Chapter 11 Intermolecular Forces Liquids and Solids Jennie
















- Slides: 67

Chapter 11: Intermolecular Forces, Liquids, and Solids Jennie L. Borders

Section 11. 1 – A Molecular Comparison of Gases, Liquids, and Solids • Intramolecular forces within a molecule influence molecular shape, bond energies, and chemical behavior. • The physical properties of molecular liquids and solids are due largely to intermolecular forces, the forces that exist between molecules.

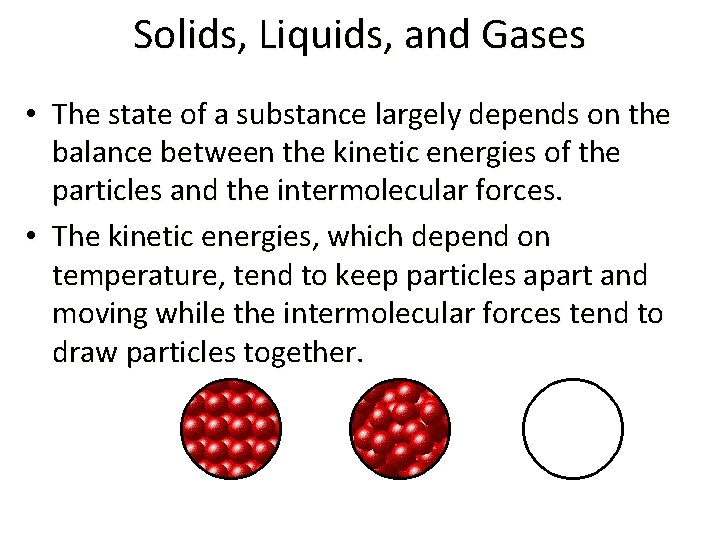
Solids, Liquids, and Gases • The state of a substance largely depends on the balance between the kinetic energies of the particles and the intermolecular forces. • The kinetic energies, which depend on temperature, tend to keep particles apart and moving while the intermolecular forces tend to draw particles together.

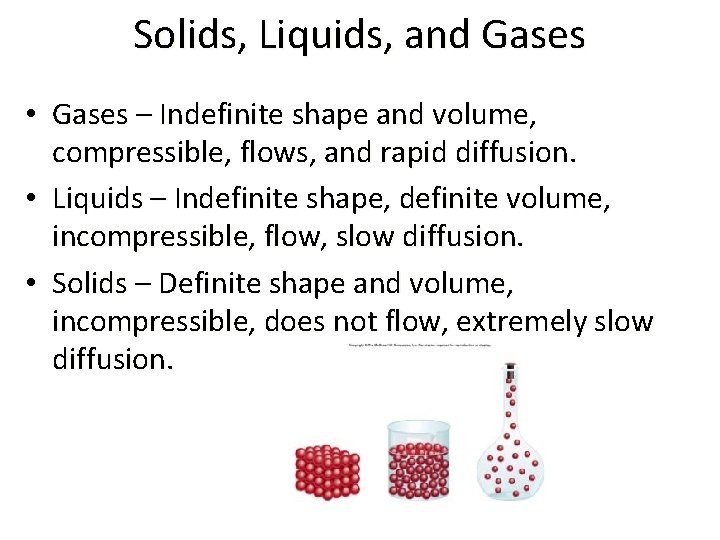
Solids, Liquids, and Gases • Gases – Indefinite shape and volume, compressible, flows, and rapid diffusion. • Liquids – Indefinite shape, definite volume, incompressible, flow, slow diffusion. • Solids – Definite shape and volume, incompressible, does not flow, extremely slow diffusion.

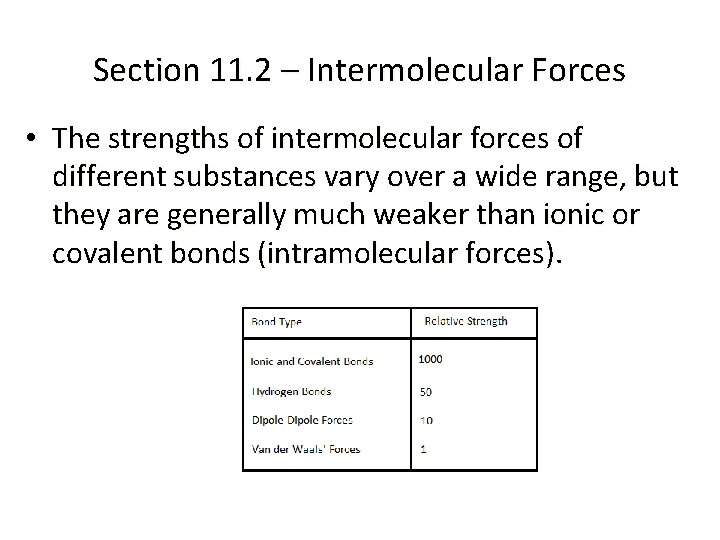
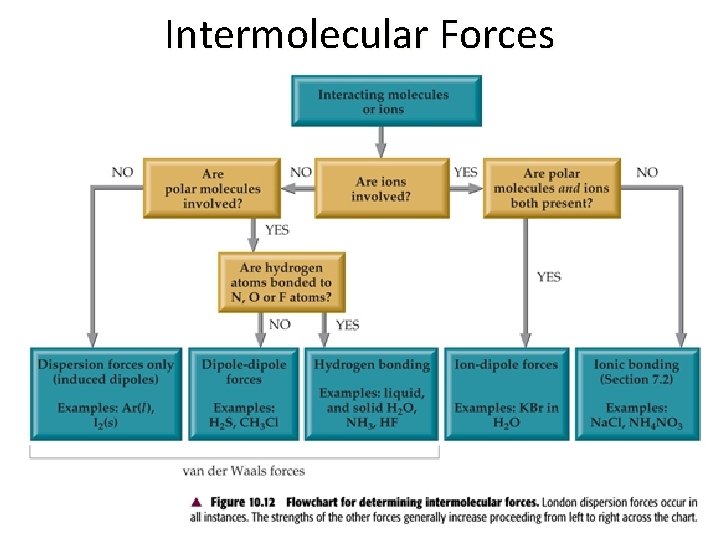
Section 11. 2 – Intermolecular Forces • The strengths of intermolecular forces of different substances vary over a wide range, but they are generally much weaker than ionic or covalent bonds (intramolecular forces).

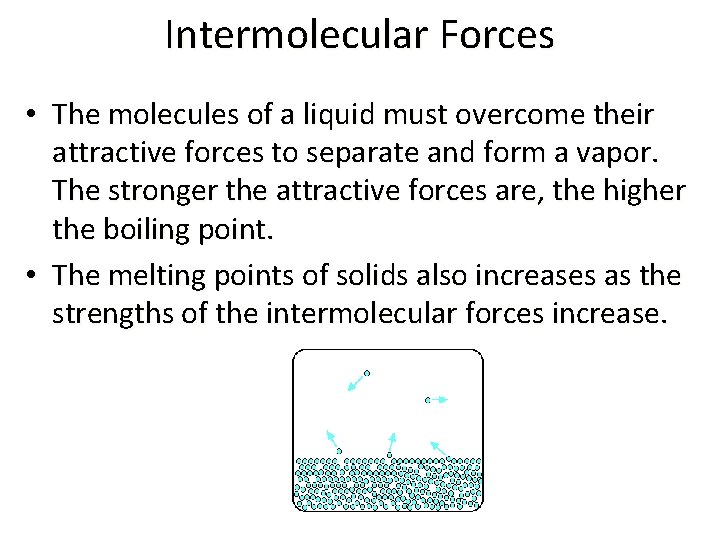
Intermolecular Forces • The molecules of a liquid must overcome their attractive forces to separate and form a vapor. The stronger the attractive forces are, the higher the boiling point. • The melting points of solids also increases as the strengths of the intermolecular forces increase.

