Chapter 11 Energy in Thermal Processes 1 Heat







- Slides: 19

Chapter 11 Energy in Thermal Processes 1. Heat and Internal Energy 2. Specific Heat 3. Calorimetry 4. Latent Heat and Phase Change 5. Energy Transfer

James Prescott Joule n n 1818 – 1889 British physicist Conservation of Energy Relationship between heat and other forms of energy transfer

Heat and Internal Energy n n What is Heat? What are the units of Heat? What is Internal Energy? Energy Transfer

Specific Heat n n n What is specific Heat? Units of specific Heat and specific Heat

A Consequence of Different Specific Heats n n n Water has a high specific heat compared to land On a hot day, the air above the land warms faster The warmer air flows upward and cooler air moves toward the beach

Calorimeter n n What is a Calorimeter? How do I use it?

Calorimeter - Example The temperature of a 0. 05 kg ingot of metal is raised to 200. 0 °C, and the ingot is then dropped into a light, insulated beaker containing 0. 40 kg of water initially at 20. 0 °C. If the final equilibrium temperature of the mixed system is 22. 4 °C, find the specific heat of the metal.

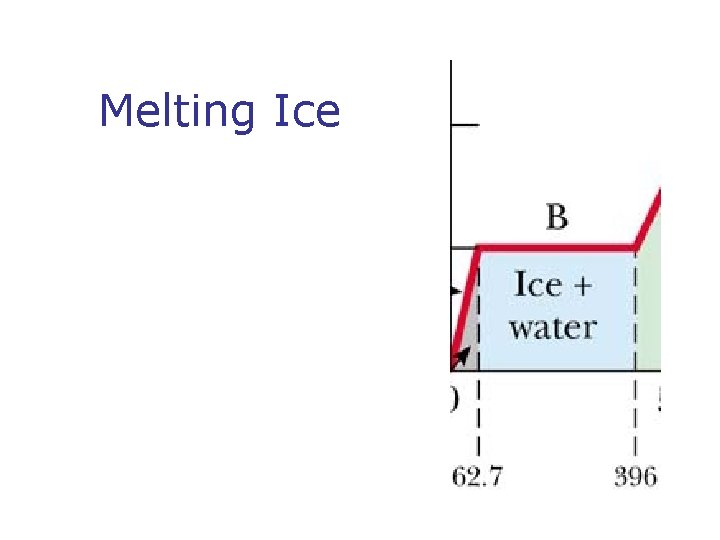
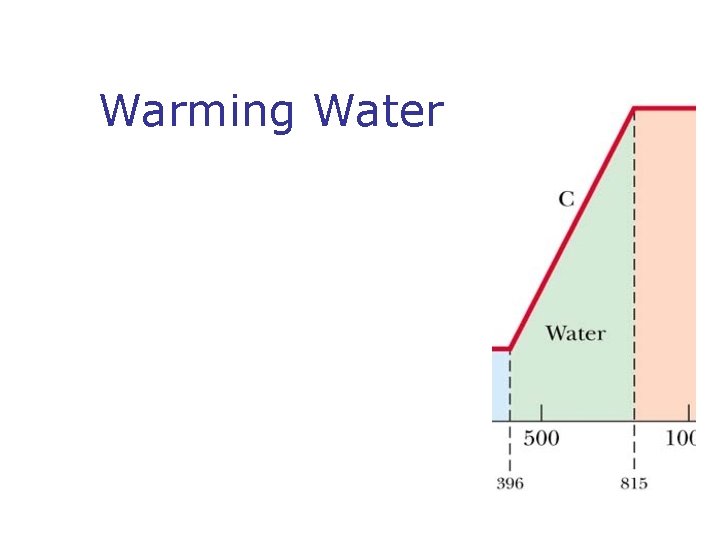
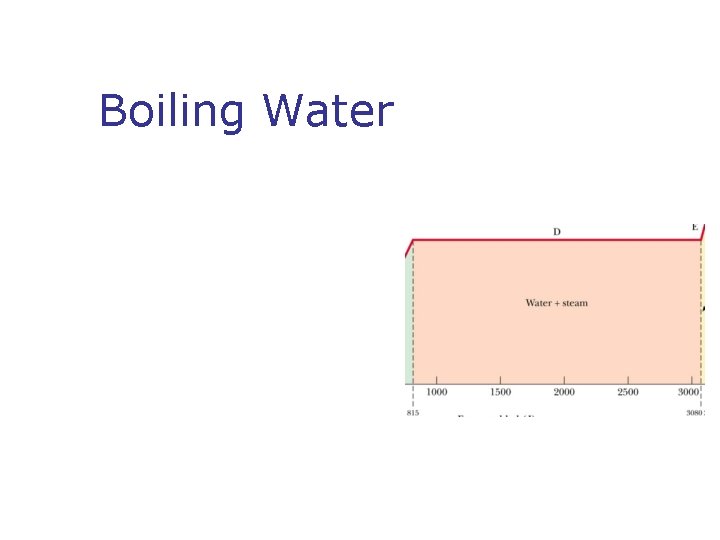
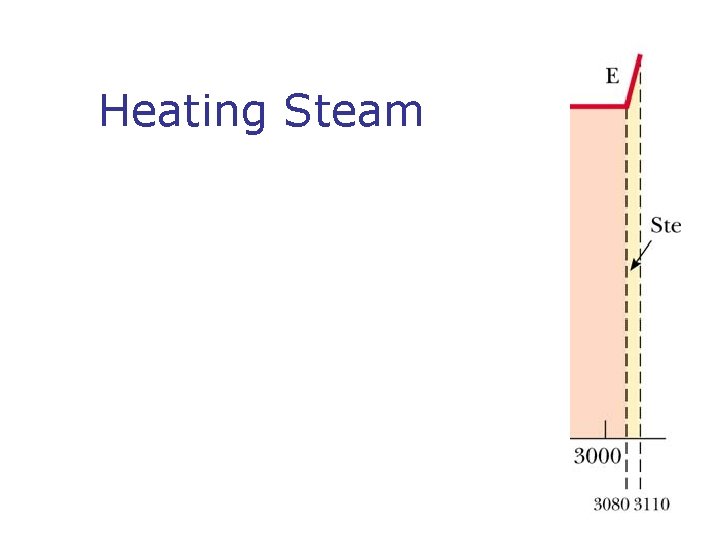
Phase Changes n n What is a phase change? Types of phases change n n n Solid to liquid – melting Liquid to gas – boiling What is changing during Phases change? What is latent heat? SI units of latent heat What is sublimation?

