Chapter 11 Chemical Reactions Physical Changes Physical Change









- Slides: 31

Chapter 11 Chemical Reactions

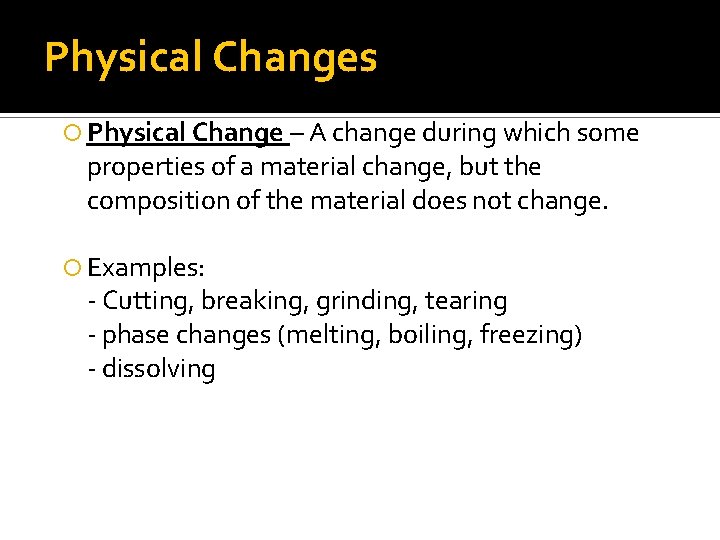
Physical Changes Physical Change – A change during which some properties of a material change, but the composition of the material does not change. Examples: - Cutting, breaking, grinding, tearing - phase changes (melting, boiling, freezing) - dissolving

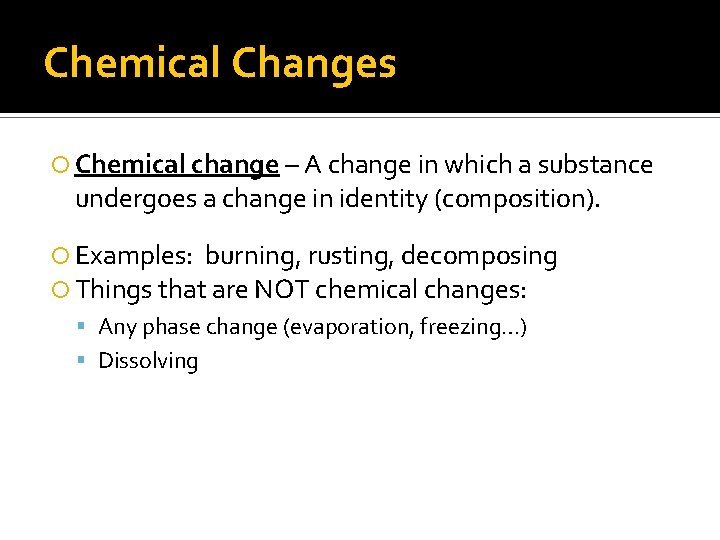
Chemical Changes Chemical change – A change in which a substance undergoes a change in identity (composition). Examples: burning, rusting, decomposing Things that are NOT chemical changes: Any phase change (evaporation, freezing…) Dissolving

Signs of Chemical Changes Several signs that a chemical change has occurred: 1) Heat, light or gas given off 2) Change in color or odor 3) A precipitate (solid product) is formed (It will look cloudy. ) 4) Sound 5) Bubbles (except boiling)

When signs of a chemical change can be deceiving. Boiling - phase change from liquid to gas. It is NOT a reaction. But you will see bubbles. Diluting – adding water to lower the concentration. It will alter the color, but it is NOT a reaction. Diluting is NOT a chemical change. Two clear liquids making a yellow precipitate IS a chemical reaction