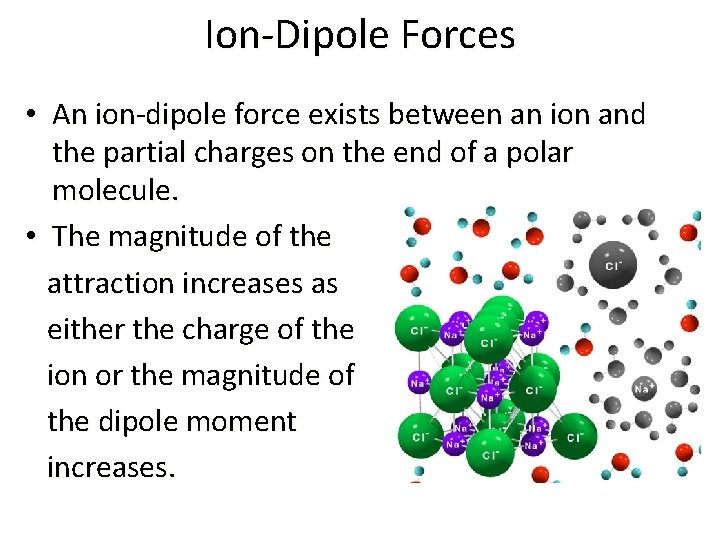
Ion-Dipole Forces • An ion-dipole force exists between an ion and the partial charges on the end of a polar molecule. • The magnitude of the attraction increases as either the charge of the ion or the magnitude of the dipole moment increases.

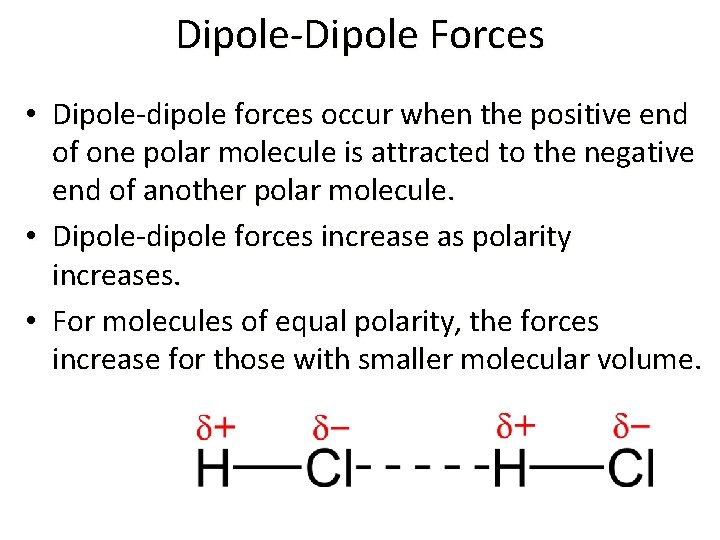
Dipole-Dipole Forces • Dipole-dipole forces occur when the positive end of one polar molecule is attracted to the negative end of another polar molecule. • Dipole-dipole forces increase as polarity increases. • For molecules of equal polarity, the forces increase for those with smaller molecular volume.

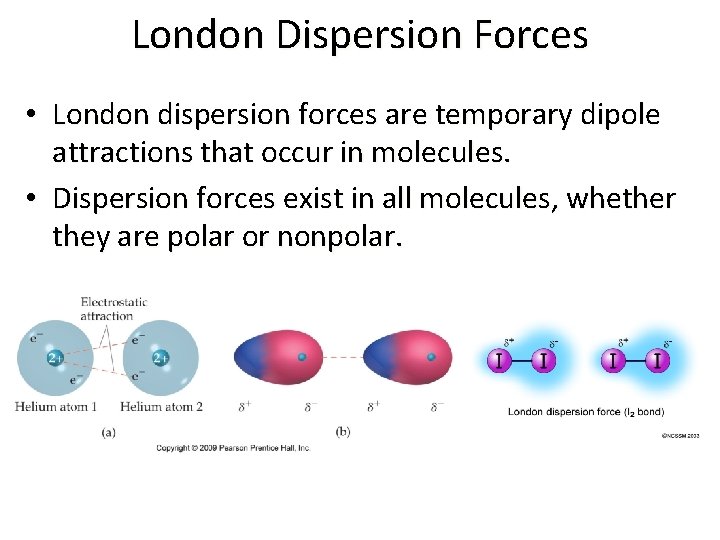
London Dispersion Forces • London dispersion forces are temporary dipole attractions that occur in molecules. • Dispersion forces exist in all molecules, whether they are polar or nonpolar.

Polarizability • Polarizability refers to the ease with which the electron distribution in a molecule is distorted. • Molecules with higher polarizability have larger dispersion forces. • In general, larger molecules tend to have greater polarizabilities because they have a greater number of electrons, and their electrons are farther from the nuclei. • Dispersion forces tend to increase in strength with increasing molecular weight.

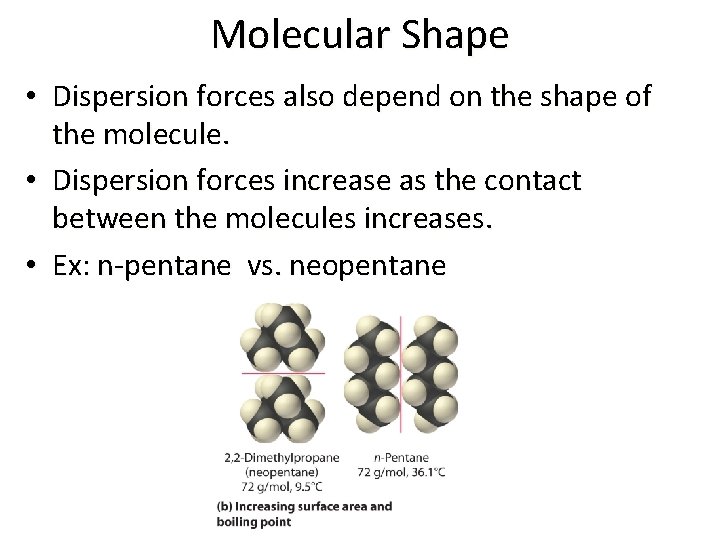
Molecular Shape • Dispersion forces also depend on the shape of the molecule. • Dispersion forces increase as the contact between the molecules increases. • Ex: n-pentane vs. neopentane

Intermolecular Forces • When comparing the strengths of intermolecular attractions in two substances, consider the following generalizations: 1. For molecules of similar molecular weights and shapes, dispersion forces are equal unless there is a difference in the polarity. 2. For molecules of different molecular weights, the substance with more mass has the strongest attractions.

Sample Exercise 11. 1 • The dipole moments of CH 3 CN is 3. 9 D and CH 3 I is 1. 62 D. a. Which of these substances has greater dipole attractions among its molecules? b. Which of these substances has greater London dispersion attractions?

Sample Exercise 11. 1 con’t c. The boiling points of CH 3 CN and CH 3 I are 354. 8 K and 315. 6 K, respectively. Which substance has the greater overall attractive forces?

Practice Exercise • Of Br 2, HCl, HBr, and N 2, which is likely to have a. the largest intermolecular dispersion forces? b. the largest dipole-dipole attractive forces?

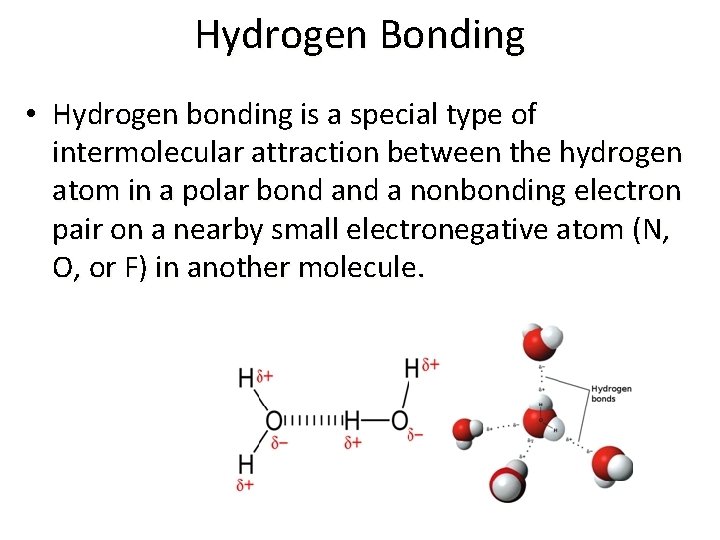
Hydrogen Bonding • Hydrogen bonding is a special type of intermolecular attraction between the hydrogen atom in a polar bond a nonbonding electron pair on a nearby small electronegative atom (N, O, or F) in another molecule.

Sample Exercise 11. 2 • In which of the following substances is hydrogen bonding likely to play an important role in determining physical properties: methane (CH 4), hydrazine (H 2 NNH 2), methyl fluoride (CH 3 F), or hydrogen sulfide (H 2 S)?

Practice Exercise • In which of the following substances is significant hydrogen bonding possible: methylene chloride (CH 2 Cl 2), phosphine (PH 3), hydrogen peroxide (HOOH), or acetone (CH 3 COCH 3)?

