Chapter 11 Intermolecular Forces Liquids Solids Overview u





- Slides: 43

Chapter 11 Intermolecular Forces, Liquids & Solids

Overview u u u u Liquids & Solids Intermolecular Forces Liquids Phase Changes Vapor Pressure Phase Diagrams Solids -- Structure Bonding Types in Solids

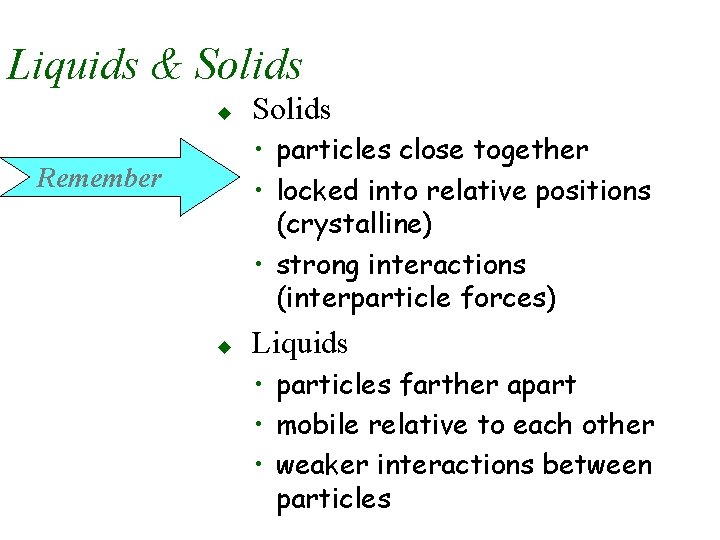
Liquids & Solids u Solids • particles close together • locked into relative positions (crystalline) • strong interactions (interparticle forces) Remember u Liquids • particles farther apart • mobile relative to each other • weaker interactions between particles

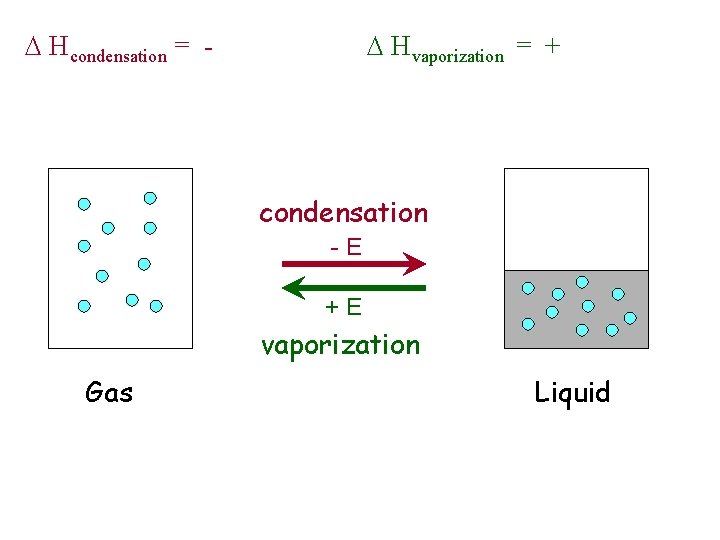
D Hcondensation = - D Hvaporization = + condensation -E +E vaporization Gas Liquid

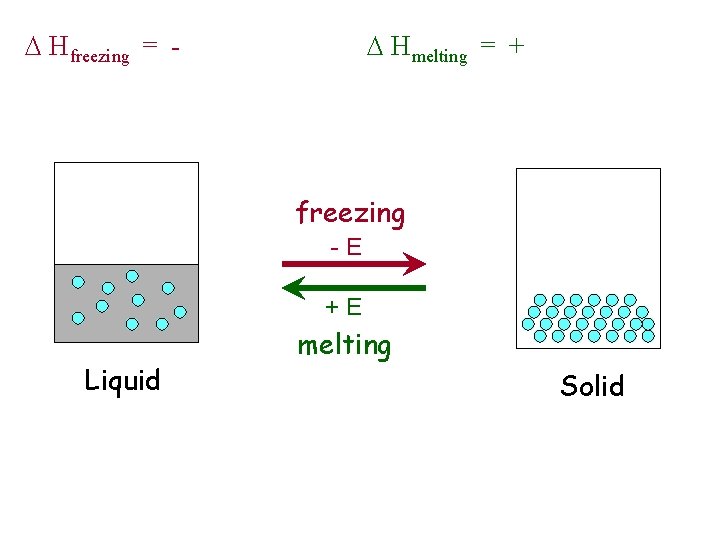
D Hfreezing = - D Hmelting = + freezing -E +E Liquid melting Solid

