Chapter 11 Introduction to Organic Chemistry Alkanes 11
- Slides: 19

Chapter 11 Introduction to Organic Chemistry: Alkanes 11. 3 Alkanes with Substituents General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 1

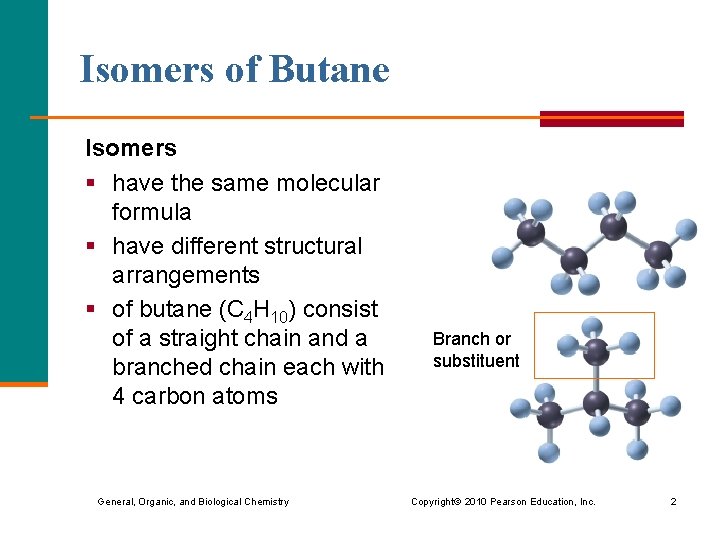
Isomers of Butane Isomers § have the same molecular formula § have different structural arrangements § of butane (C 4 H 10) consist of a straight chain and a branched chain each with 4 carbon atoms Branch or substituent General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 2

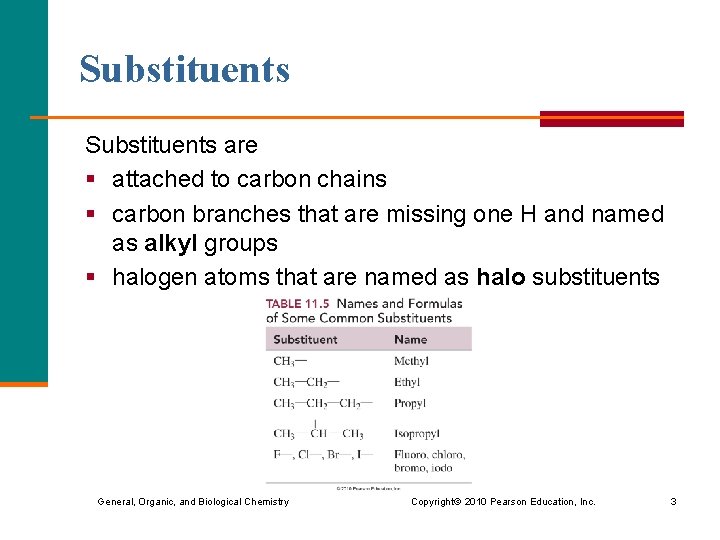
Substituents are § attached to carbon chains § carbon branches that are missing one H and named as alkyl groups § halogen atoms that are named as halo substituents General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 3

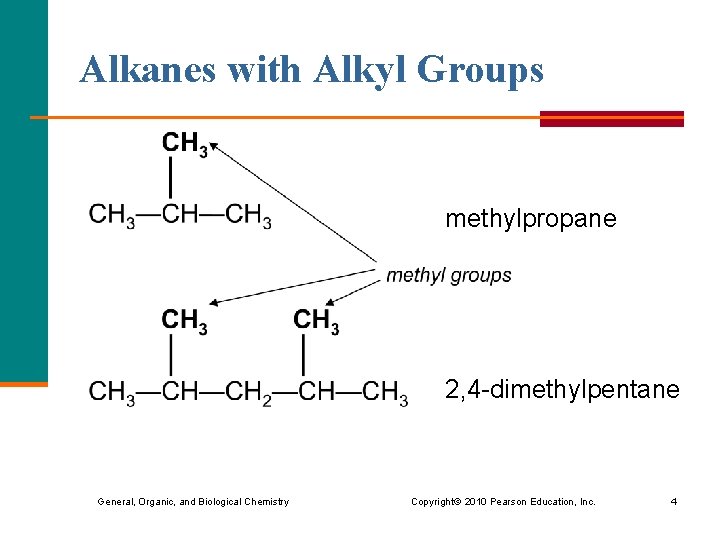
Alkanes with Alkyl Groups methylpropane 2, 4 -dimethylpentane General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 4