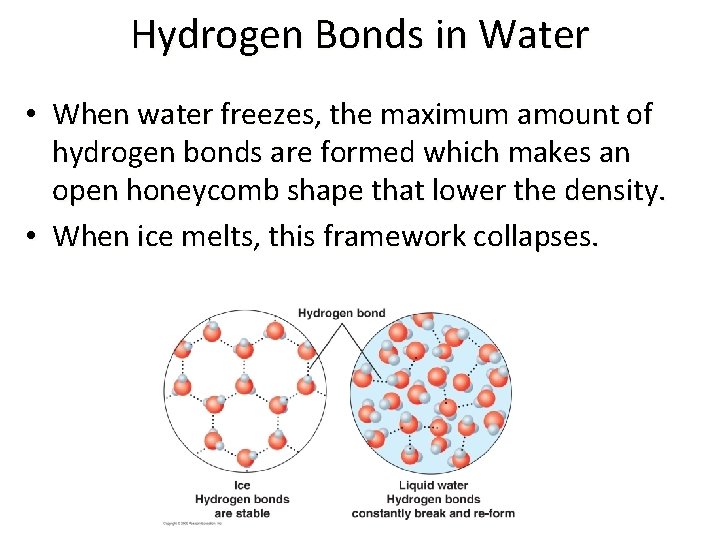
Hydrogen Bonds in Water • In most substances the molecules in the solid are more closely packed than in a liquid. Thus, the solid phase is denser than the liquid phase. • However, ice is less dense than water due to the hydrogen bonds formed in the crystalline structure.

Hydrogen Bonds in Water • When water freezes, the maximum amount of hydrogen bonds are formed which makes an open honeycomb shape that lower the density. • When ice melts, this framework collapses.

Comparing Intermolecular Forces • Dispersion forces are found in all substances, increase with molar mass, and depend on molecular shapes. • Dipole-dipole forces add to the effect of dispersion forces and are found in polar molecules. • Hydrogen bonding adds to the effect of dispersion forces and tends to be the strongest intermolecular attraction.

Strengths of Intermolecular Forces • None of these intermolecular attractions is as strong as ordinary ionic and covalent bonds. • It is important to realize that the effects of all these attractions are additive. ß Bonds Intermolecular Forces

Intermolecular Forces

Sample Exercise 11. 3 • List the substances Ba. Cl 2, H 2, CO, HF, and Ne in order of increasing boiling points.

Practice Exercise a. Identify the intermolecular attractions present in the following substances: CH 3, CH 3 OH, and CH 3 CH 2 OH. b. Select which substance has the highest boiling point.

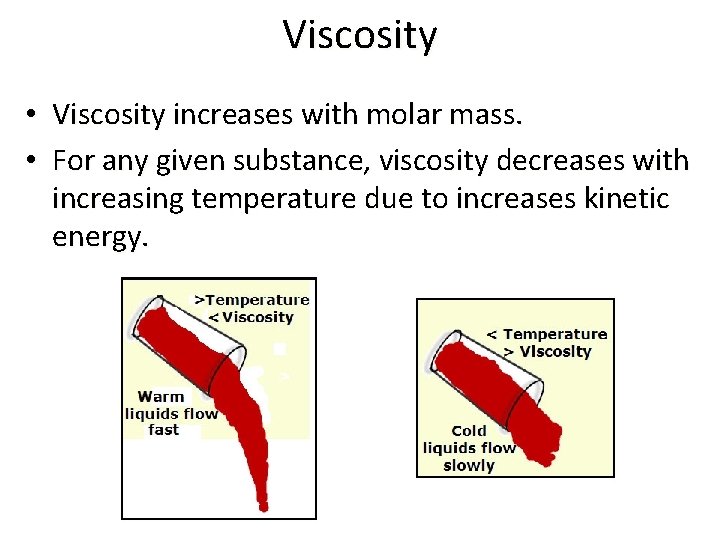
Section 11. 3 – Some Properties of Liquids • The resistance of a liquid to flow is called if viscosity. The greater the liquid’s viscosity, the more slowly it flows. • Viscosity is related to intermolecular forces and the likelihood of molecules to become entangled.

Viscosity • Viscosity increases with molar mass. • For any given substance, viscosity decreases with increasing temperature due to increases kinetic energy.

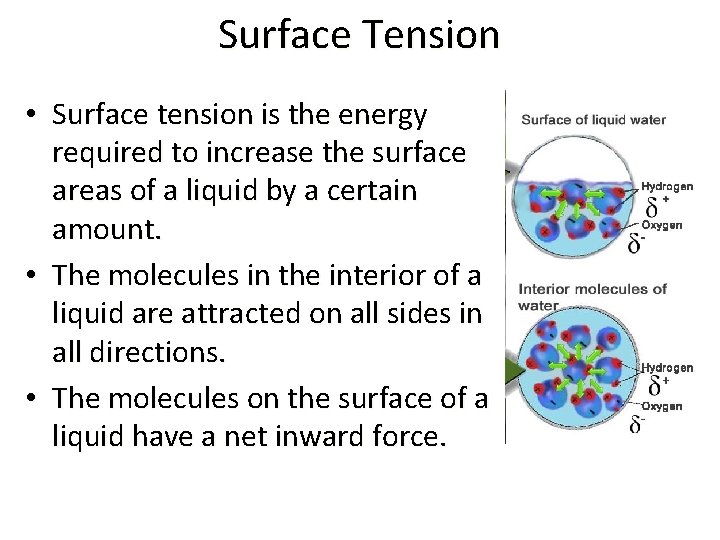
Surface Tension • Surface tension is the energy required to increase the surface areas of a liquid by a certain amount. • The molecules in the interior of a liquid are attracted on all sides in all directions. • The molecules on the surface of a liquid have a net inward force.

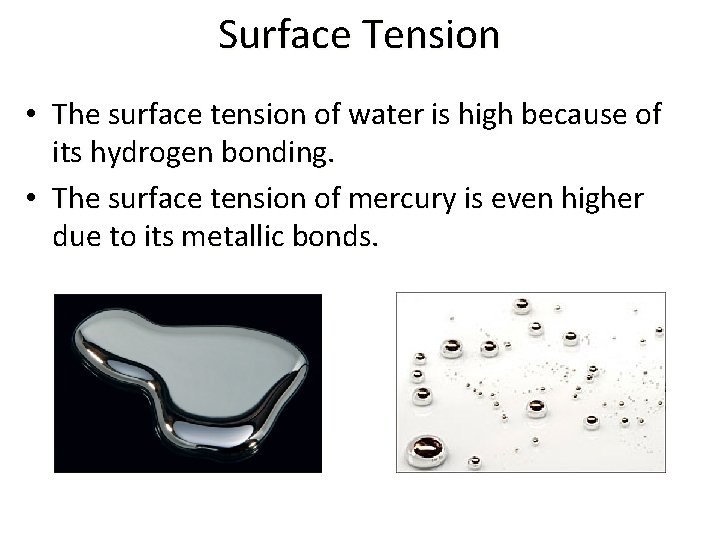
Surface Tension • The surface tension of water is high because of its hydrogen bonding. • The surface tension of mercury is even higher due to its metallic bonds.

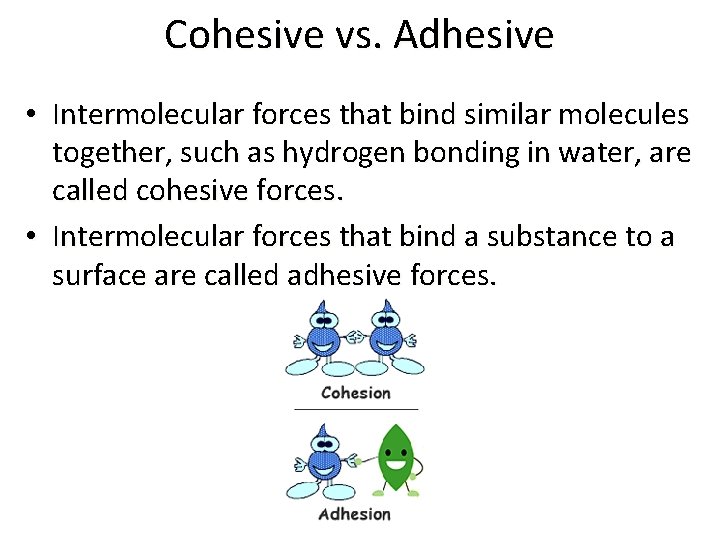
Cohesive vs. Adhesive • Intermolecular forces that bind similar molecules together, such as hydrogen bonding in water, are called cohesive forces. • Intermolecular forces that bind a substance to a surface are called adhesive forces.

