Chapter 11 Intermolecular Forces Liquids and Solids 11





















- Slides: 40

Chapter 11 – Intermolecular Forces, Liquids and Solids

11. 3 – Intermolecular Forces***** n Strengths of intermolecular forces of different substances varies n n n Generally weaker than ionic or covalent bonds Less energy needed to vaporize (or evaporate) a liquid or melt a solid than to break the covalent bonds in molecules In other words: Compound stay intact when melting or boiling, just breaking intermolecular forces

n Many properties of liquids reflect strength of the intermolecular forces n Such as boiling point n n Lower intermolecular forces = lower melting and boiling points Example: n Because the forces between HCl molecules are so weak, HCL boils at very low temperature n -85ºC at atmospheric pressure

Boiling/Melting n n A liquid boils when bubbles of its vapor form within the liquid Molecules in the liquid must overcome their attractive forces to do vaporize The stronger the forces, the higher the temperature at which the liquid boils Same general principle applies to melting

Types of Intermolecular Forces n Three types exist between neutral molecules n n In order of decreasing (so strongest to weakest) hydrogen-bonding forces n n dipole-dipole forces London dispersion forces/dispersion forces There is one other force that mostly applies to solutions n n a. k. a. van der Waals forces ion-dipole force All of these forces tend to be less than 15% as strong as covalent or ionic bonds

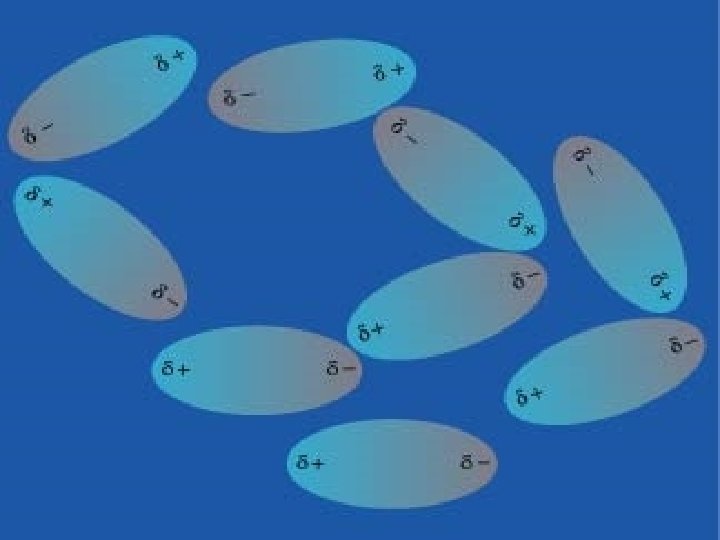
Dipole-Dipole Forces n Neutral POLAR molecules attract each other when the positive end of one molecule is near the negative end of another n These dipole-dipole forces only work when the polar molecules are very close together n Weaker than ion-dipole forces


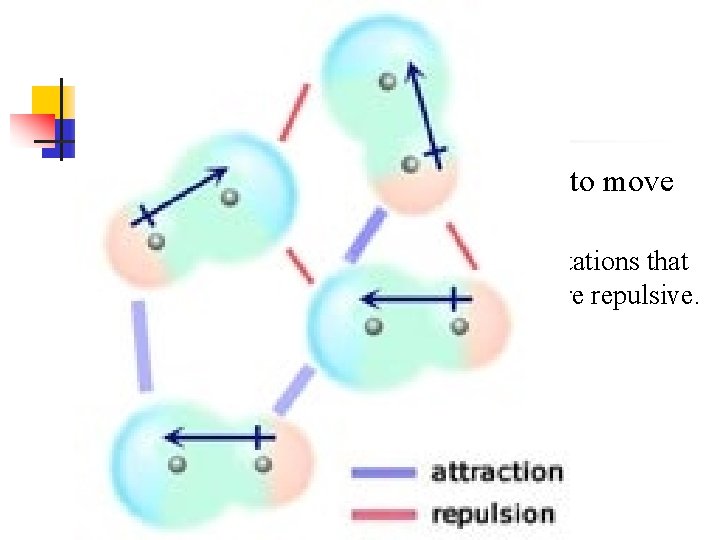
n In liquids polar molecules are free to move with respect to one another n Different configurations create orientations that are attractive, and orientations that are repulsive.