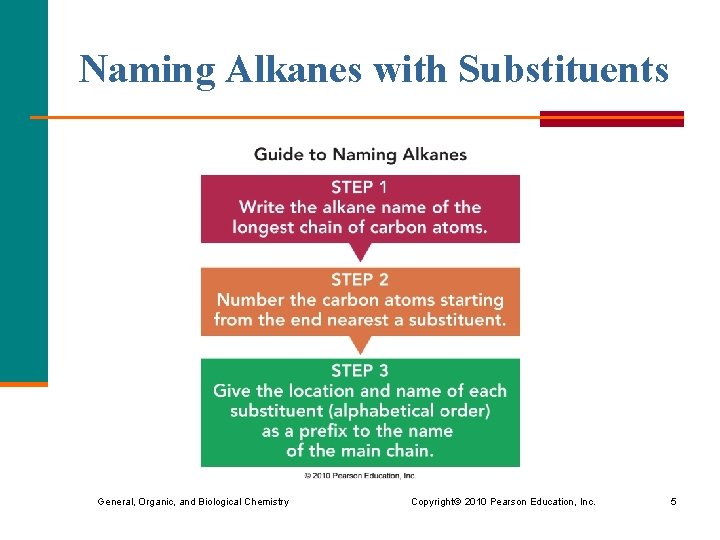
Naming Alkanes with Substituents General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 5

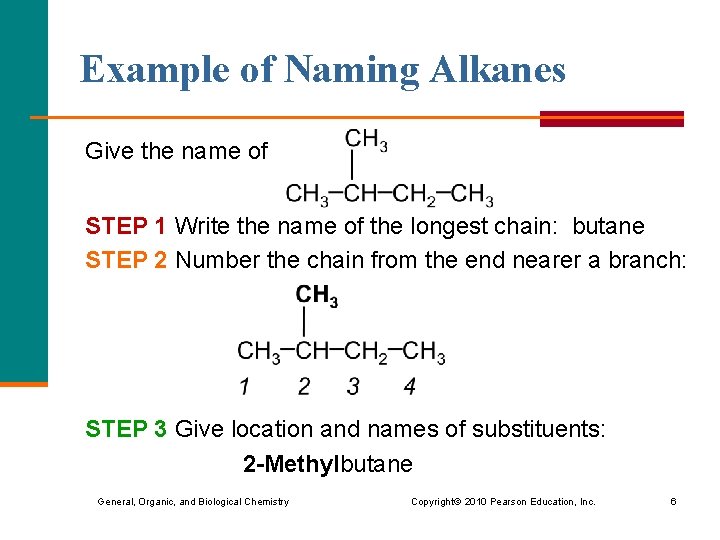
Example of Naming Alkanes Give the name of STEP 1 Write the name of the longest chain: butane STEP 2 Number the chain from the end nearer a branch: STEP 3 Give location and names of substituents: 2 -Methylbutane General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 6