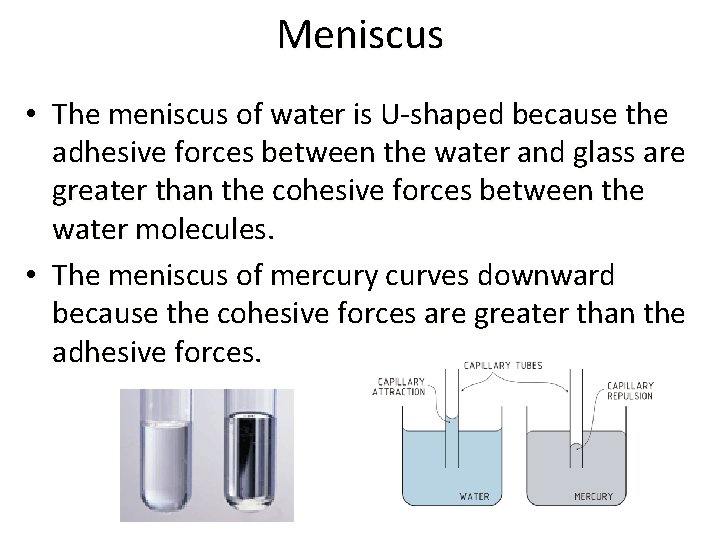
Meniscus • The meniscus of water is U-shaped because the adhesive forces between the water and glass are greater than the cohesive forces between the water molecules. • The meniscus of mercury curves downward because the cohesive forces are greater than the adhesive forces.

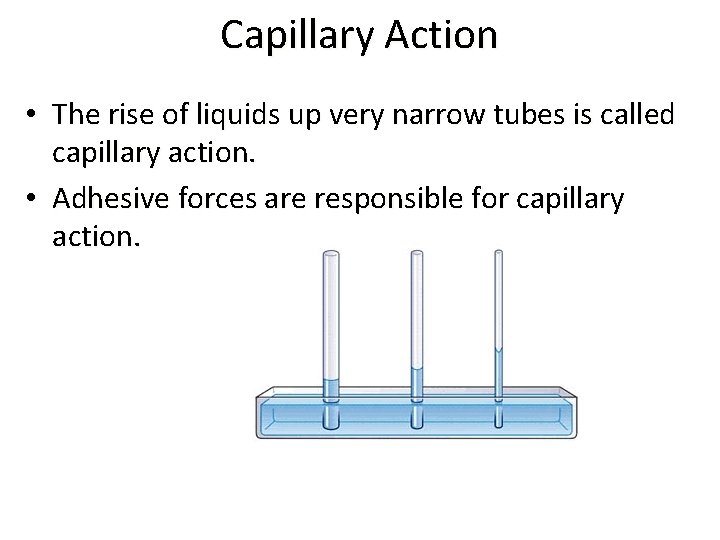
Capillary Action • The rise of liquids up very narrow tubes is called capillary action. • Adhesive forces are responsible for capillary action.

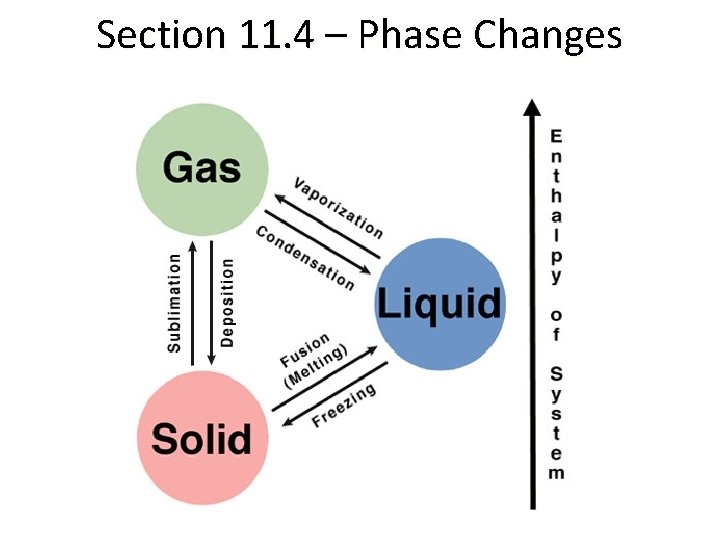
Section 11. 4 – Phase Changes

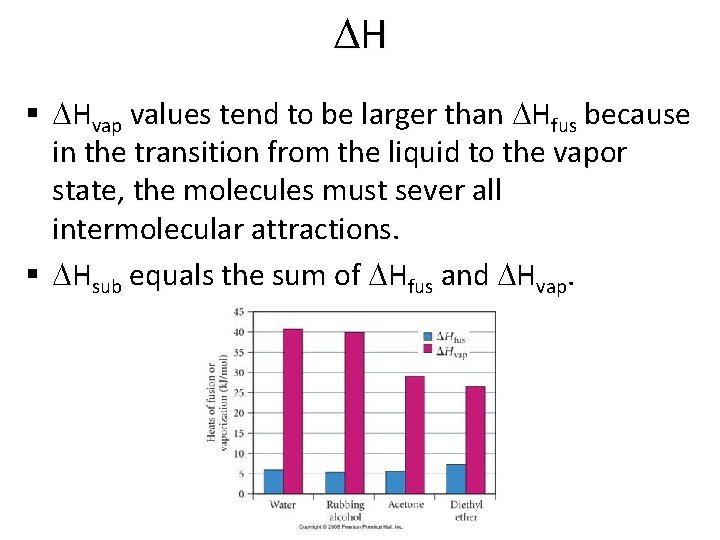
DH § DHvap values tend to be larger than DHfus because in the transition from the liquid to the vapor state, the molecules must sever all intermolecular attractions. § DHsub equals the sum of DHfus and DHvap.

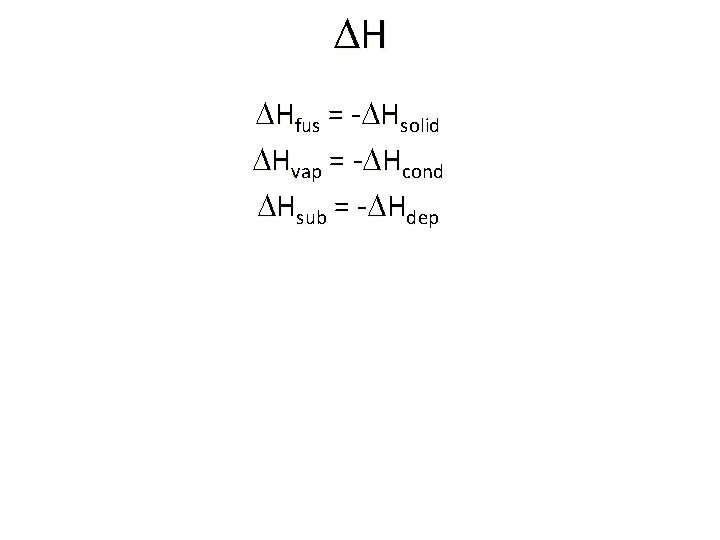
DH DHfus = -DHsolid DHvap = -DHcond DHsub = -DHdep