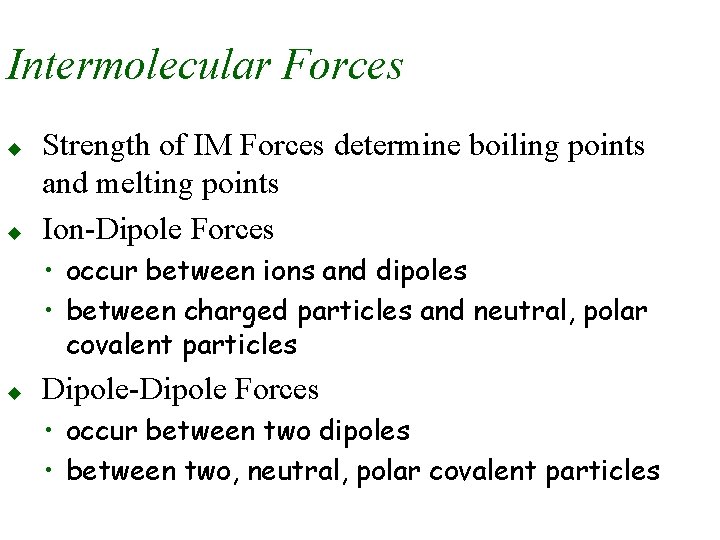
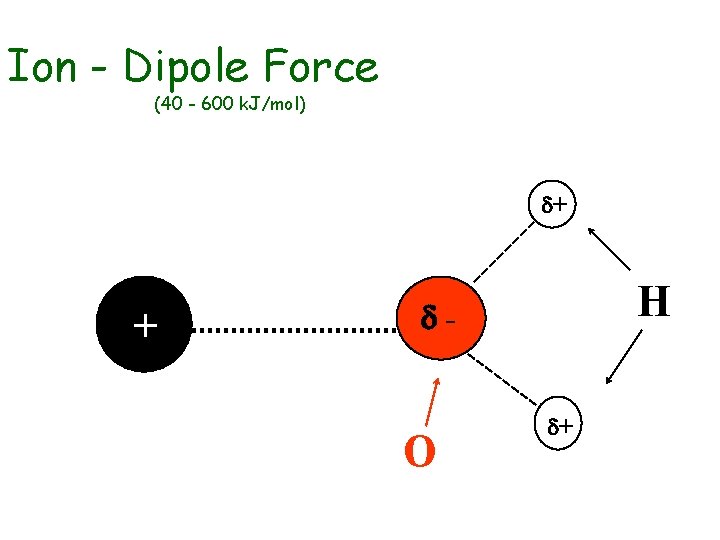
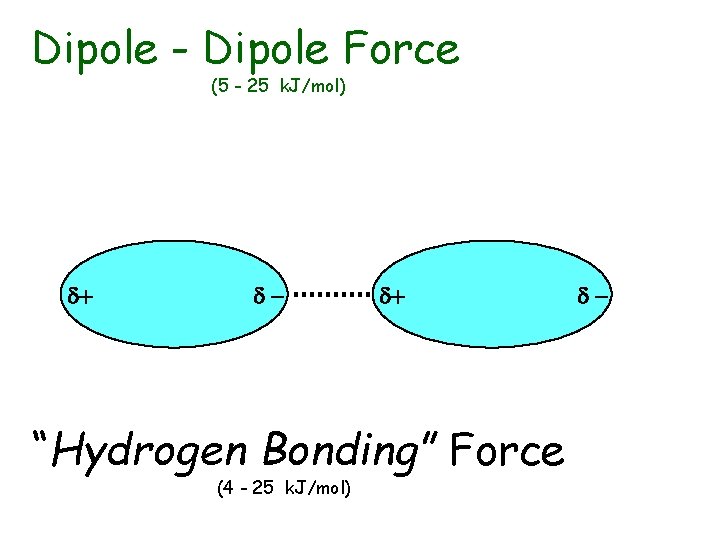
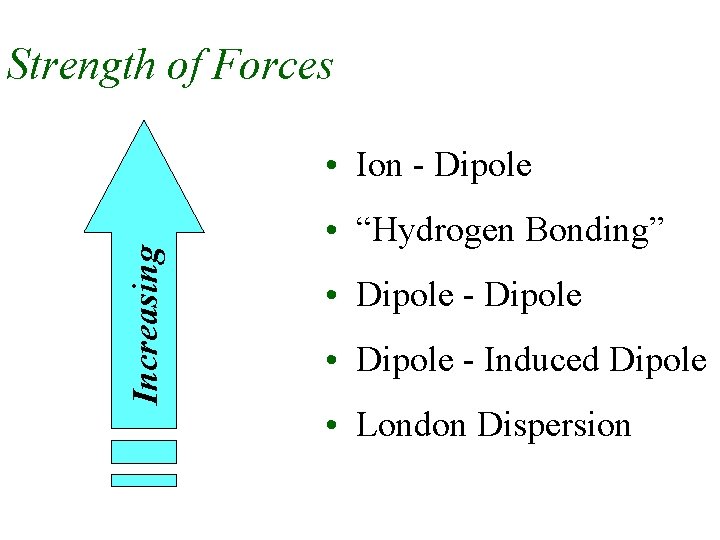
Intermolecular Forces u u Strength of IM Forces determine boiling points and melting points Ion-Dipole Forces • occur between ions and dipoles • between charged particles and neutral, polar covalent particles u Dipole-Dipole Forces • occur between two dipoles • between two, neutral, polar covalent particles

Ion - Dipole Force (40 - 600 k. J/mol) d+ + H d- O d+

Dipole - Dipole Force (5 - 25 k. J/mol) d+ d- d+ “Hydrogen Bonding” Force (4 - 25 k. J/mol) d-

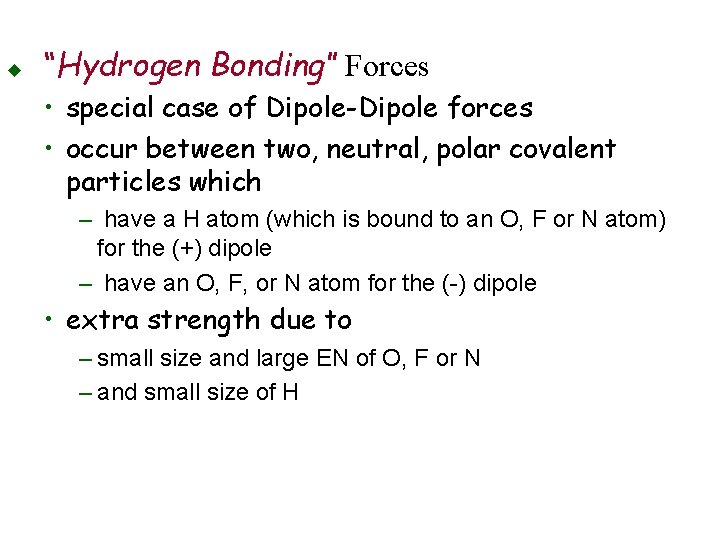
u “Hydrogen Bonding” Forces • special case of Dipole-Dipole forces • occur between two, neutral, polar covalent particles which – have a H atom (which is bound to an O, F or N atom) for the (+) dipole – have an O, F, or N atom for the (-) dipole • extra strength due to – small size and large EN of O, F or N – and small size of H

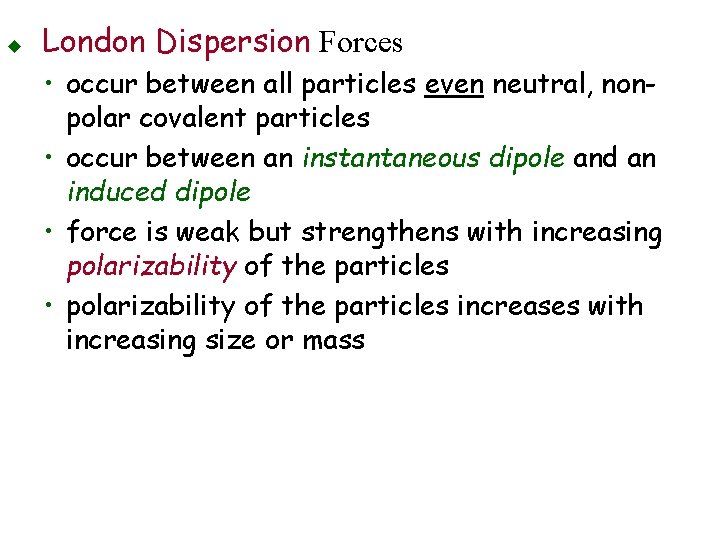
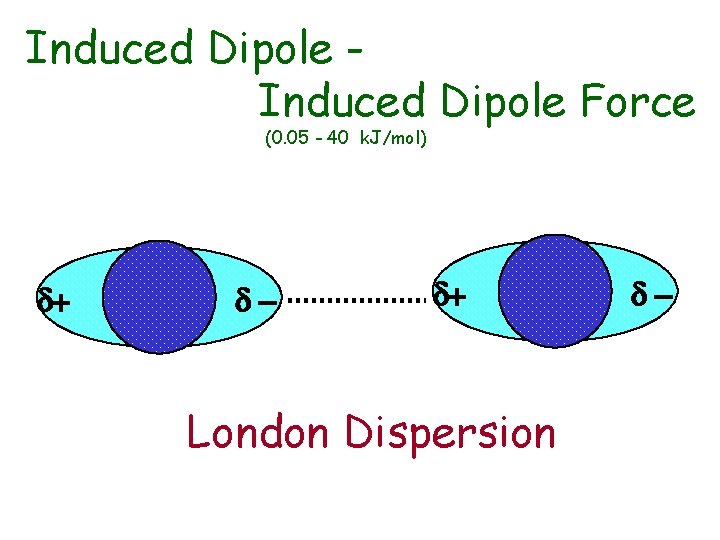
u London Dispersion Forces • occur between all particles even neutral, nonpolar covalent particles • occur between an instantaneous dipole and an induced dipole • force is weak but strengthens with increasing polarizability of the particles • polarizability of the particles increases with increasing size or mass