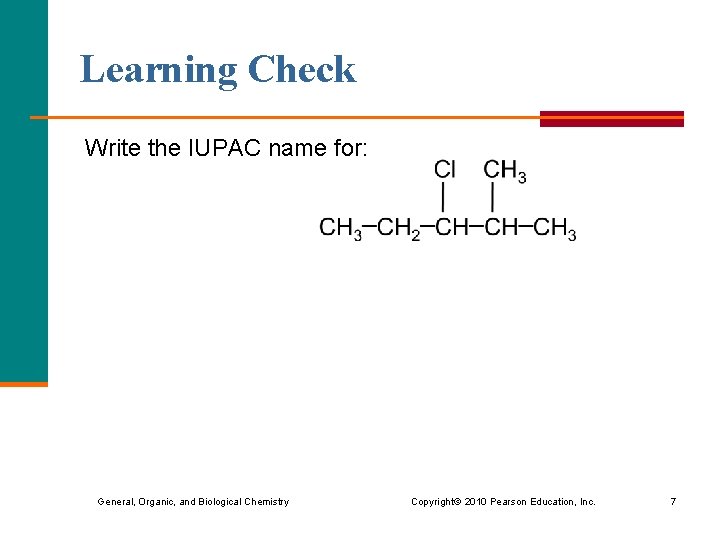
Learning Check Write the IUPAC name for: General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 7

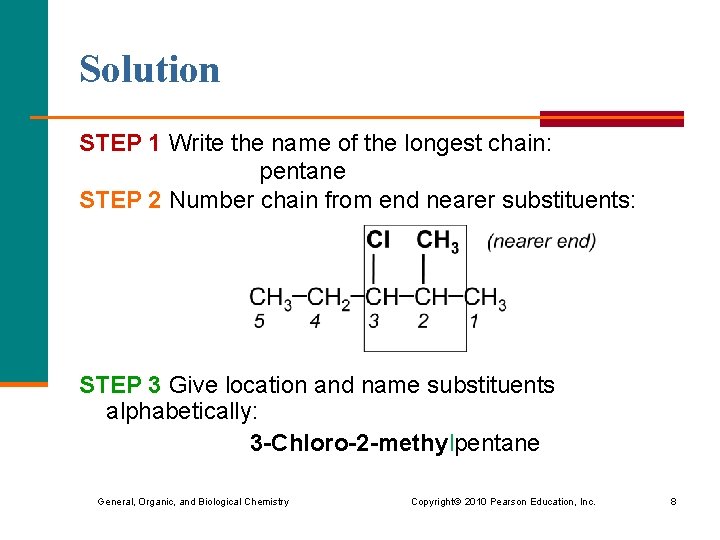
Solution STEP 1 Write the name of the longest chain: pentane STEP 2 Number chain from end nearer substituents: STEP 3 Give location and name substituents alphabetically: 3 -Chloro-2 -methylpentane General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 8

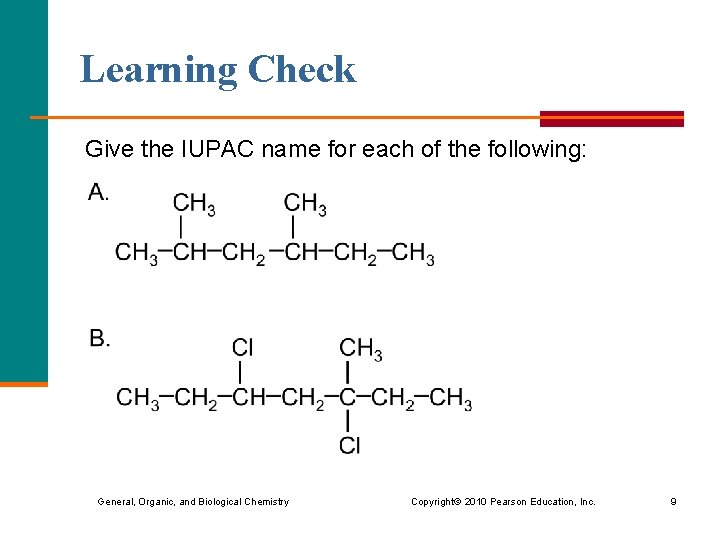
Learning Check Give the IUPAC name for each of the following: General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 9