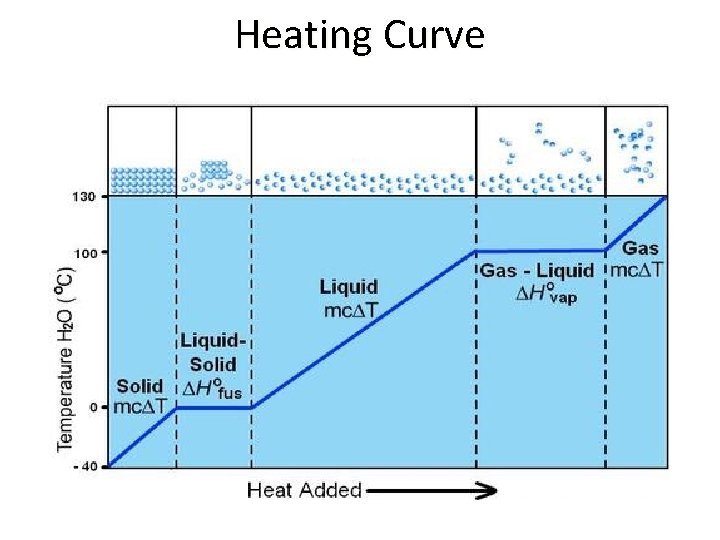
Heating Curve

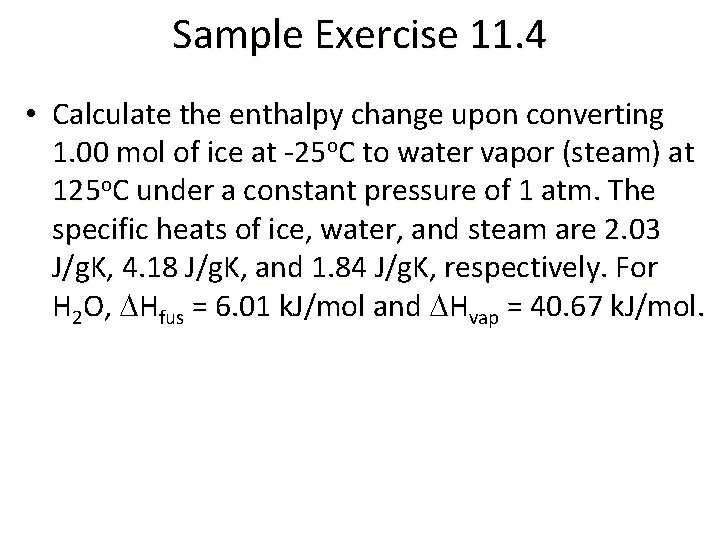
Sample Exercise 11. 4 • Calculate the enthalpy change upon converting 1. 00 mol of ice at -25 o. C to water vapor (steam) at 125 o. C under a constant pressure of 1 atm. The specific heats of ice, water, and steam are 2. 03 J/g. K, 4. 18 J/g. K, and 1. 84 J/g. K, respectively. For H 2 O, DHfus = 6. 01 k. J/mol and DHvap = 40. 67 k. J/mol.

Practice Exercise • What is the enthalpy change during the process in which 100. 0 g of water at 50. 0 o. C is cooled to ice at -30. 0 o. C? (Cooling a substance has the opposite effect of heating it. )

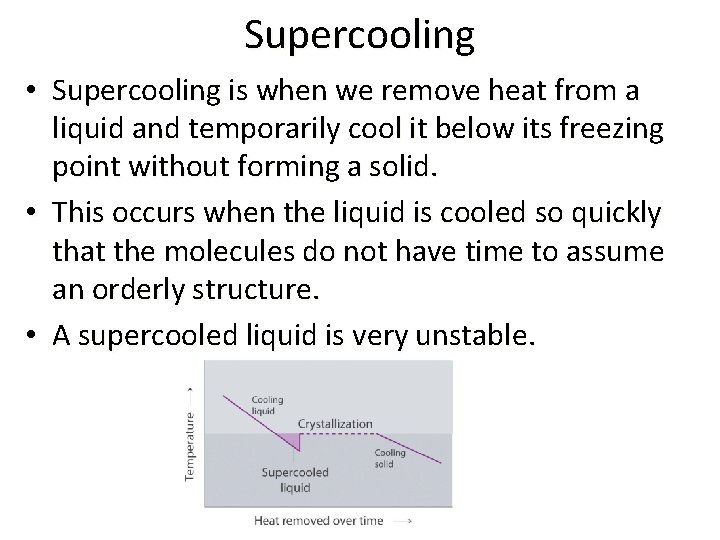
Supercooling • Supercooling is when we remove heat from a liquid and temporarily cool it below its freezing point without forming a solid. • This occurs when the liquid is cooled so quickly that the molecules do not have time to assume an orderly structure. • A supercooled liquid is very unstable.

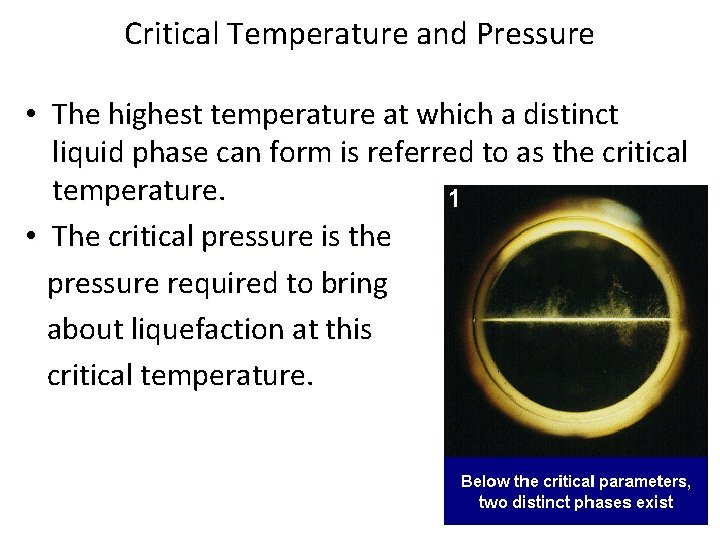
Critical Temperature and Pressure • The highest temperature at which a distinct liquid phase can form is referred to as the critical temperature. • The critical pressure is the pressure required to bring about liquefaction at this critical temperature.

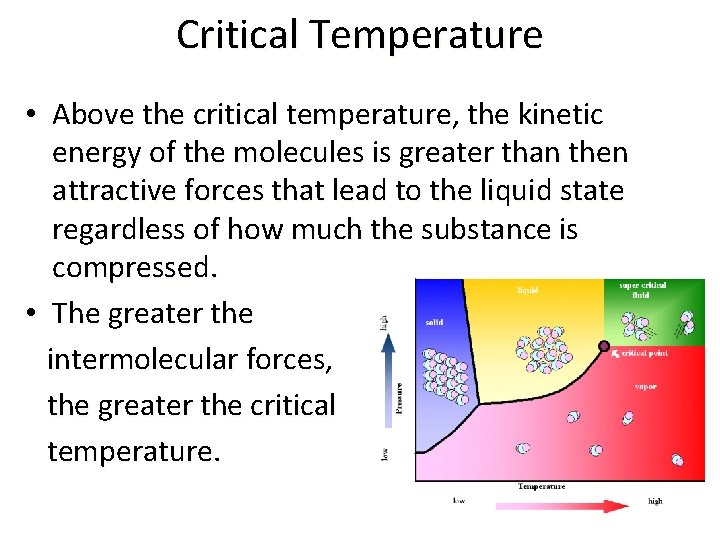
Critical Temperature • Above the critical temperature, the kinetic energy of the molecules is greater than then attractive forces that lead to the liquid state regardless of how much the substance is compressed. • The greater the intermolecular forces, the greater the critical temperature.

Section 11. 5 – Vapor Pressure • Vapor pressure is the pressure exerted by a vapor in equilibrium with its liquid or solid phase. • At any instant some of the molecules on the surface of a liquid possess sufficient kinetic energy to overcome the attractive forces and escape to the gas phase.

Vapor Pressure • The weaker the intermolecular forces, the larger the number of particles that can escape and the higher the vapor pressure. • If the container is closed, the rate of evaporation will eventually equal the rate of condensation and equilibrium will be reached.

Volatility • Substances with high vapor pressure (such as gasoline) evaporate more quickly than substance with low vapor pressure. • Liquids that evaporate quickly are said to be volatile. • Vapor pressure increases with increasing temperature.

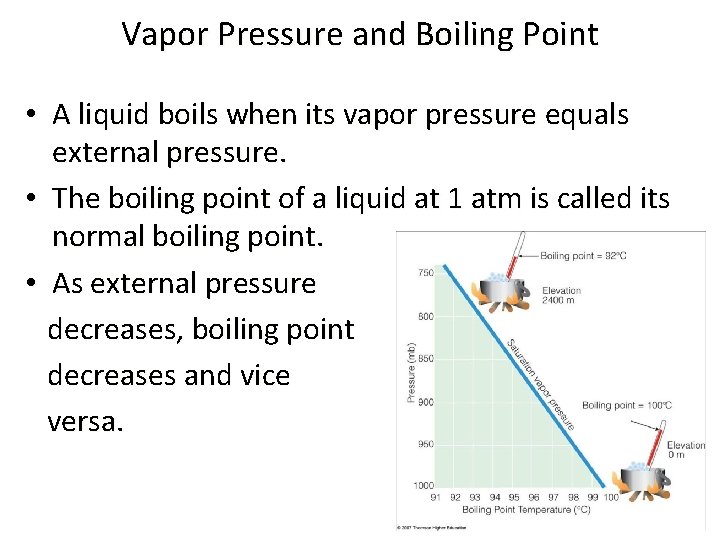
Vapor Pressure and Boiling Point • A liquid boils when its vapor pressure equals external pressure. • The boiling point of a liquid at 1 atm is called its normal boiling point. • As external pressure decreases, boiling point decreases and vice versa.

