Chapter 11 Introduction to Organic Chemistry Alkanes 11
- Slides: 12

Chapter 11 Introduction to Organic Chemistry: Alkanes 11. 5 Functional Groups General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 1

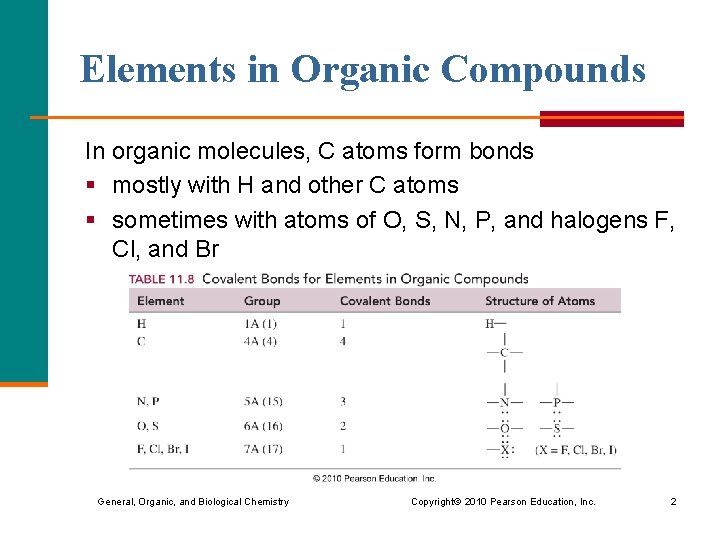
Elements in Organic Compounds In organic molecules, C atoms form bonds § mostly with H and other C atoms § sometimes with atoms of O, S, N, P, and halogens F, Cl, and Br General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 2

Functional Groups Functional groups § are a characteristic feature of organic molecules that behave in a predictable way § are composed of an atom or group of atoms § are groups that replace a H in the corresponding alkane § provide a way to classify families of organic compounds General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 3

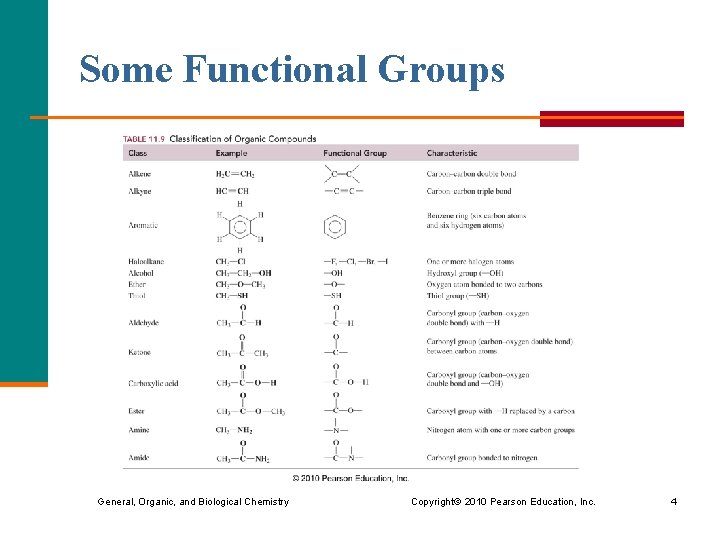
Some Functional Groups General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 4

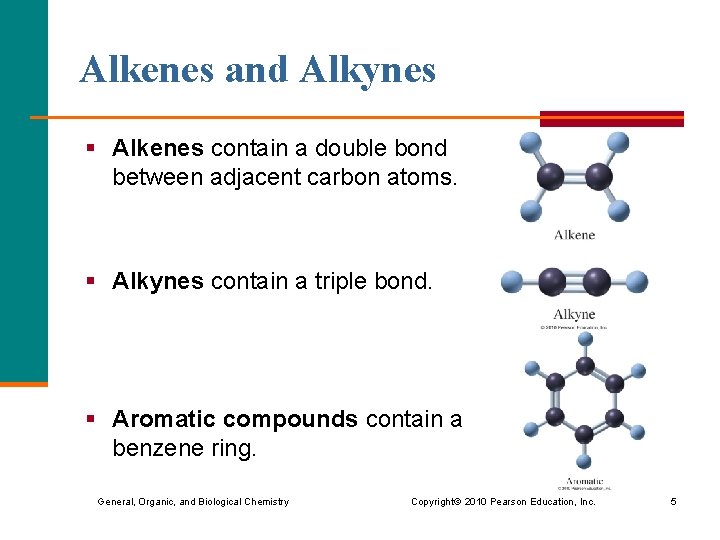
Alkenes and Alkynes § Alkenes contain a double bond between adjacent carbon atoms. § Alkynes contain a triple bond. § Aromatic compounds contain a benzene ring. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 5