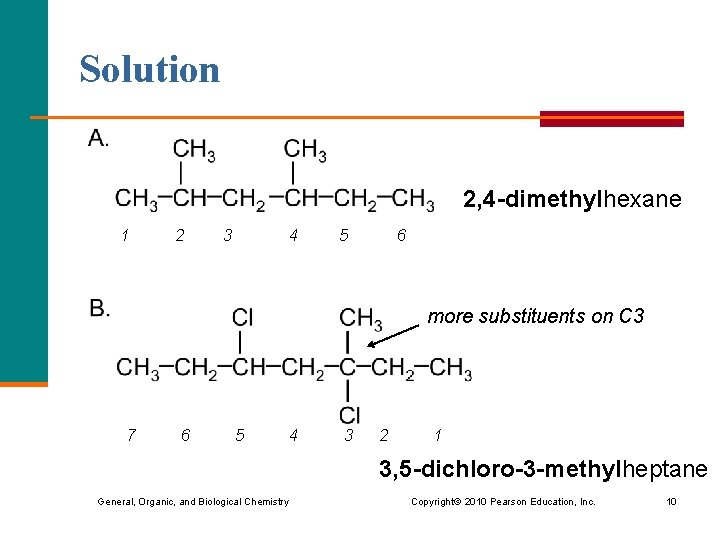
Solution 2, 4 -dimethylhexane 1 2 3 4 5 6 more substituents on C 3 7 6 5 4 3 2 1 3, 5 -dichloro-3 -methylheptane General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 10

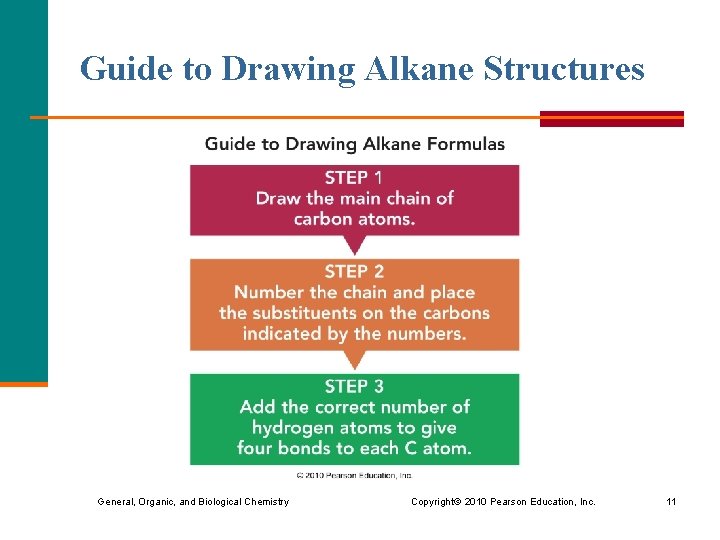
Guide to Drawing Alkane Structures General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 11

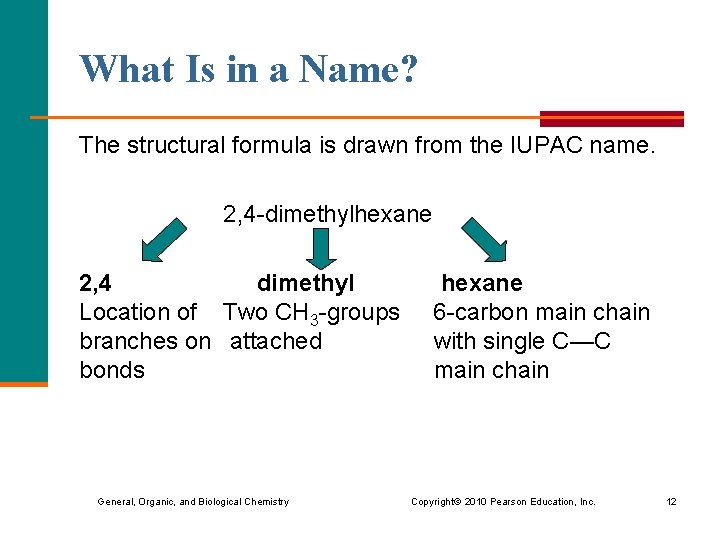
What Is in a Name? The structural formula is drawn from the IUPAC name. 2, 4 -dimethylhexane 2, 4 dimethyl hexane Location of Two CH 3 -groups 6 -carbon main chain branches on attached with single C—C bonds main chain General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 12