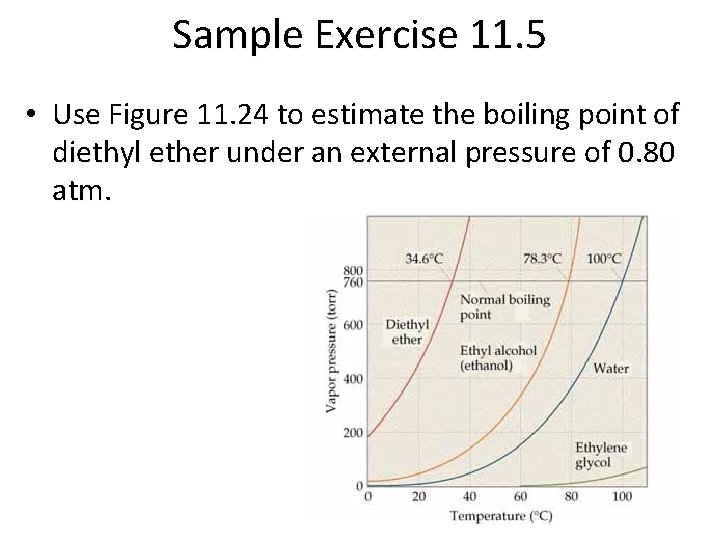
Sample Exercise 11. 5 • Use Figure 11. 24 to estimate the boiling point of diethyl ether under an external pressure of 0. 80 atm.

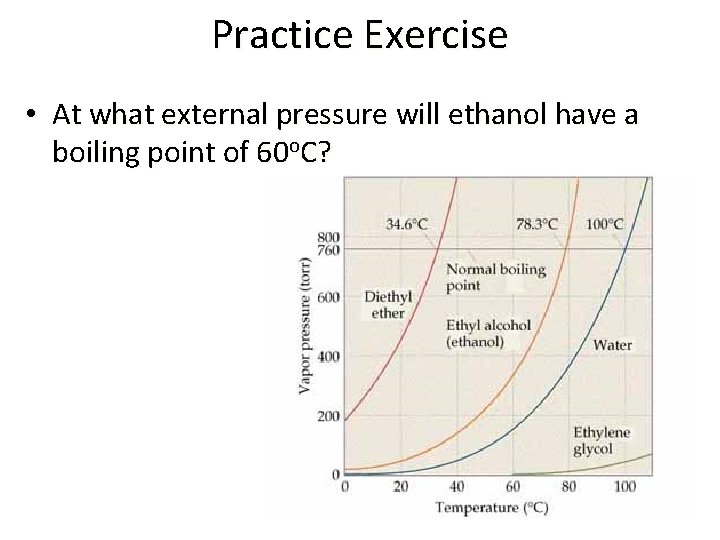
Practice Exercise • At what external pressure will ethanol have a boiling point of 60 o. C?

Section 11. 6 – Phase Diagrams

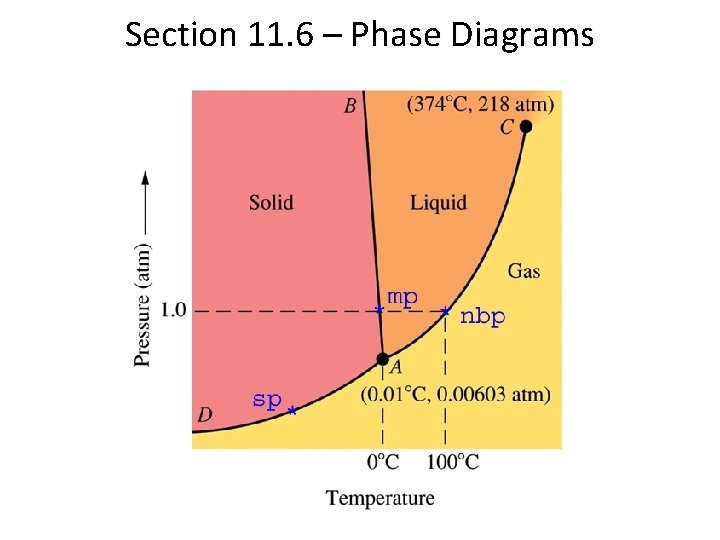
Phase Diagrams • A line on a phase diagram indicates the equilibrium between two phases. • The triple point is where all three phases exist at a certain temperature and pressure. • The critical point is the point at which the liquid and gas phase become indistinguishable. Beyond the critical point, a substance is referred to as a supercritical fluid.

Phase Diagrams

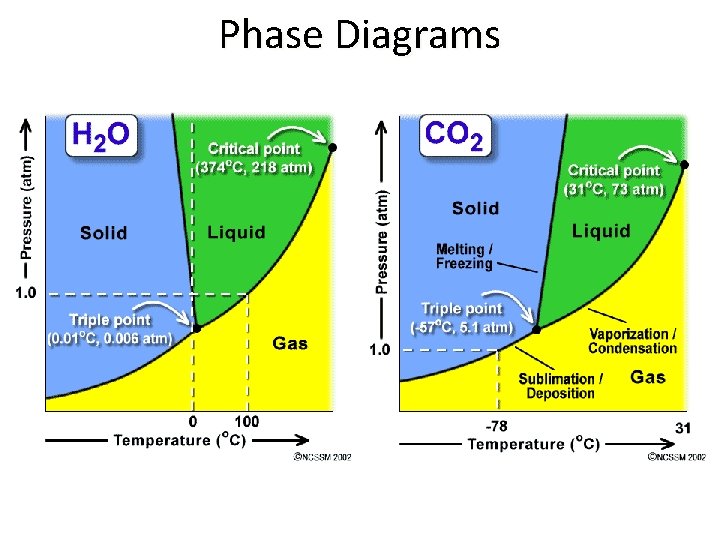
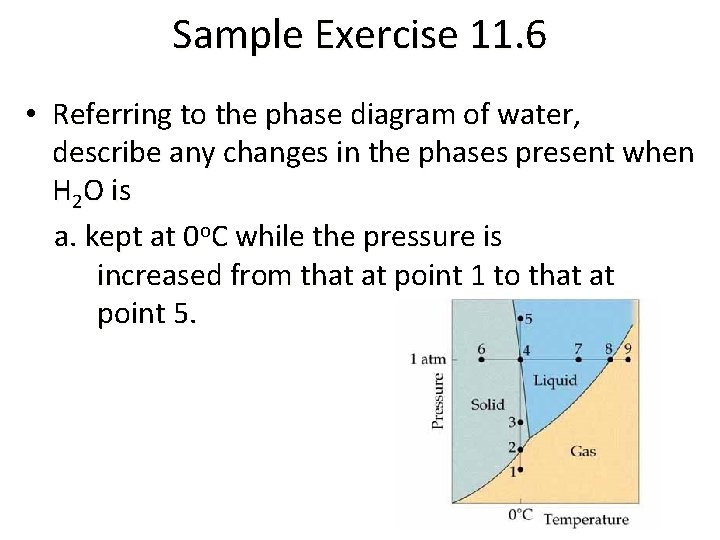
Phase Diagrams • The solid-liquid equilibrium line for CO 2 slants to the right with increasing pressure, telling you that melting point increases with increasing pressure. • The solid-liquid equilibrium line for H 2 O slants to the left with increasing pressure, telling you that melting point decreases with increasing pressure. This atypical behavior is due to the fact that liquid water is less dense than ice.

Sample Exercise 11. 6 • Referring to the phase diagram of water, describe any changes in the phases present when H 2 O is a. kept at 0 o. C while the pressure is increased from that at point 1 to that at point 5.