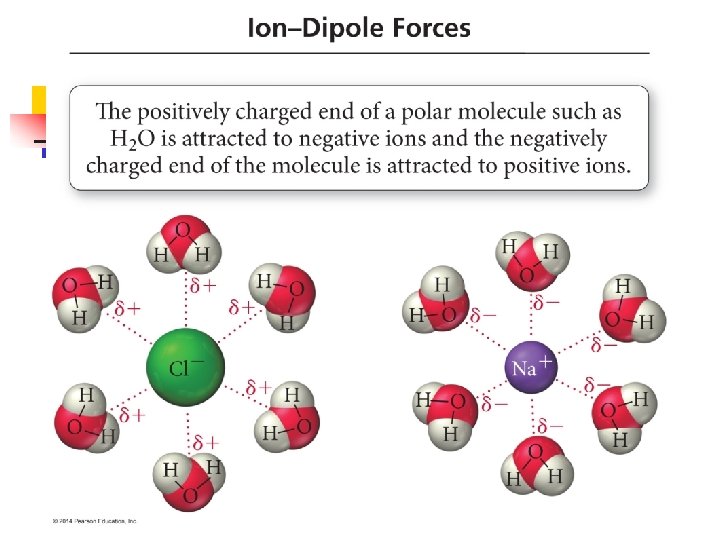
Ion-Dipole Forces n n n Pretty simple Exists between an ion and a partial charge on a polar molecule. Increases as either the charge of the ion increases, or the magnitude of the dipole moment increases


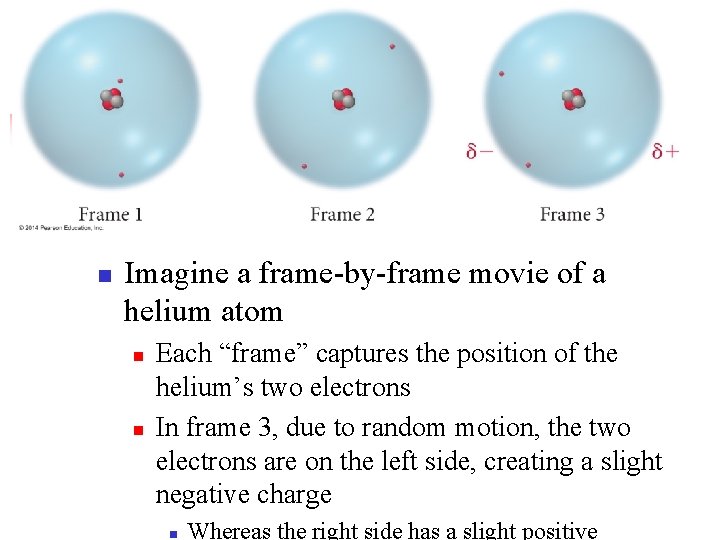
London Dispersion Force (aka Dispersion Force) n n This is caused as the valence electrons move around a molecule, they can randomly create temporary dipoles. These temporary dipoles act similar to a normal dipole, providing a weak intermolecular force between two molecules.

n Imagine a frame-by-frame movie of a helium atom n n Each “frame” captures the position of the helium’s two electrons In frame 3, due to random motion, the two electrons are on the left side, creating a slight negative charge n Whereas the right side has a slight positive