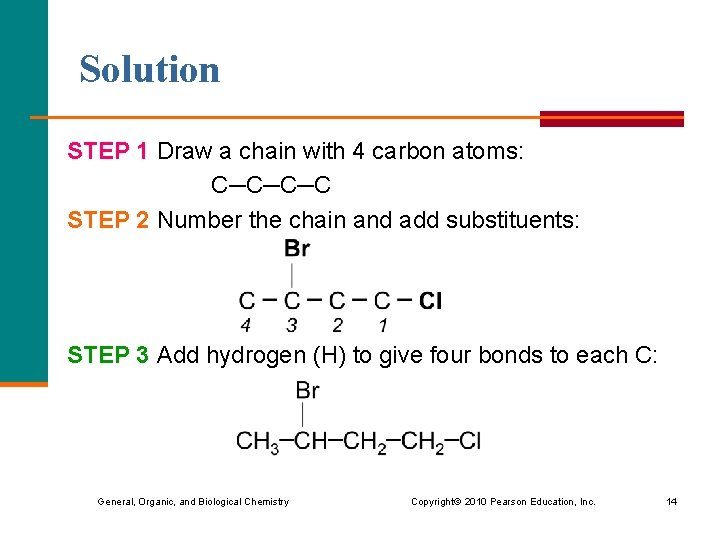
Learning Check Draw the condensed structural formula for 3 -bromo-1 -chlorobutane. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 13

Solution STEP 1 Draw a chain with 4 carbon atoms: C─C─C─C STEP 2 Number the chain and add substituents: STEP 3 Add hydrogen (H) to give four bonds to each C: General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 14

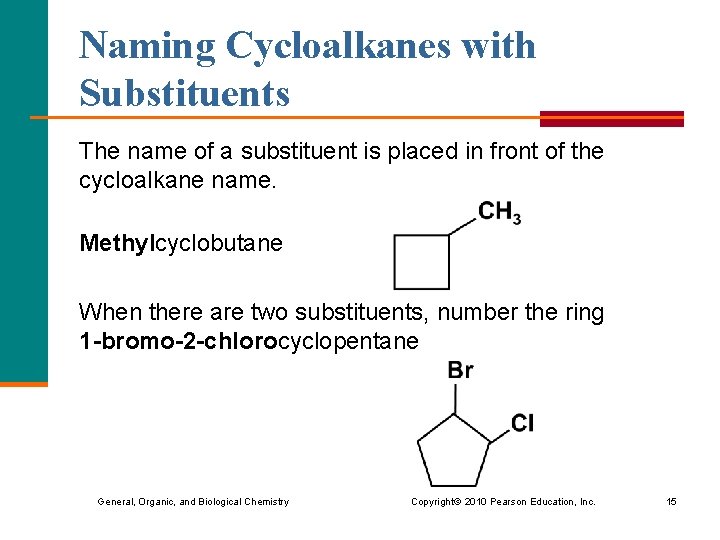
Naming Cycloalkanes with Substituents The name of a substituent is placed in front of the cycloalkane name. Methylcyclobutane When there are two substituents, number the ring 1 -bromo-2 -chlorocyclopentane General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 15