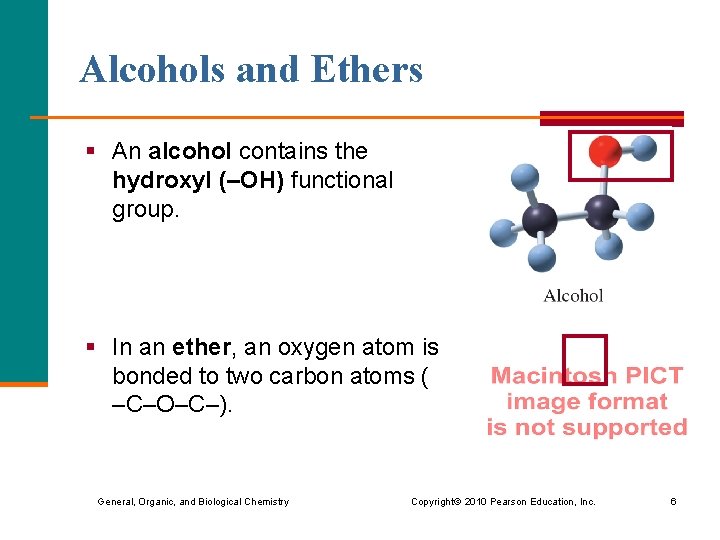
Alcohols and Ethers § An alcohol contains the hydroxyl (–OH) functional group. § In an ether, an oxygen atom is bonded to two carbon atoms ( –C–O–C–). General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 6

Thiols § In a thiol, the functional group –SH is bonded to a carbon atom. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 7

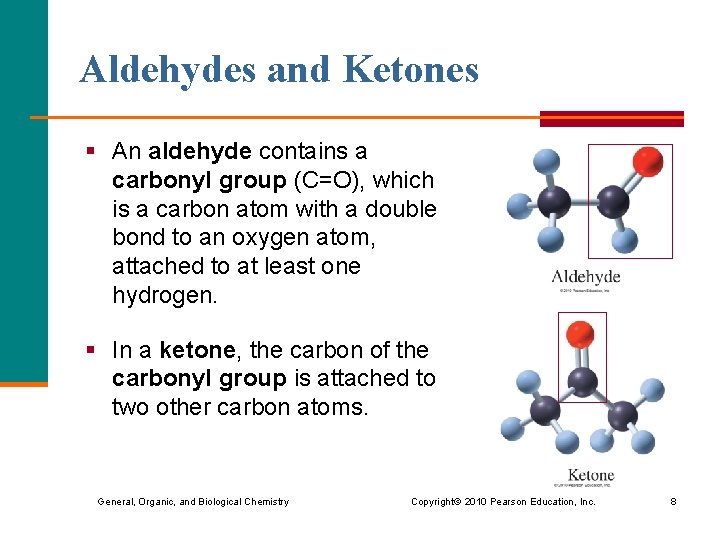
Aldehydes and Ketones § An aldehyde contains a carbonyl group (C=O), which is a carbon atom with a double bond to an oxygen atom, attached to at least one hydrogen. § In a ketone, the carbon of the carbonyl group is attached to two other carbon atoms. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 8

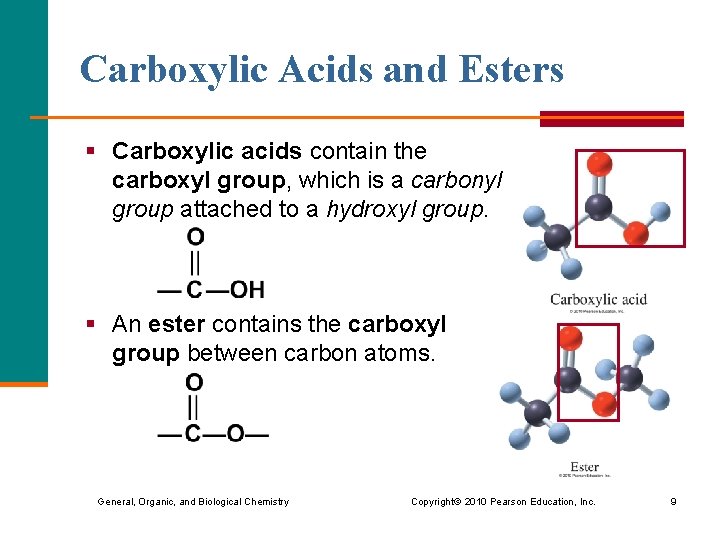
Carboxylic Acids and Esters § Carboxylic acids contain the carboxyl group, which is a carbonyl group attached to a hydroxyl group. § An ester contains the carboxyl group between carbon atoms. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 9