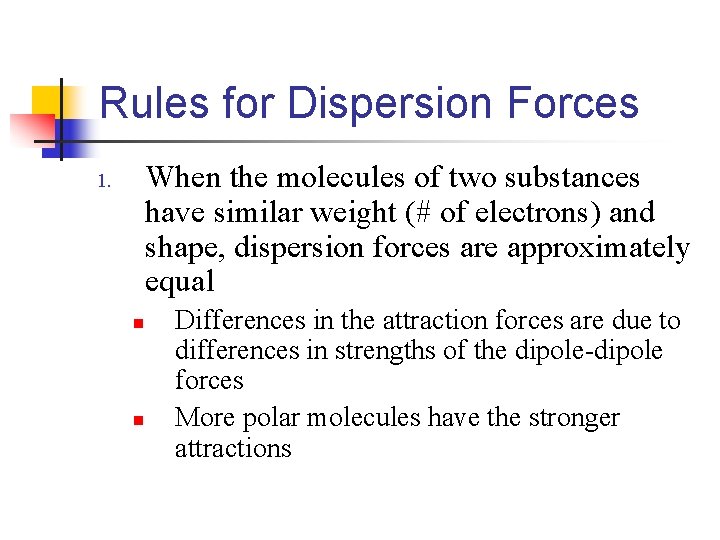
Rules for Dispersion Forces When the molecules of two substances have similar weight (# of electrons) and shape, dispersion forces are approximately equal 1. n n Differences in the attraction forces are due to differences in strengths of the dipole-dipole forces More polar molecules have the stronger attractions

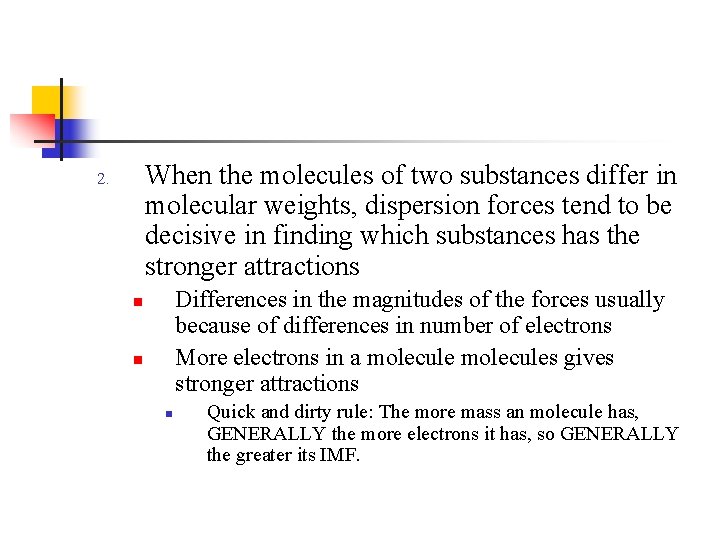
When the molecules of two substances differ in molecular weights, dispersion forces tend to be decisive in finding which substances has the stronger attractions 2. Differences in the magnitudes of the forces usually because of differences in number of electrons More electrons in a molecules gives stronger attractions n n n Quick and dirty rule: The more mass an molecule has, GENERALLY the more electrons it has, so GENERALLY the greater its IMF.

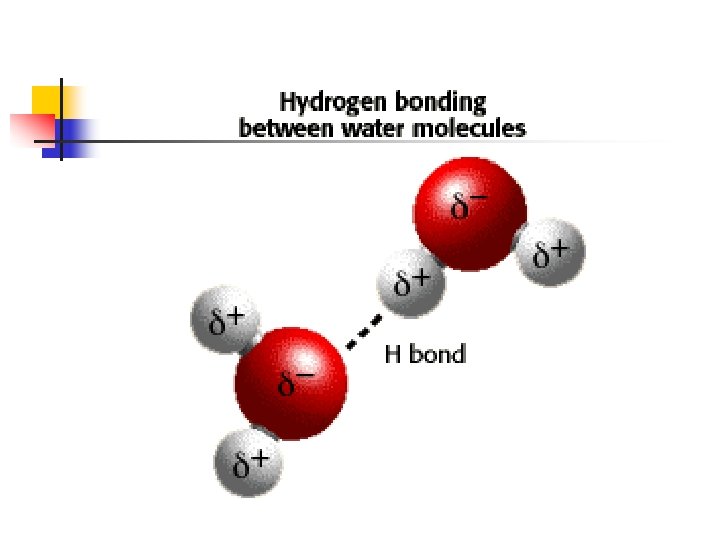
Hydrogen Bonding n Hydrogen bonding is a special type of intermolecular force n Always between a H atom in a polar bond an unshared electron pair on a nearby, small, electronegative ion or atom n n Usually a H-F, H-O or H-N bond Usually a F, O or N atom in another molecule