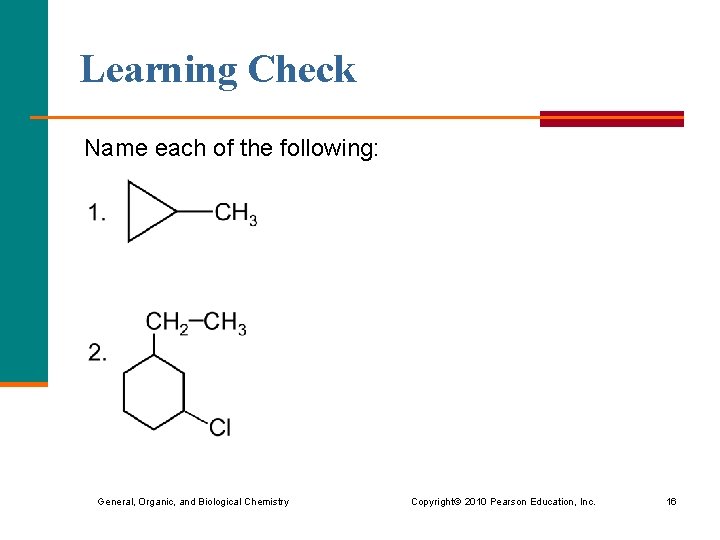
Learning Check Name each of the following: General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 16

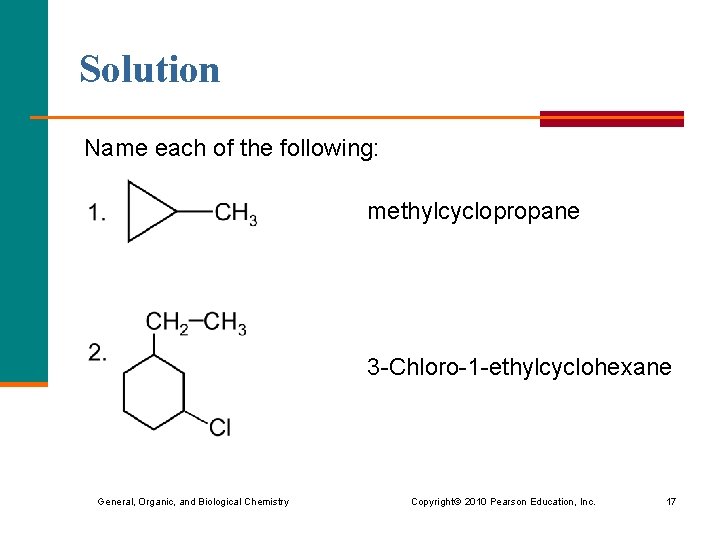
Solution Name each of the following: methylcyclopropane 3 -Chloro-1 -ethylcyclohexane General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 17

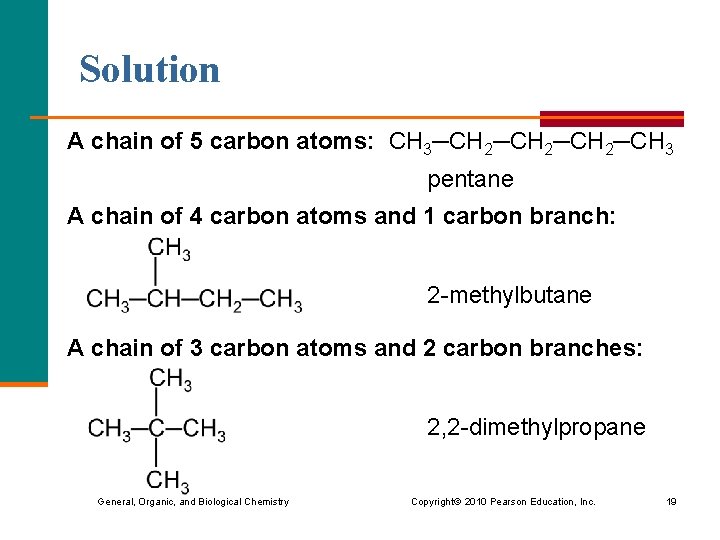
Learning Check Write three isomers of C 5 H 12, and name each. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 18

Solution A chain of 5 carbon atoms: CH 3─CH 2─CH 3 pentane A chain of 4 carbon atoms and 1 carbon branch: 2 -methylbutane A chain of 3 carbon atoms and 2 carbon branches: 2, 2 -dimethylpropane General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 19