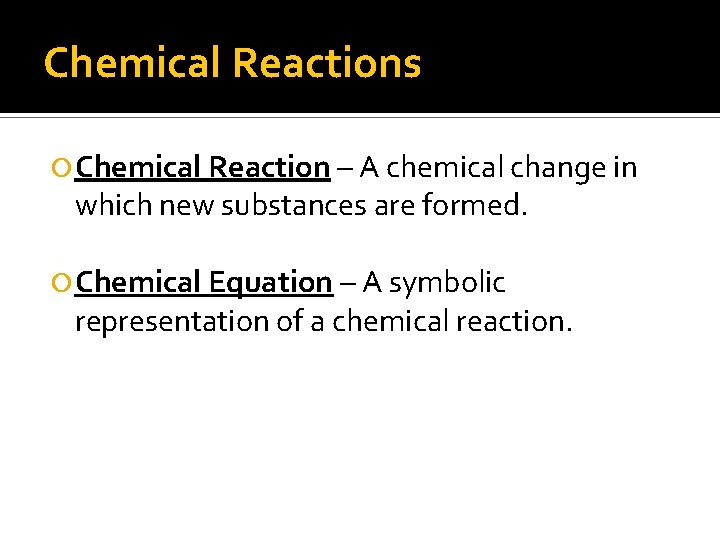
Chemical Reactions Chemical Reaction – A chemical change in which new substances are formed. Chemical Equation – A symbolic representation of a chemical reaction.

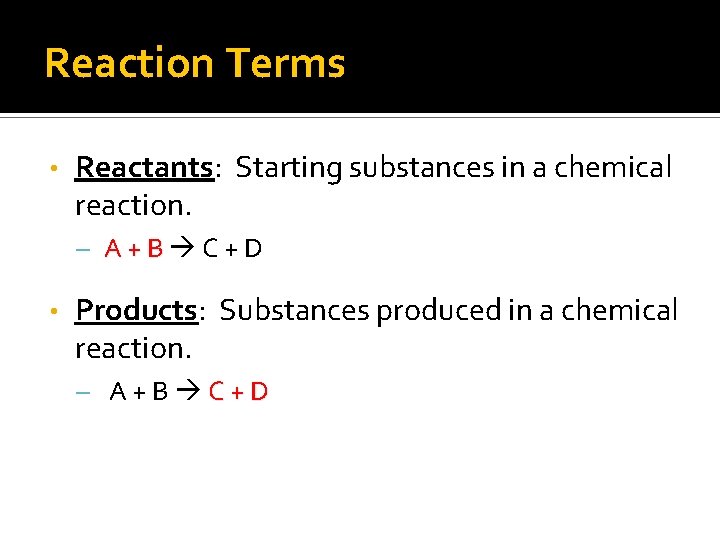
Reaction Terms • Reactants: Starting substances in a chemical reaction. – A+B C+D • Products: Substances produced in a chemical reaction. – A+B C+D

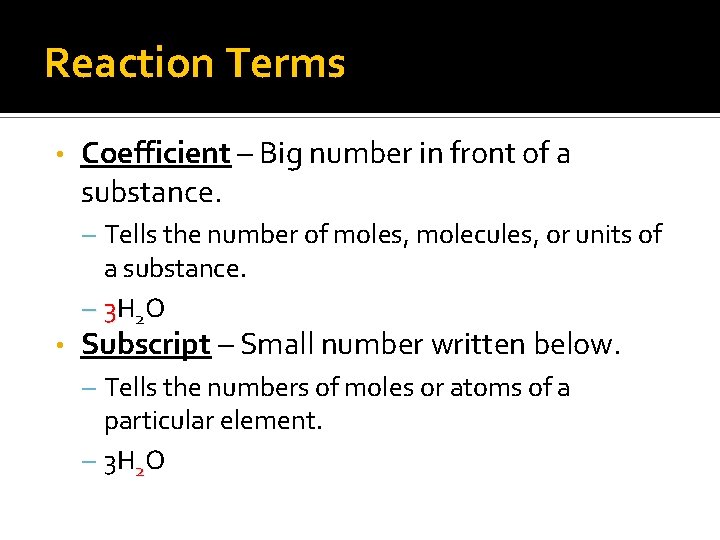
Reaction Terms • Coefficient – Big number in front of a substance. – Tells the number of moles, molecules, or units of a substance. – 3 H 2 O • Subscript – Small number written below. – Tells the numbers of moles or atoms of a particular element. – 3 H 2 O