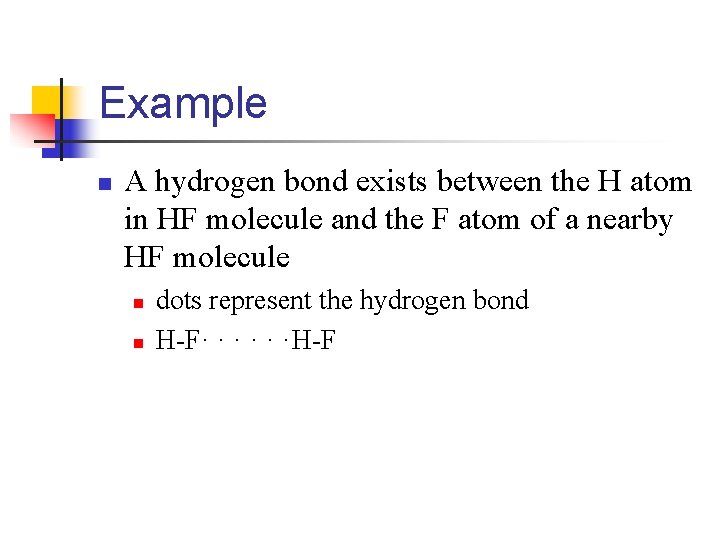
Example n A hydrogen bond exists between the H atom in HF molecule and the F atom of a nearby HF molecule n n dots represent the hydrogen bond H-F· · · ·H-F

n The hydrogen bond is a unique form of a dipole attraction n Because F, N and O are so electronegative, bonds are VERY polar n n H has no inner core of electrons n n n H on positive end Positive side has partially exposed nucleus Gives a large dipole effect (since not just electron density, but actual nuclear charge) Also, since H is so small, it can approach an electronegative atom very closely


n n Hydrogen bonds still weaker than ordinary bonds Stronger than dipole-dipole or dispersion forces

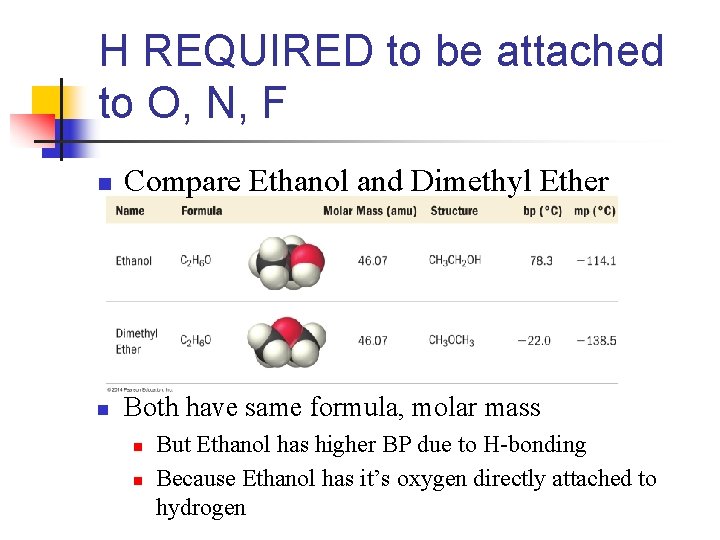
H REQUIRED to be attached to O, N, F n Compare Ethanol and Dimethyl Ether n Both have same formula, molar mass n n But Ethanol has higher BP due to H-bonding Because Ethanol has it’s oxygen directly attached to hydrogen