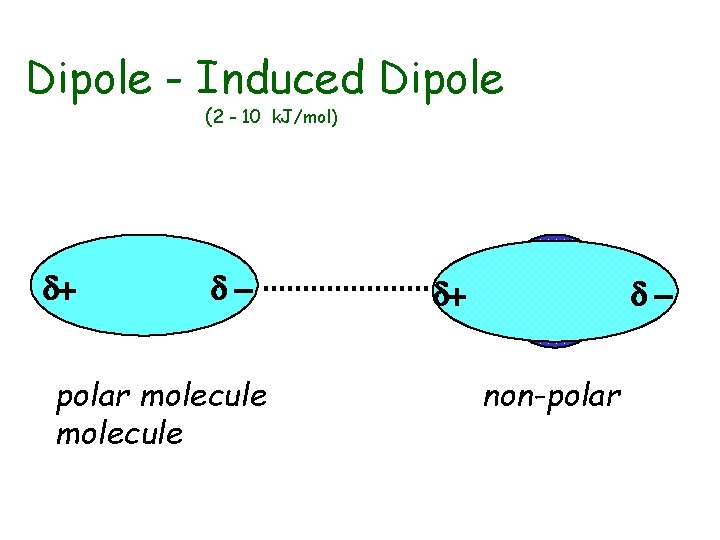
Dipole - Induced Dipole (2 - 10 k. J/mol) d+ d- polar molecule d+ dnon-polar

Induced Dipole Force (0. 05 - 40 k. J/mol) d+ d- d+ London Dispersion d-

Strength of Forces Increasing • Ion - Dipole • “Hydrogen Bonding” • Dipole - Dipole • Dipole - Induced Dipole • London Dispersion

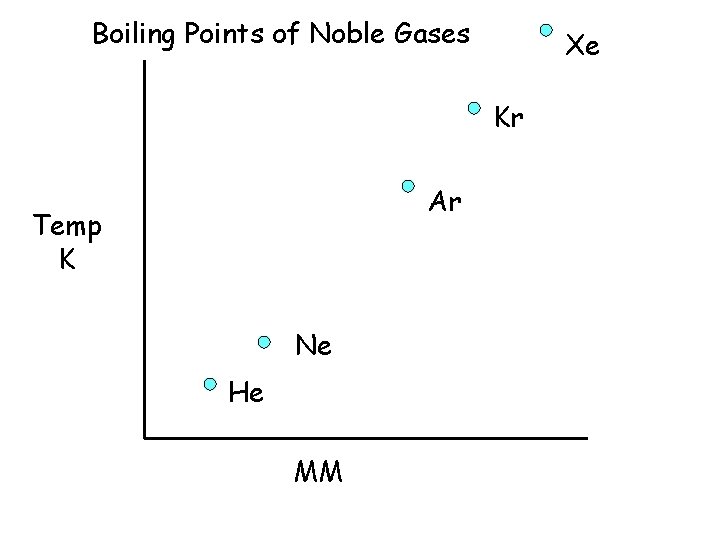
Boiling Points of Noble Gases Xe 166. 1 Kr 120. 9 Ar 87. 5 Temp K Ne 27. 3 He 4. 6 MM

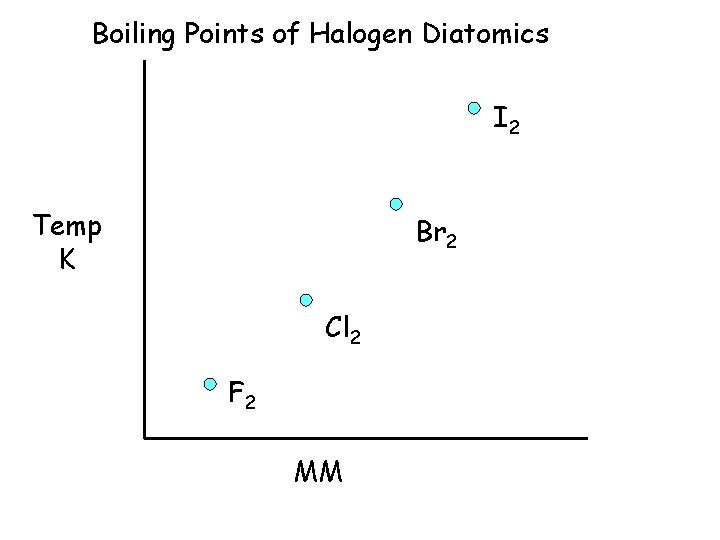
Boiling Points of Halogen Diatomics I 2 457. 6 Temp K Br 2 332. 0 Cl 2 238. 6 F 2 85. 1 MM

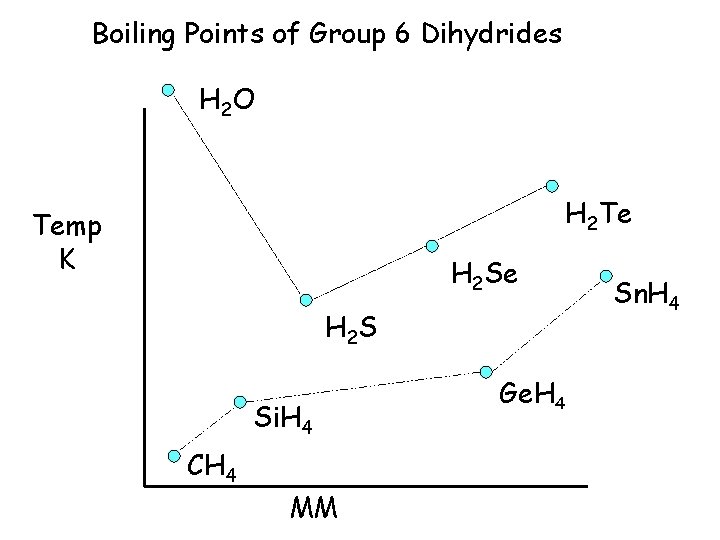
Boiling Points of Group 6 Dihydrides H 2 O 373 H 2 Te 271 Temp K H 2 Se 231 H 2 S 212 Si. H 4 161 CH 4 109 MM Sn. H 4 Ge. H 4 184