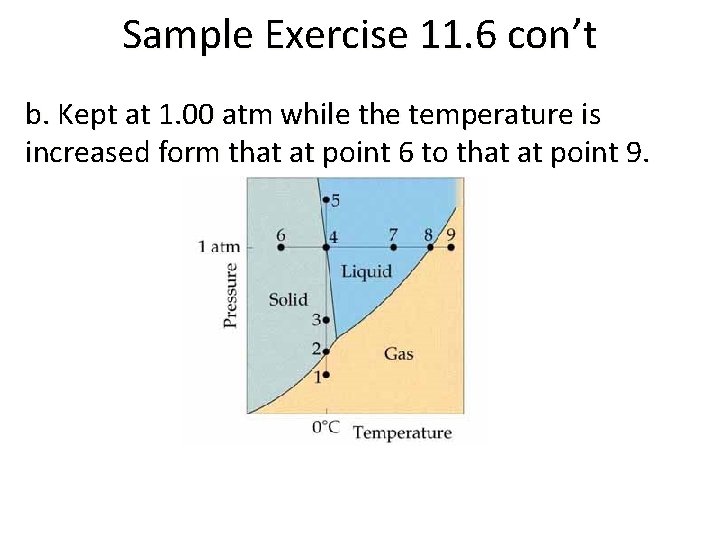
Sample Exercise 11. 6 con’t b. Kept at 1. 00 atm while the temperature is increased form that at point 6 to that at point 9.

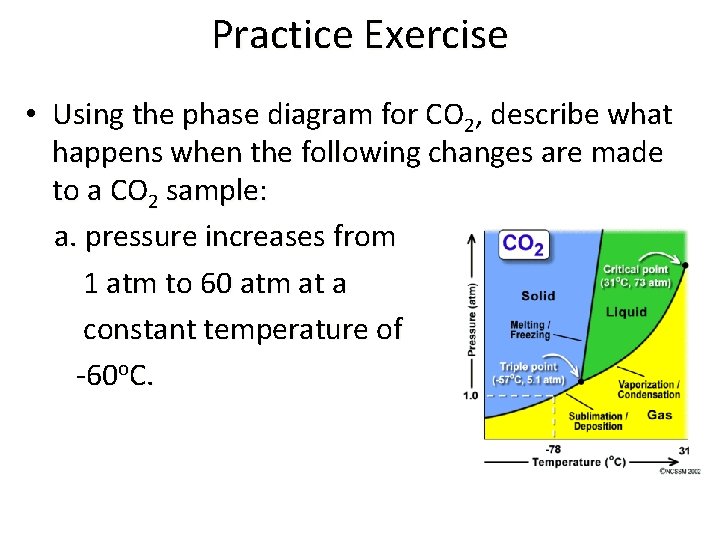
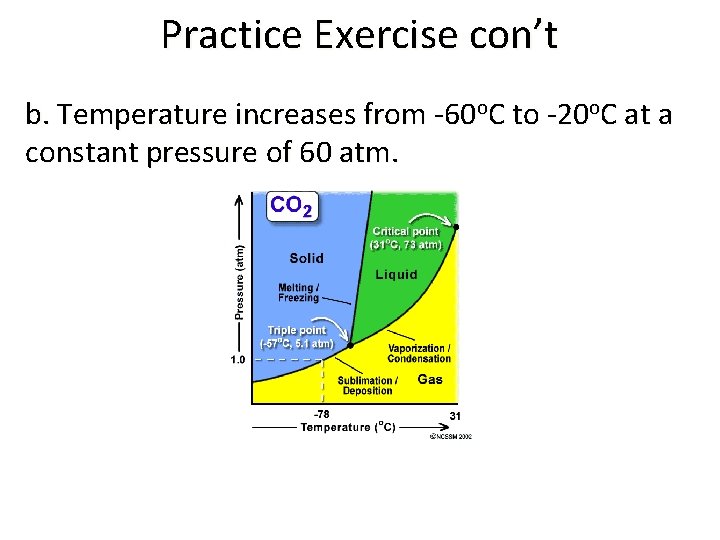
Practice Exercise • Using the phase diagram for CO 2, describe what happens when the following changes are made to a CO 2 sample: a. pressure increases from 1 atm to 60 atm at a constant temperature of -60 o. C.

Practice Exercise con’t b. Temperature increases from -60 o. C to -20 o. C at a constant pressure of 60 atm.

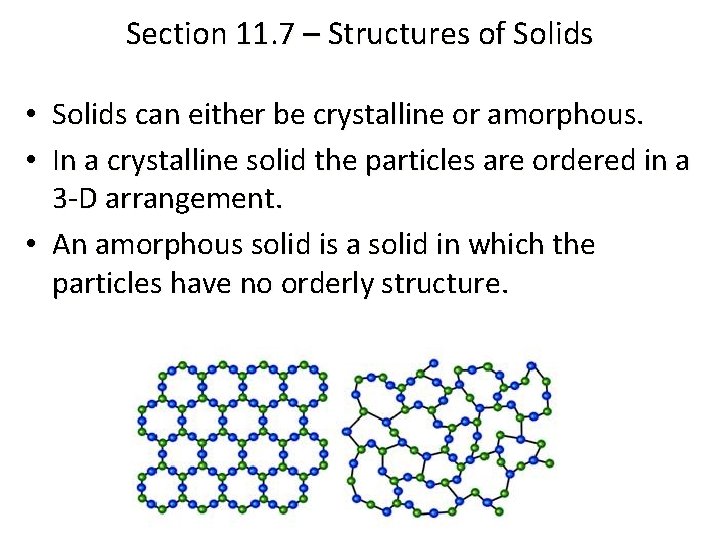
Section 11. 7 – Structures of Solids • Solids can either be crystalline or amorphous. • In a crystalline solid the particles are ordered in a 3 -D arrangement. • An amorphous solid is a solid in which the particles have no orderly structure.

Amorphous Solids • Since the particles of an amorphous solid lack any long-range order intermolecular forces vary throughout the sample. • Thus, amorphous solids do not melt at specific temperatures. Instead, they soften over a temperature range.

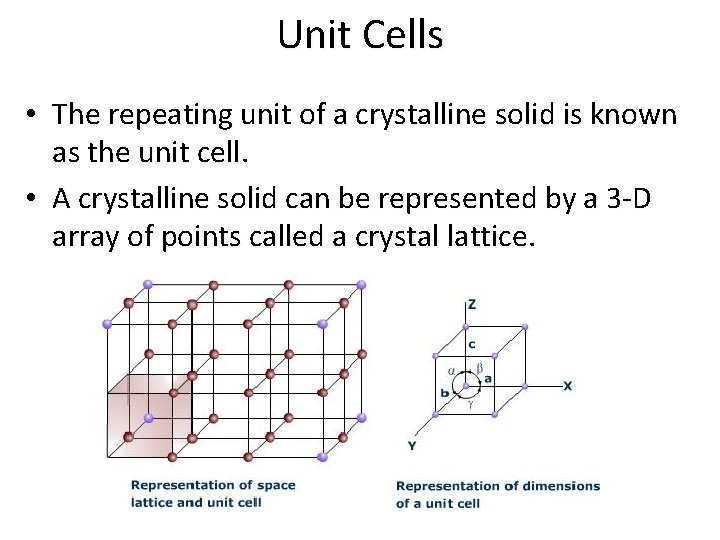
Unit Cells • The repeating unit of a crystalline solid is known as the unit cell. • A crystalline solid can be represented by a 3 -D array of points called a crystal lattice.

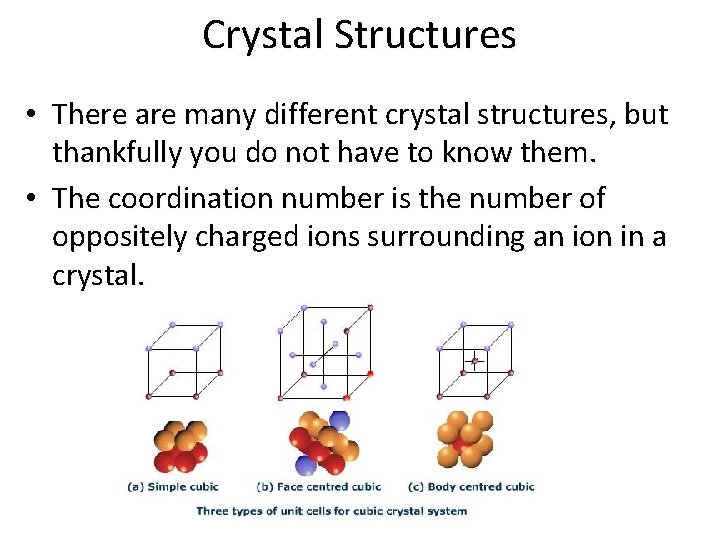
Crystal Structures • There are many different crystal structures, but thankfully you do not have to know them. • The coordination number is the number of oppositely charged ions surrounding an ion in a crystal.