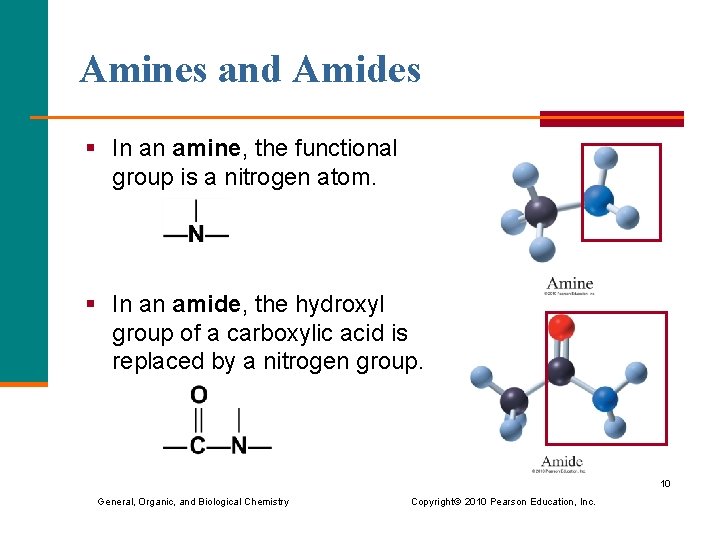
Amines and Amides § In an amine, the functional group is a nitrogen atom. § In an amide, the hydroxyl group of a carboxylic acid is replaced by a nitrogen group. 10 General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc.

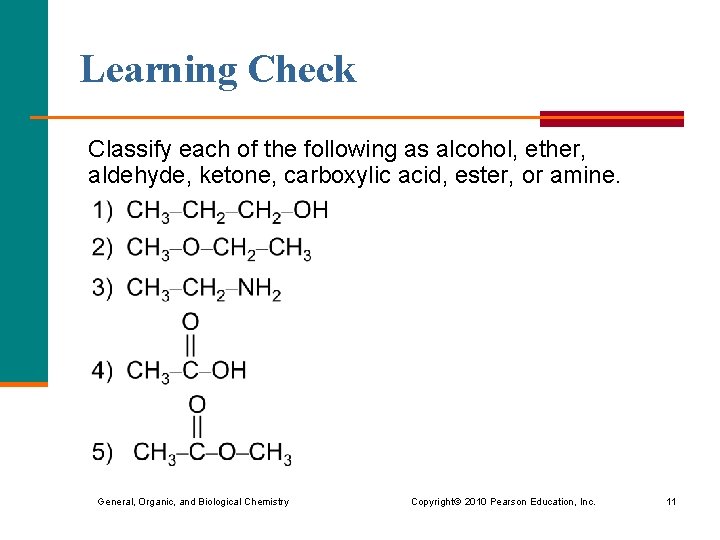
Learning Check Classify each of the following as alcohol, ether, aldehyde, ketone, carboxylic acid, ester, or amine. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 11

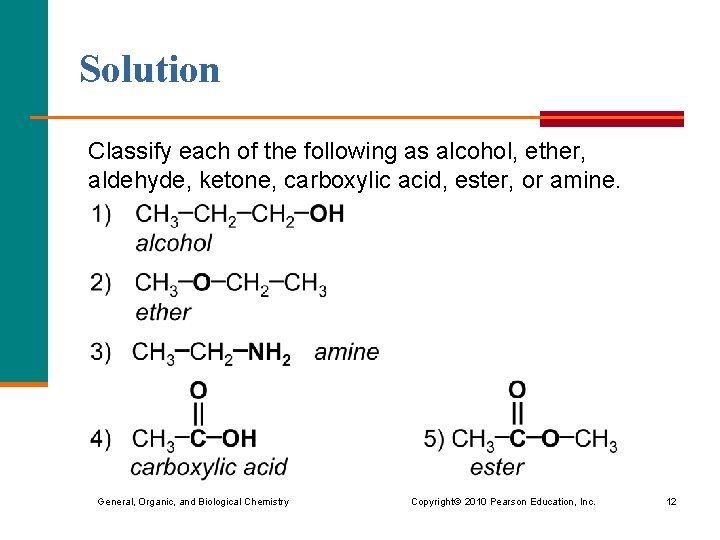
Solution Classify each of the following as alcohol, ether, aldehyde, ketone, carboxylic acid, ester, or amine. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 12