What Does it Mean? u u “hydration” is very important in the solvation process & compound formation stronger the interaction, the more energy is released, more exothermic the strength of the interaction determines the state of the substance unusual properties of water are due to “hydrogen bonding”

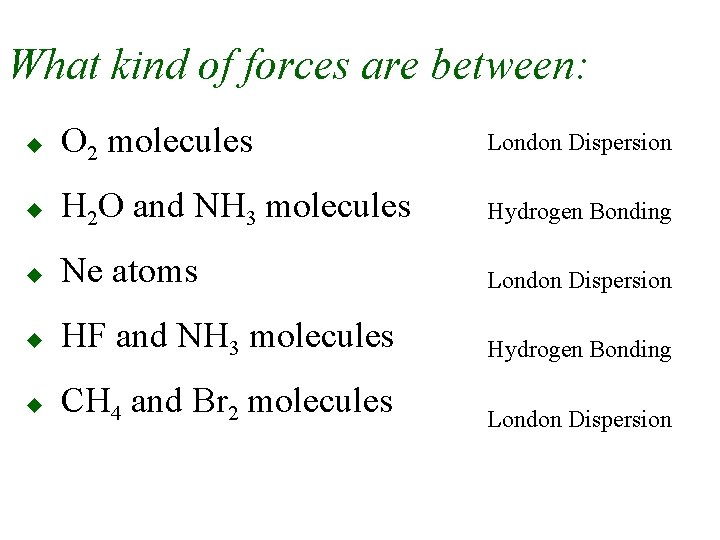
What kind of forces are between: u O 2 molecules London Dispersion u H 2 O and NH 3 molecules Hydrogen Bonding u Ne atoms London Dispersion u HF and NH 3 molecules Hydrogen Bonding u CH 4 and Br 2 molecules London Dispersion

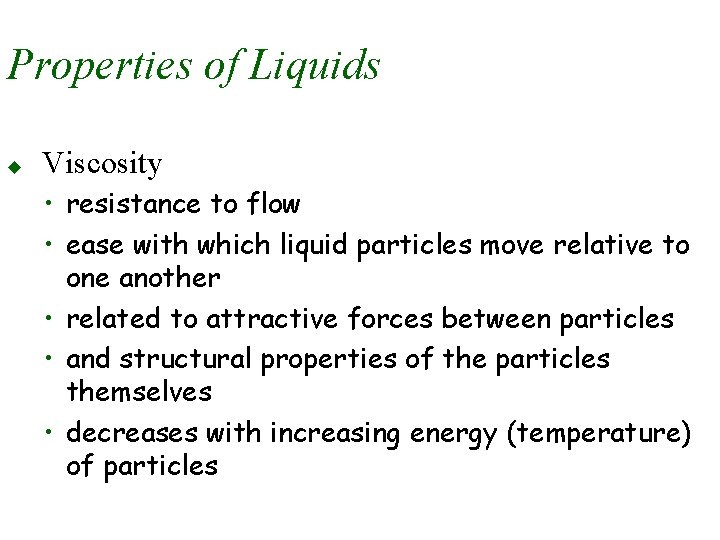
Properties of Liquids u Viscosity • resistance to flow • ease with which liquid particles move relative to one another • related to attractive forces between particles • and structural properties of the particles themselves • decreases with increasing energy (temperature) of particles

u Surface Tension • energy required to increase the surface area of a liquid by a unit amount • a sphere produces the minimum surface area • competition between cohesive forces vs adhesive forces – cohesive forces tend to minimize surface area – adhesive forces tend to maximize surface area • high surface tension reflects strong cohesive forces • capillary action -- low surface tension, strong adhesive forces

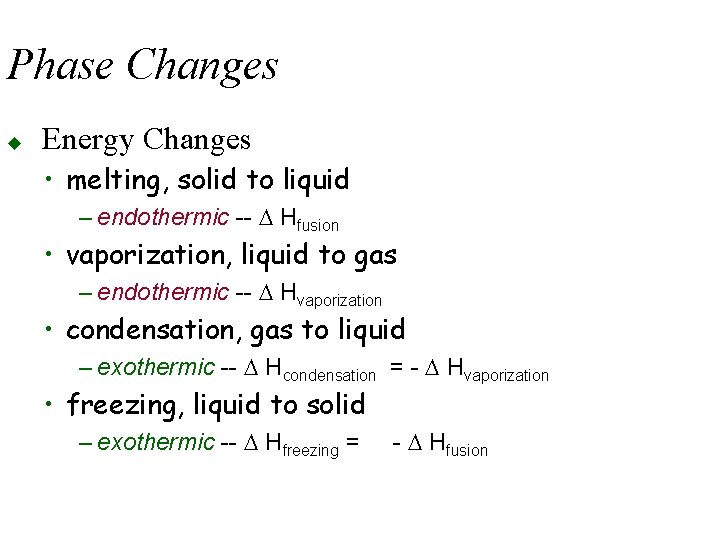
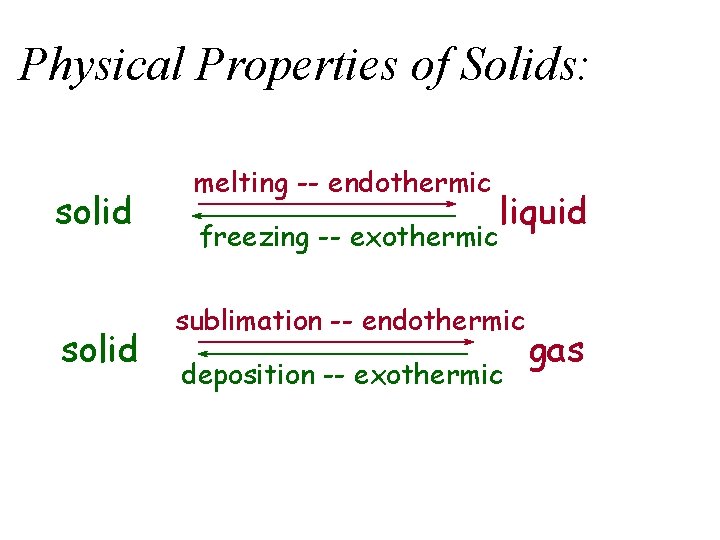
Phase Changes u Energy Changes • melting, solid to liquid – endothermic -- D Hfusion • vaporization, liquid to gas – endothermic -- D Hvaporization • condensation, gas to liquid – exothermic -- D Hcondensation = - D Hvaporization • freezing, liquid to solid – exothermic -- D Hfreezing = - D Hfusion