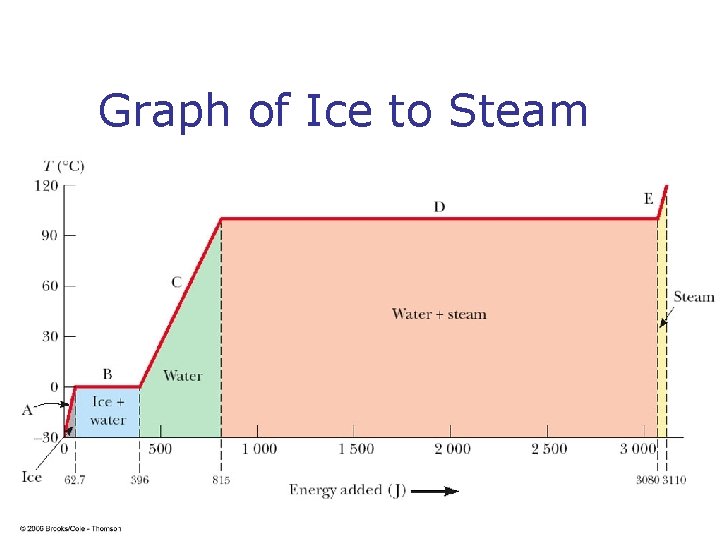
Graph of Ice to Steam

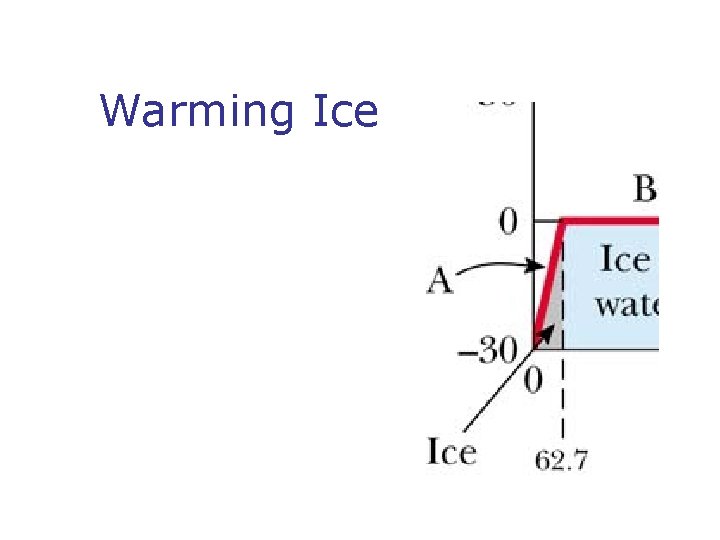
Warming Ice

Melting Ice

Warming Water

Boiling Water

Heating Steam

Extra Credit A iron horseshoe of mass mi initially at temperature Ti °C is dropped into a bucket containing mw kg of water at temperature Tw °C. Find using energy conservation an expression of the final equilibrium temperature Tf? Neglect any energy transfer to or from the surrounding. Let cw be the specific heat of water and ci be the specific heat of iron.