Comparing Intermolecular Forces n Dispersion forces are found in all substances n n Strengths increase with increasing molecular weight Strength increases with longer molecules

n Dipole-dipole forces adds to dispersion forces n n Found only in polar molecules Hydrogen bonds require H atoms bonded to F, O or N n Also adds to dispersion forces

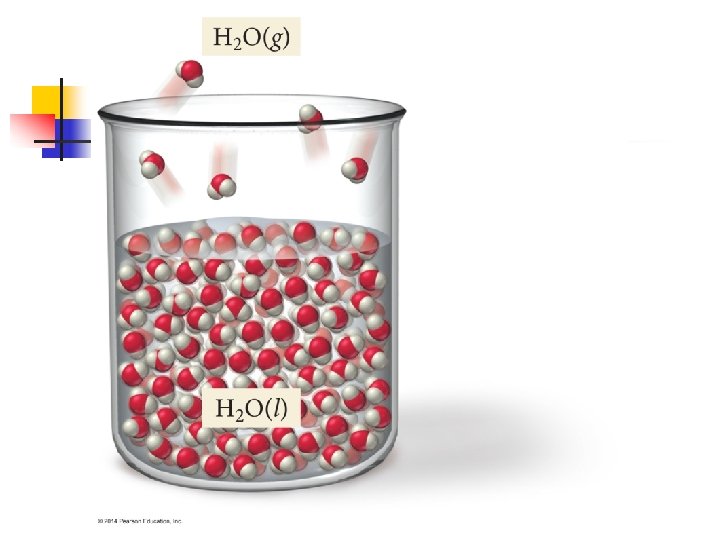
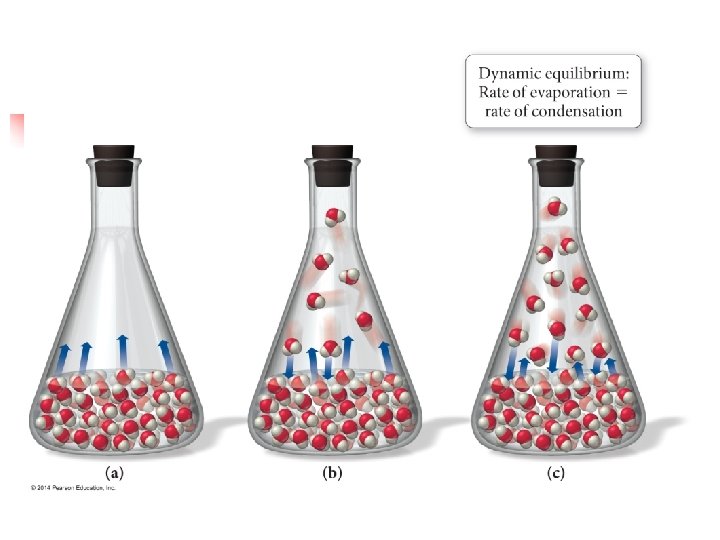
11. 5 – Vapor Pressure* n n Molecules can escape from the surface of a liquid to the gas phase by evaporation. Suppose we place an amount of ethanol (C 2 H 5 OH) in an evacuated, closed container. n n Ethanol will quickly begin to evaporate So pressure exerted by the vapor above the liquid will increase Eventually, the pressure of the vapor will become a constant value This is called the vapor pressure of the substance


Explaining Vapor Pressure n n Molecules of a liquid move at various speeds At any instant, some of the molecules at the surface of the liquid get enough kinetic energy to overcome the attractive forces of their neighbors n n Thus escaping into the gas phase The weaker the attractive forces, the more particles that can escape, and therefore, more vapor pressure

n n At any given temperature the movement of molecules from liquid to gas goes on continuously. As the number of gaseous molecules increase, the probability increases that a molecule in the gas phase will hit the liquid surface and be recaptured by the liquid Eventually, rate at which molecules return to liquid equals the rate at which they escape So we get a steady number of molecules in the gas phase

Dynamic Equilibrium n The condition when two opposing processes are occurring at the same time, and at the same rate, is called dynamic equilibrium n n n A liquid and vapor are in equilibrium when evaporation and condensation occurs at equal rates Often appears like nothing is happening, but no net change n n Often referred to simply as equilibrium But particles are constantly changing from liquid gas and from gas liquid The vapor pressure of a liquid is the pressure exerted by its vapor when vapor and liquid are in equilibrium.