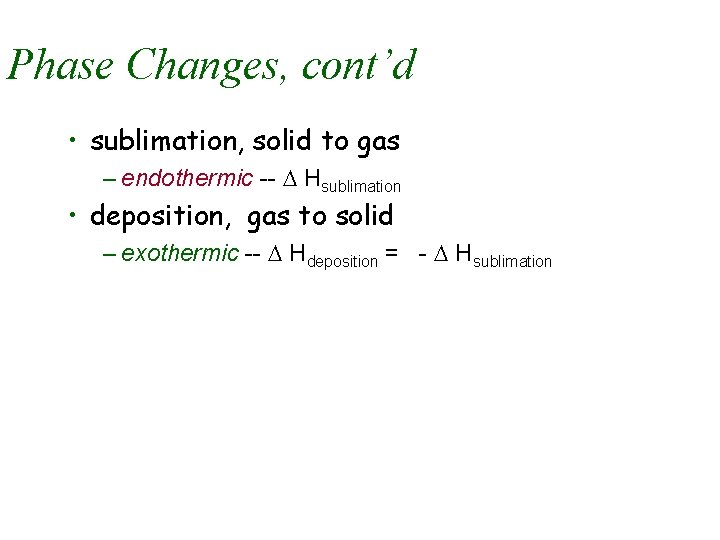
Phase Changes, cont’d • sublimation, solid to gas – endothermic -- D Hsublimation • deposition, gas to solid – exothermic -- D Hdeposition = - D Hsublimation

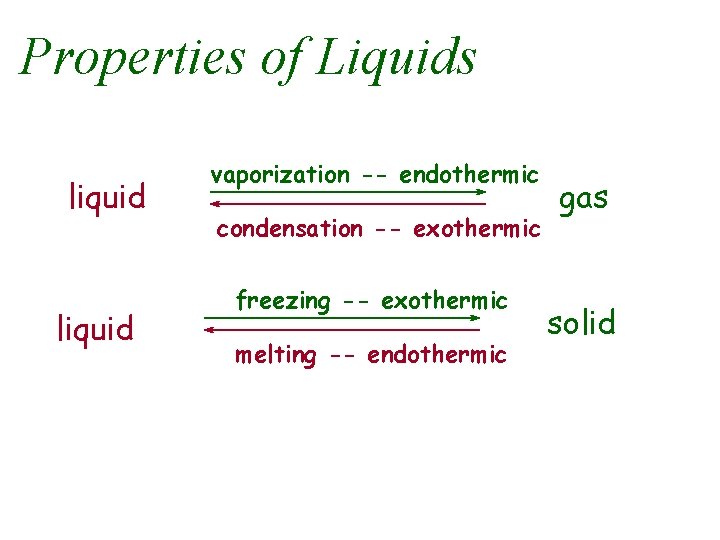
Properties of Liquids liquid vaporization -- endothermic condensation -- exothermic freezing -- exothermic melting -- endothermic gas solid

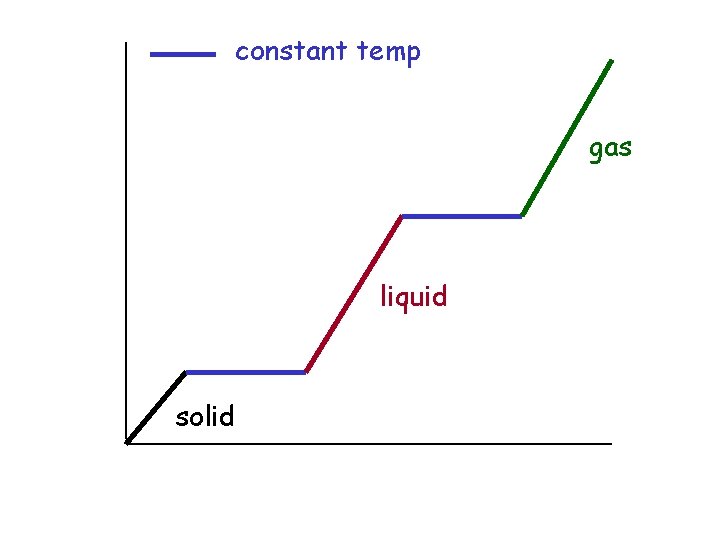
constant temp liquid + gas in equilibrium gas Temp solid + liquid in equilibrium liquid solid Time

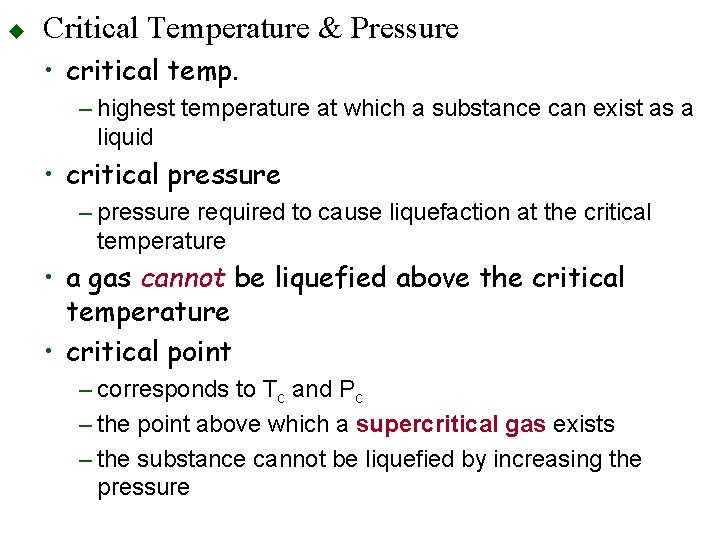
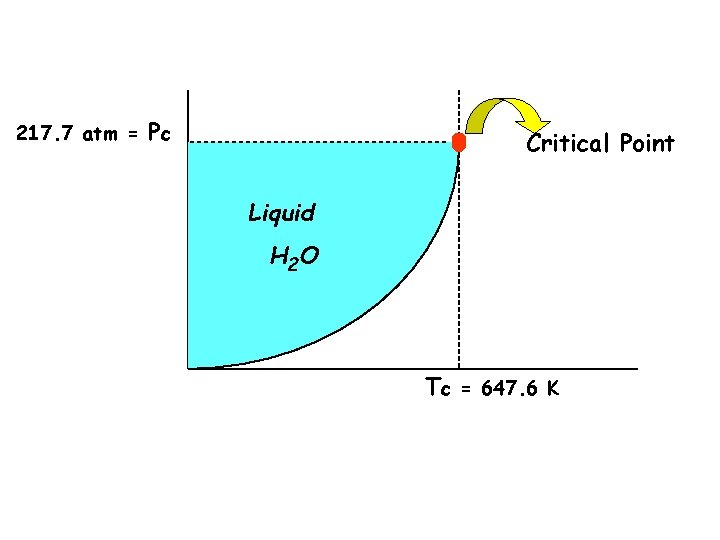
u Critical Temperature & Pressure • critical temp. – highest temperature at which a substance can exist as a liquid • critical pressure – pressure required to cause liquefaction at the critical temperature • a gas cannot be liquefied above the critical temperature • critical point – corresponds to Tc and Pc – the point above which a supercritical gas exists – the substance cannot be liquefied by increasing the pressure

217. 7 atm = Pc Critical Point Liquid Pressure Vapor H 2 O Temperature Tc = 647. 6 K