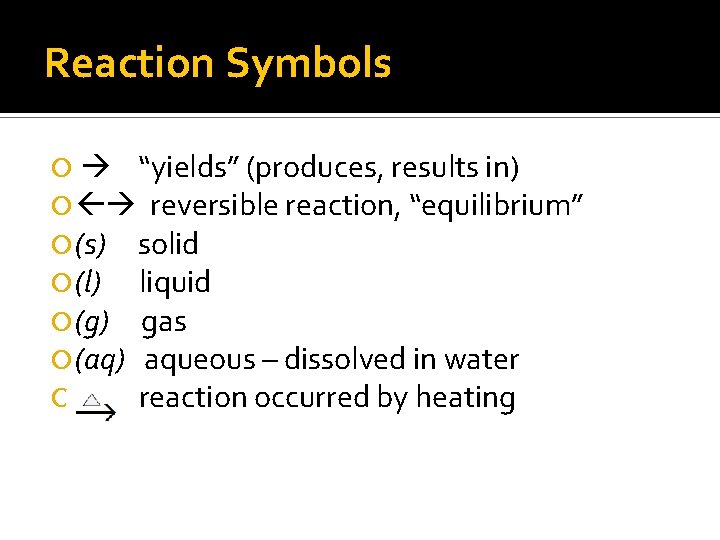
Reaction Symbols “yields” (produces, results in) reversible reaction, “equilibrium” (s) solid (l) liquid (g) gas (aq) aqueous – dissolved in water reaction occurred by heating

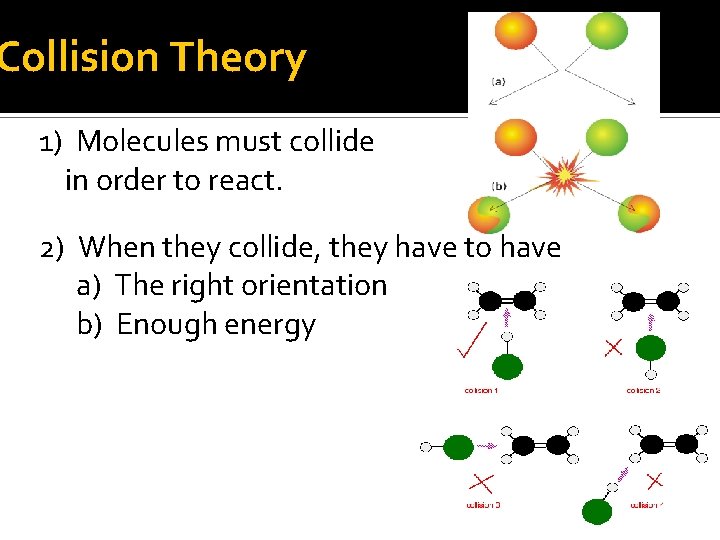
Collision Theory 1) Molecules must collide in order to react. 2) When they collide, they have to have a) The right orientation b) Enough energy