Volatility, Vapor Pressure and Temperature n n n Substances with high vapor pressure evaporate more quickly than substances with low vapor pressure Liquids that evaporate easily are said to be volatile As temperature increases, particles move more, and will evaporate more. n n Vapor pressure will increase as temperature increases Non-linear progression

Vapor Pressure and Boiling Point n A liquid boils when vapor pressure equals the external pressure acting on the surface of the liquid n n The temperature at which a liquid boils increases with increasing external pressure The boiling point of a liquid at 1 atm pressure is called its normal boiling point

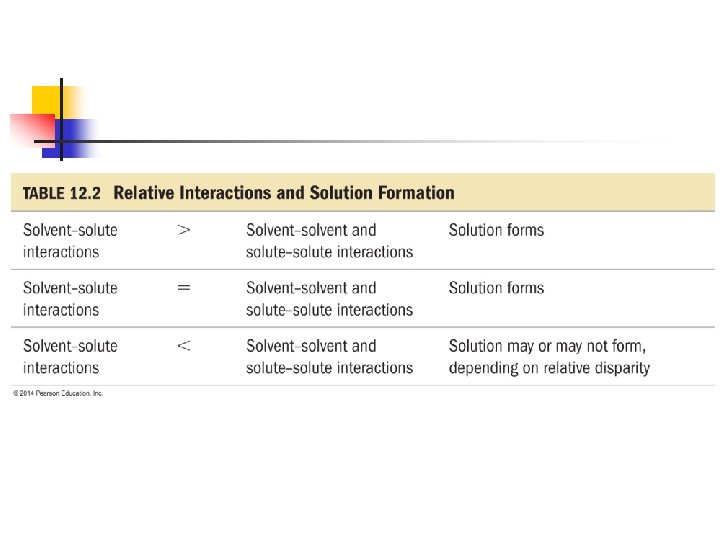
12. 2 – The Solution Process n n Any of the types of intermolecular forces can operate between solute and solvent particles in a solution. There’s three types of interactions that must be taken into consideration to determine if something will dissolve in a particular solvent.

Solute-Solute n This attraction must be OVERCOME in order to disperse solute through the solvent. n Energy is needed (endothermic) to break apart these attractions between solvent particles.

Solvent-Solvent n This attraction must also be overcome to make room for the solute particles in the solvent. n Energy is needed (endothermic) to break apart these attractions between solvent particles.

Solvent-Solute n This is an intermolecular attraction between the solute and solvent. n n n Attraction of solute/solvent will be exothermic How much something dissolves depends on the relative strength of these three interactions. Solutions form when the solvent-solute strength is about the same or greater than the solute-solute AND solvent-solvent.


Example - Na. Cl n Na. Cl dissolves in water because the solventsolute interaction between polar water and ions n This attraction is greater/similar to the solute/solute (Na. Cl ions for each other) and the solvent/solvent (polar water molecules for each other) attractions

n Remember, attraction between solvent/solute must be greater/similar to the attractions between solute/solute AND solvent/solvent

Overview n In general n n The stronger the attractions between solute and solvent, the greater the solubility of that solute in that solvent. What that means n n Polar solvents can dissolve ionic/polar solutes Polar solvents will NOT dissolve nonpolar solutes n Because LDF not strong enough to overcome attractive forces of solute

Solubility and Pressure n The solubility of a gas in any solvent is increased as the partial pressure of the gas above the solvent increases.

Solubility and Temperature n n Solubility of most solid solutes in water increase as temperature increases. Solubility of gases in water decreases with increasing temperature