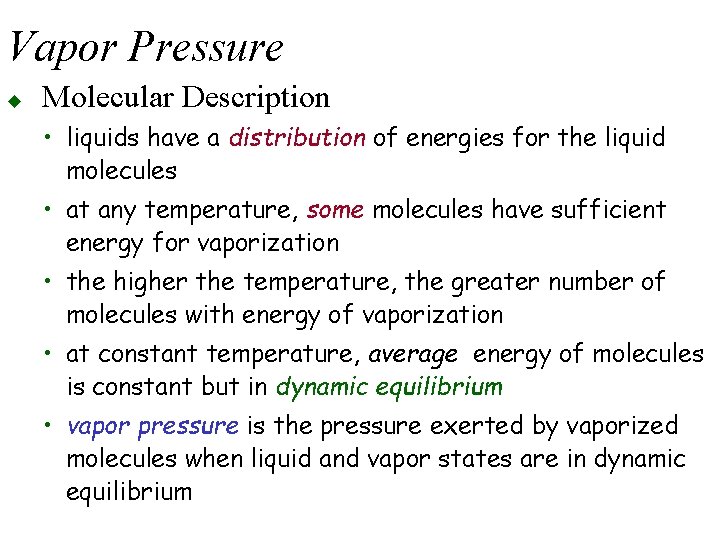
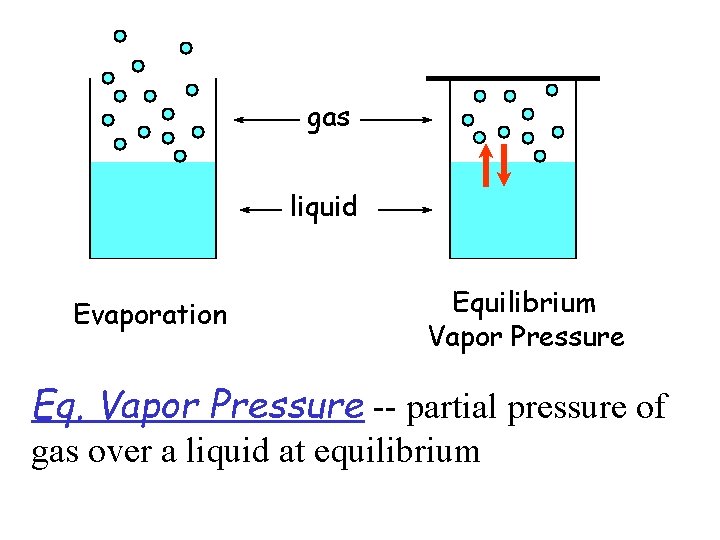
Vapor Pressure u Molecular Description • liquids have a distribution of energies for the liquid molecules • at any temperature, some molecules have sufficient energy for vaporization • the higher the temperature, the greater number of molecules with energy of vaporization • at constant temperature, average energy of molecules is constant but in dynamic equilibrium • vapor pressure is the pressure exerted by vaporized molecules when liquid and vapor states are in dynamic equilibrium

gas liquid Evaporation Equilibrium Vapor Pressure Eq. Vapor Pressure -- partial pressure of gas over a liquid at equilibrium

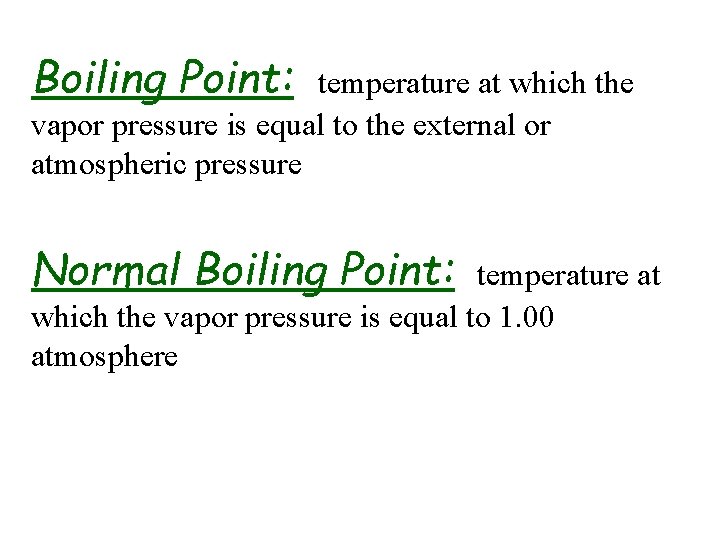
u Volatility, Vapor Pressure & Temp. • substances with high vapor pressure are volatile • in a open container, dynamic equilibrium cannot be established -- complete evaporation • higher the temperature, greater vapor pressure, greater volatility u Vapor Pressure & Boiling Point • boiling point -- temperature at which the vapor pressure = atmospheric pressure – boiling point increases with increasing external pressure, vice versa • normal boiling point -- temp. at which the vapor pressure = 1 atm

Boiling Point: temperature at which the vapor pressure is equal to the external or atmospheric pressure Normal Boiling Point: temperature at which the vapor pressure is equal to 1. 00 atmosphere

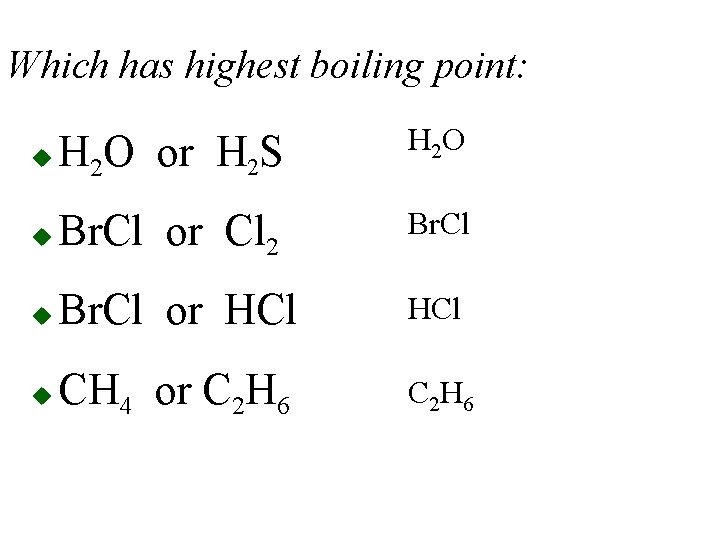
Which has highest boiling point: u H 2 O or H 2 S H 2 O u Br. Cl or Cl 2 Br. Cl u Br. Cl or HCl u CH 4 or C 2 H 6

