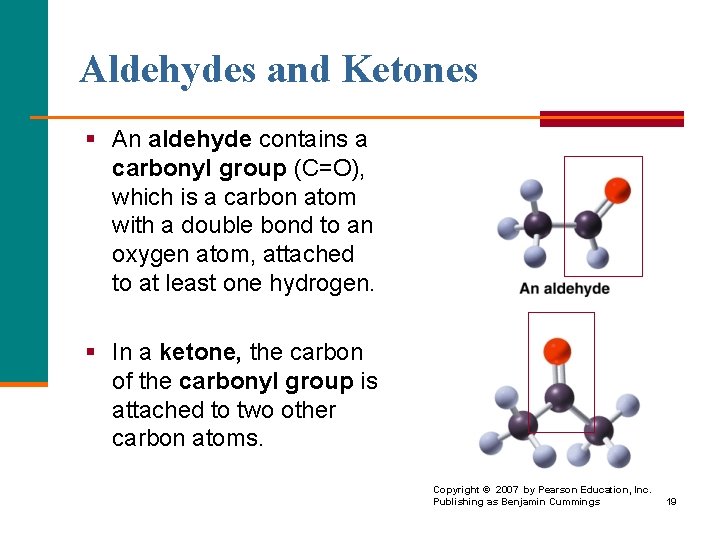
Aldehydes and Ketones § An aldehyde contains a carbonyl group (C=O), which is a carbon atom with a double bond to an oxygen atom, attached to at least one hydrogen. § In a ketone, the carbon of the carbonyl group is attached to two other carbon atoms. Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings 19

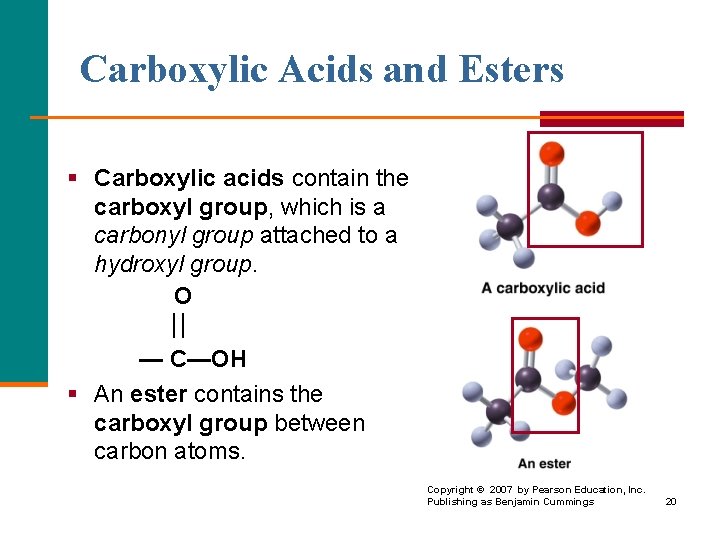
Carboxylic Acids and Esters § Carboxylic acids contain the carboxyl group, which is a carbonyl group attached to a hydroxyl group. O — C—OH § An ester contains the carboxyl group between carbon atoms. Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings 20

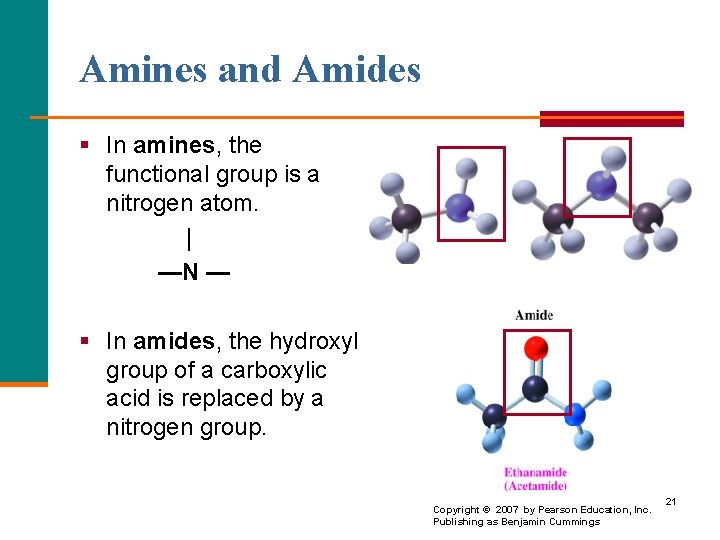
Amines and Amides § In amines, the functional group is a nitrogen atom. | —N — § In amides, the hydroxyl group of a carboxylic acid is replaced by a nitrogen group. Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings 21

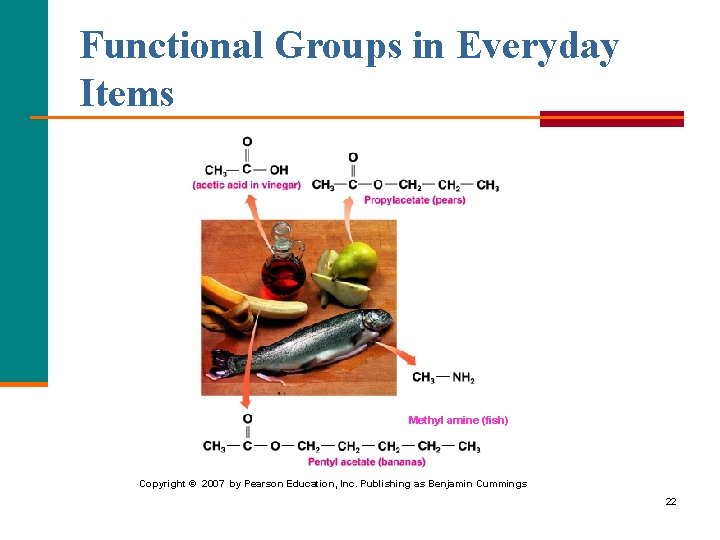
Functional Groups in Everyday Items Methyl amine (fish) Copyright © 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings 22