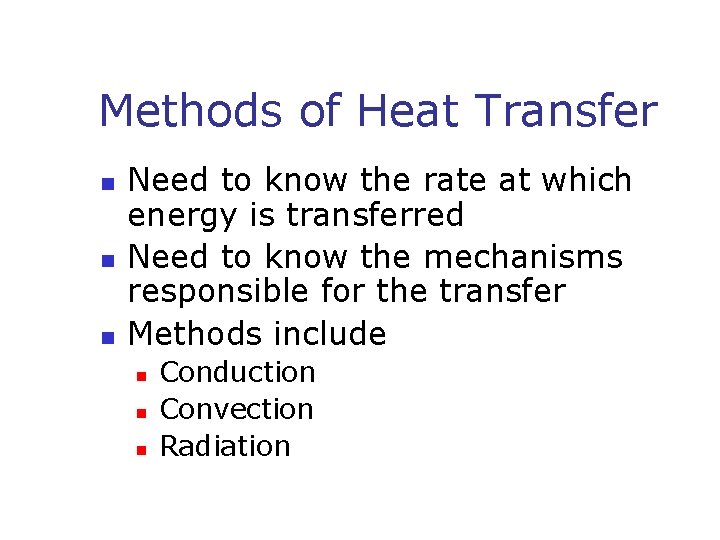
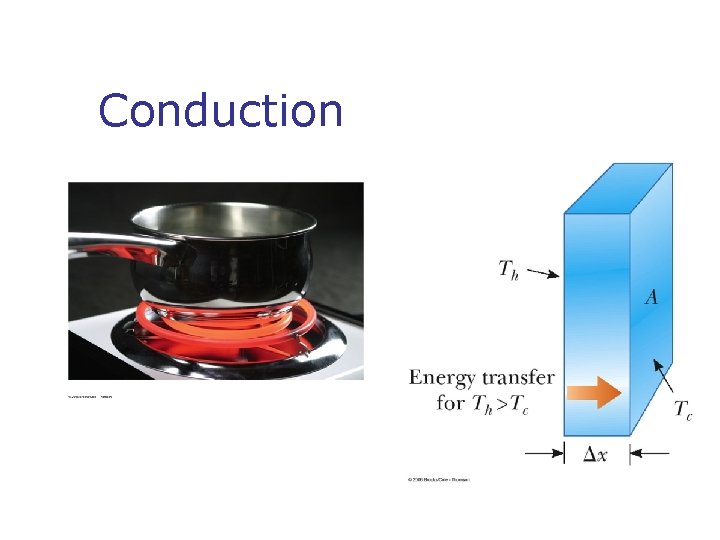
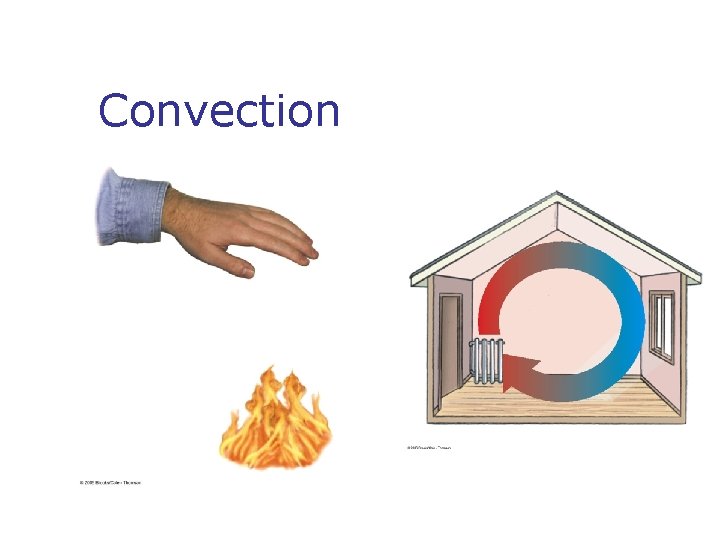
Methods of Heat Transfer n n n Need to know the rate at which energy is transferred Need to know the mechanisms responsible for the transfer Methods include n n n Conduction Convection Radiation

Conduction

Convection

Radiation