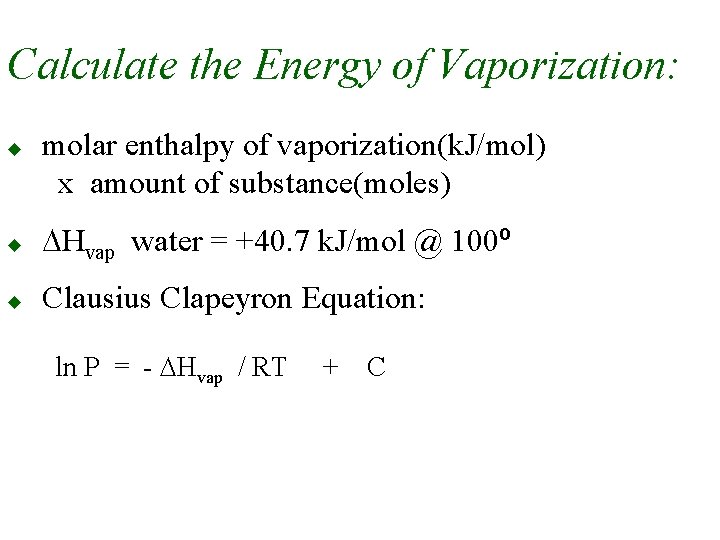
Calculate the Energy of Vaporization: u molar enthalpy of vaporization(k. J/mol) x amount of substance(moles) u DHvap water = +40. 7 k. J/mol @ 100º u Clausius Clapeyron Equation: ln P = - DHvap / RT + C

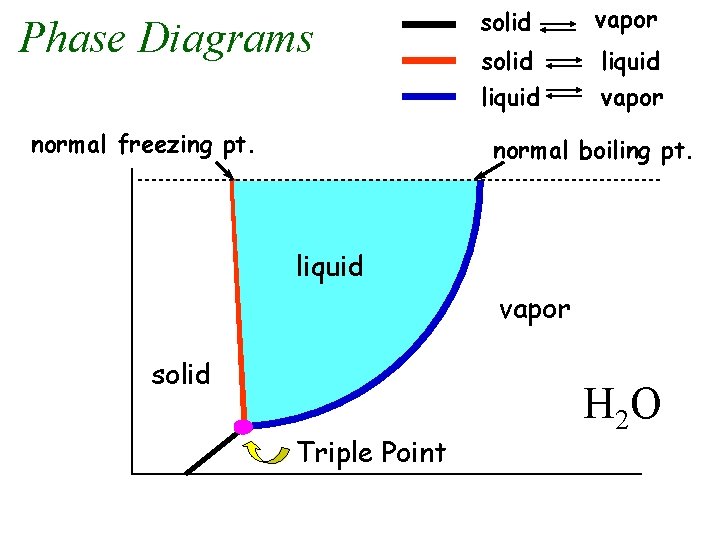
Phase Diagrams normal freezing pt. solid vapor solid liquid vapor normal boiling pt. liquid vapor Pressure solid Triple Point Temperature H 2 O

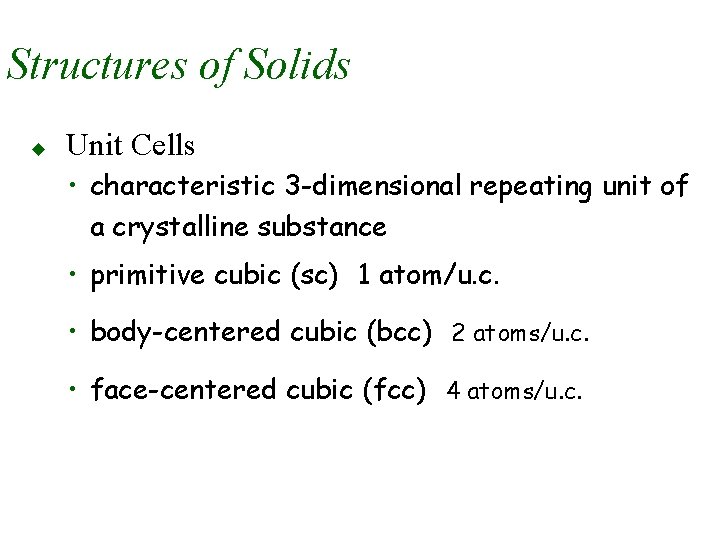
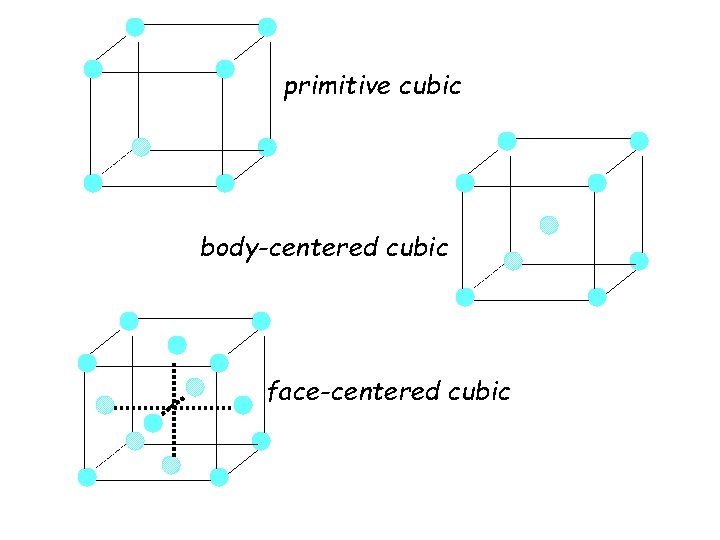
Structures of Solids u Unit Cells • characteristic 3 -dimensional repeating unit of a crystalline substance • primitive cubic (sc) 1 atom/u. c. • body-centered cubic (bcc) 2 atoms/u. c. • face-centered cubic (fcc) 4 atoms/u. c.

primitive cubic body-centered cubic face-centered cubic

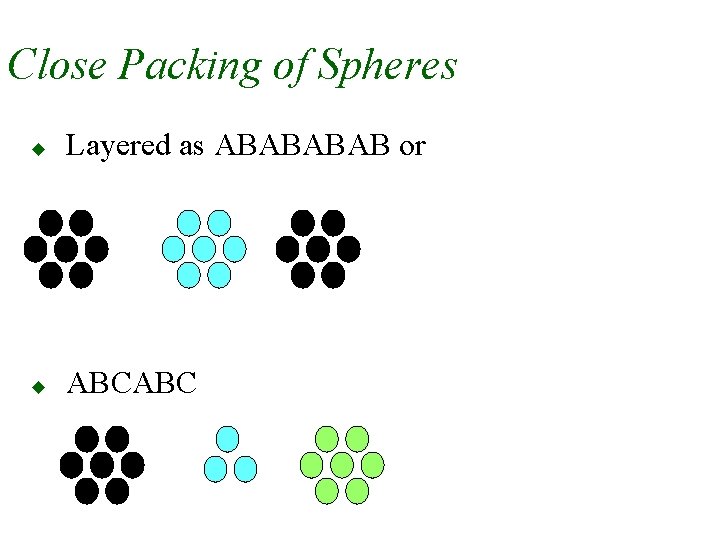
Close Packing of Spheres u Layered as ABAB or u ABCABC

Physical Properties of Solids: solid melting -- endothermic freezing -- exothermic liquid sublimation -- endothermic deposition -- exothermic gas