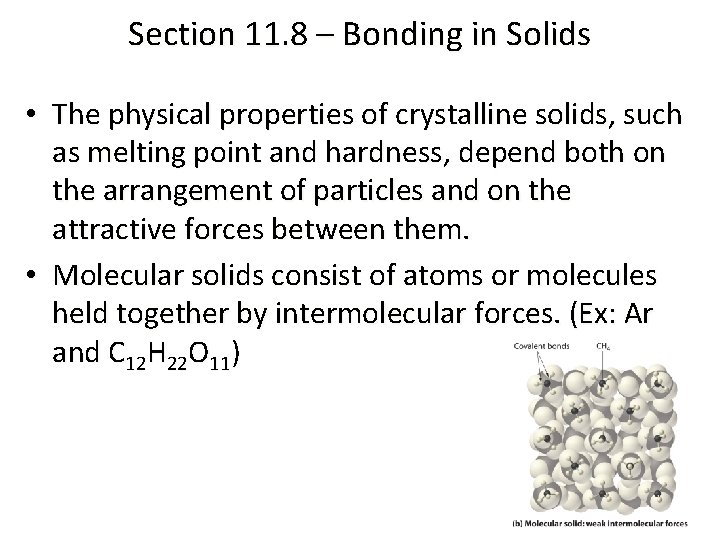
Section 11. 8 – Bonding in Solids • The physical properties of crystalline solids, such as melting point and hardness, depend both on the arrangement of particles and on the attractive forces between them. • Molecular solids consist of atoms or molecules held together by intermolecular forces. (Ex: Ar and C 12 H 22 O 11)

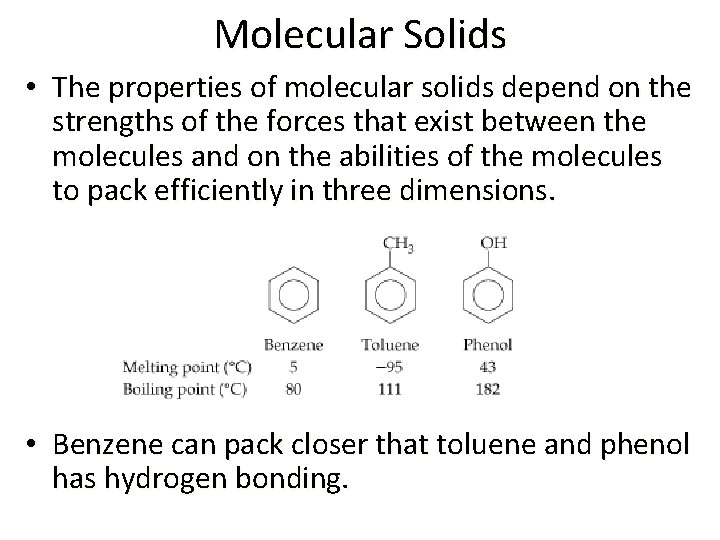
Molecular Solids • The properties of molecular solids depend on the strengths of the forces that exist between the molecules and on the abilities of the molecules to pack efficiently in three dimensions. • Benzene can pack closer that toluene and phenol has hydrogen bonding.

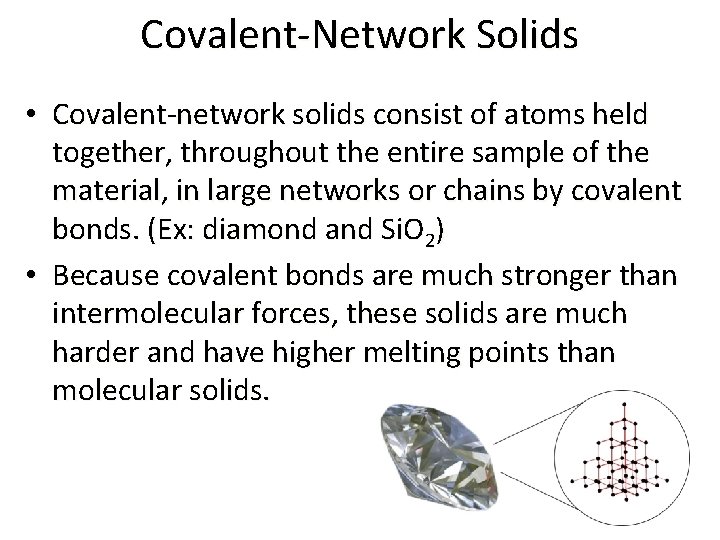
Covalent-Network Solids • Covalent-network solids consist of atoms held together, throughout the entire sample of the material, in large networks or chains by covalent bonds. (Ex: diamond and Si. O 2) • Because covalent bonds are much stronger than intermolecular forces, these solids are much harder and have higher melting points than molecular solids.

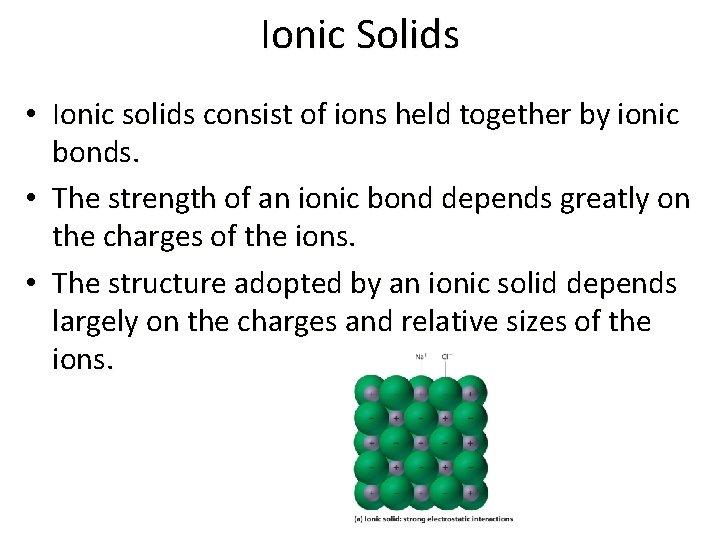
Ionic Solids • Ionic solids consist of ions held together by ionic bonds. • The strength of an ionic bond depends greatly on the charges of the ions. • The structure adopted by an ionic solid depends largely on the charges and relative sizes of the ions.

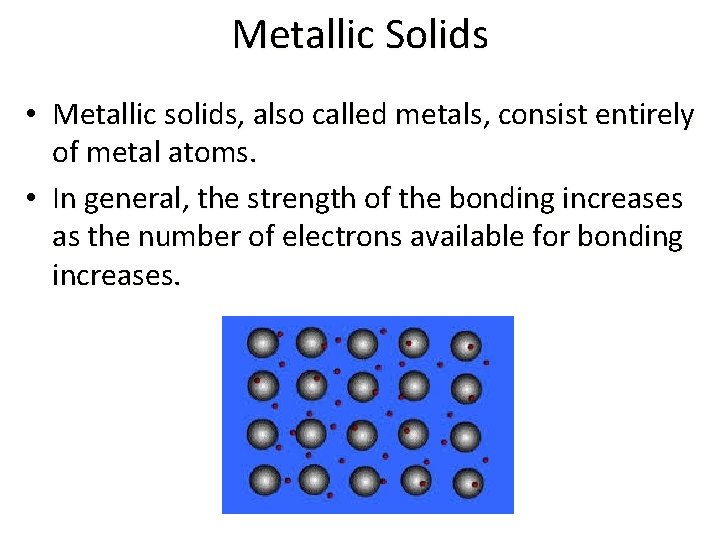
Metallic Solids • Metallic solids, also called metals, consist entirely of metal atoms. • In general, the strength of the bonding increases as the number of electrons available for bonding increases.

Sample Integrative Exercise • The substance CS 2 has a melting point of -110. 8 o. C and a boiling point of 46. 3 o. C. Its density at 20 o. C is 1. 26 g/cm 3. It is highly flammable. a. What is the name of this compound? b. List the intermolecular forces that CS 2 molecules would have with each other.

Sample Integrative Exercise c. Predict what type of crystalline solid CS 2 would form. d. Write the balanced equation for the combustion of this compound in air.

Sample Integrative Exercise e. The critical temperature and pressure for CS 2 are 552 K and 78 atm, respectively. Compare these values with those for CO 2 (Table 11. 5), and discuss the possible origins of the differences. f. Would you expect the density of CS 2 at 40 o. C to be greater or less than at 20 o. C? What accounts for the difference?