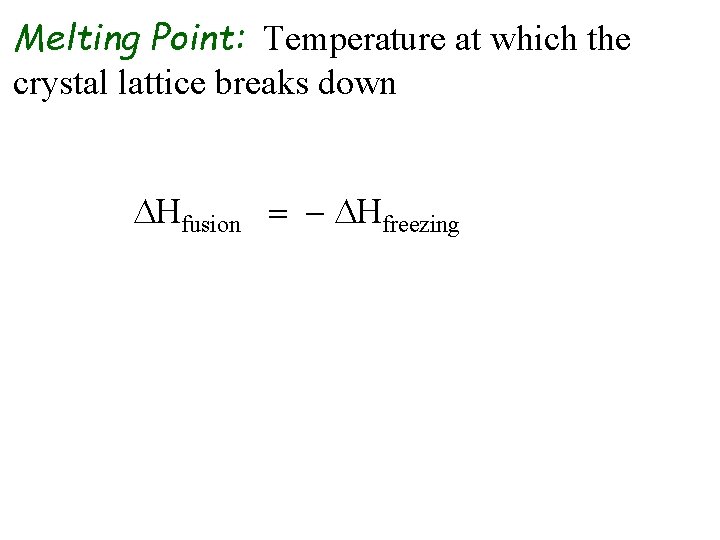
Melting Point: Temperature at which the crystal lattice breaks down DHfusion = - DHfreezing melting freezing

Which one of each pair will have the higher melting point? u Na. F or Na. I Na. F u HCl or HI HCl u O 2 or Br 2 u Na. Cl or u H 2 S or Br 2 Br. Cl H 2 O Na. Cl H 2 O

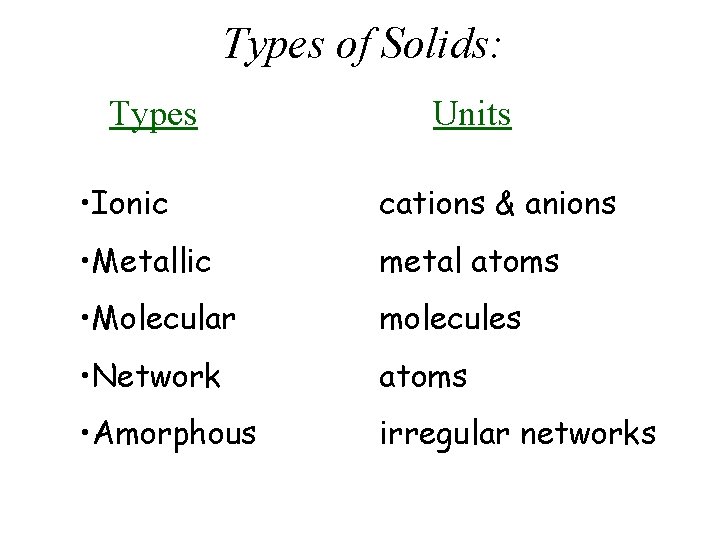
Types of Solids: Types Units • Ionic cations & anions • Metallic metal atoms • Molecular molecules • Network atoms • Amorphous irregular networks

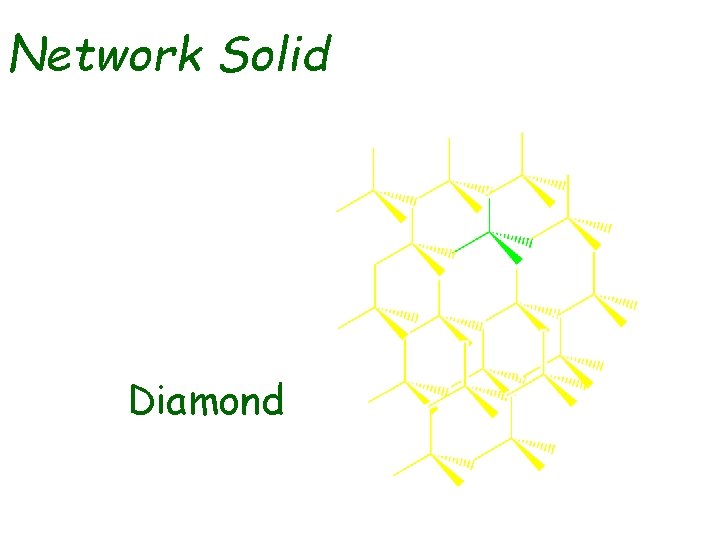
Network Solid Diamond

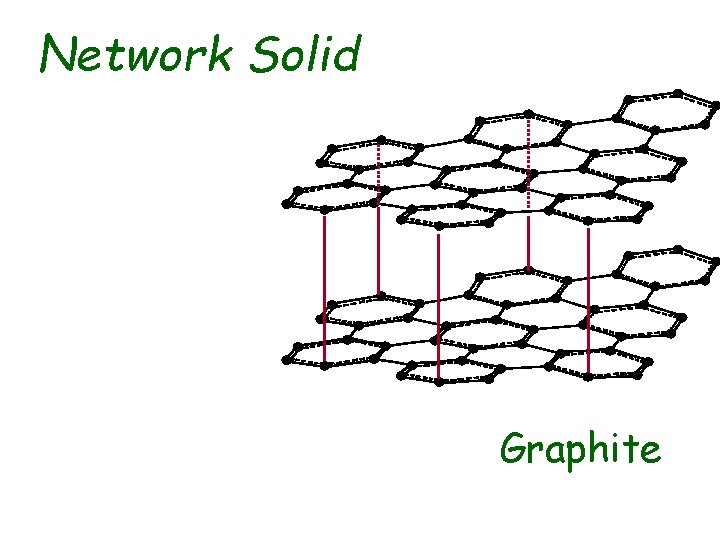
Network Solid Graphite

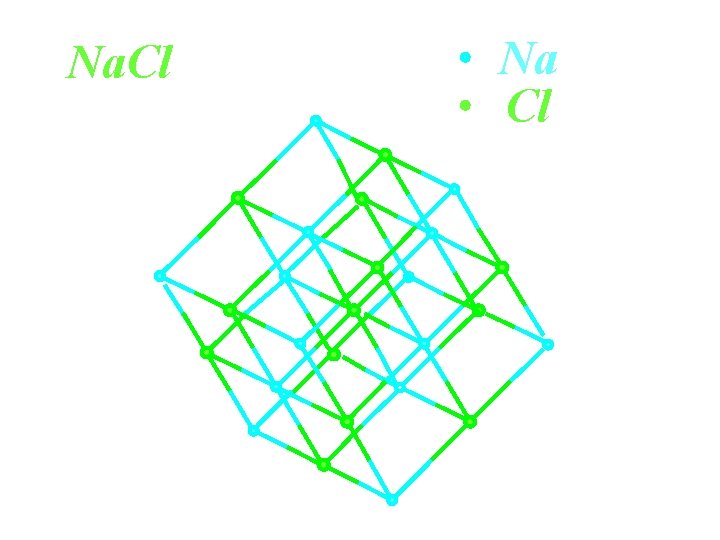
Na. Cl Na Cl