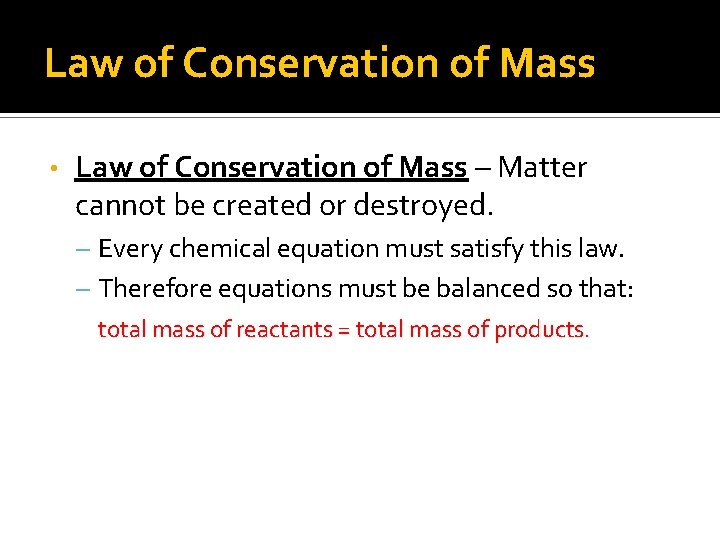
Law of Conservation of Mass • Law of Conservation of Mass – Matter cannot be created or destroyed. – Every chemical equation must satisfy this law. – Therefore equations must be balanced so that: total mass of reactants = total mass of products.

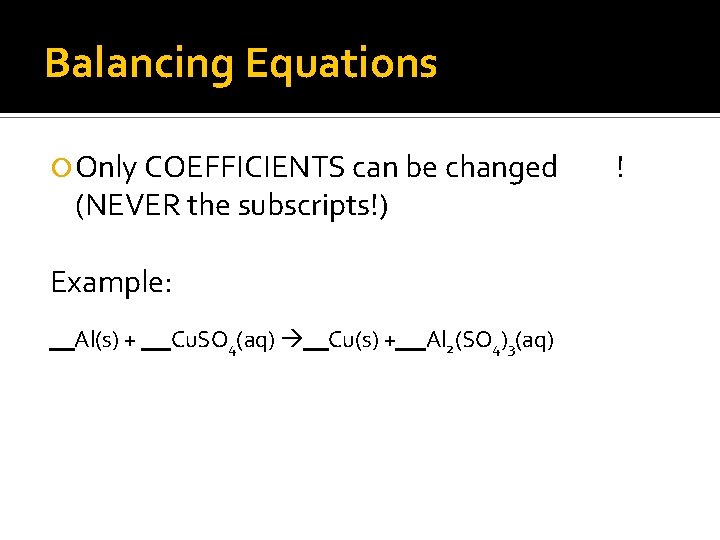
Balancing Equations Only COEFFICIENTS can be changed (NEVER the subscripts!) Example: Al(s) + Cu. SO 4(aq) Cu(s) + Al 2(SO 4)3(aq) !

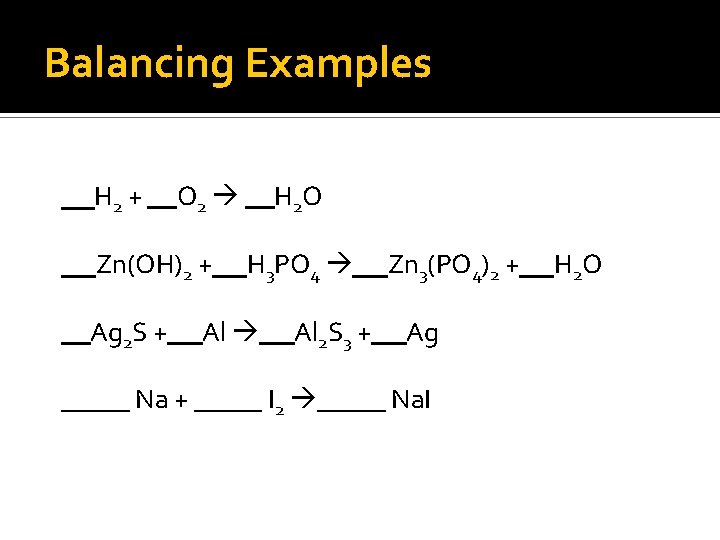
Balancing Examples H 2 + O 2 Zn(OH)2 + Ag 2 S + H 2 O H 3 PO 4 Al 2 S 3 + Zn 3(PO 4)2 + Ag _____ Na + _____ I 2 _____ Na. I H 2 O

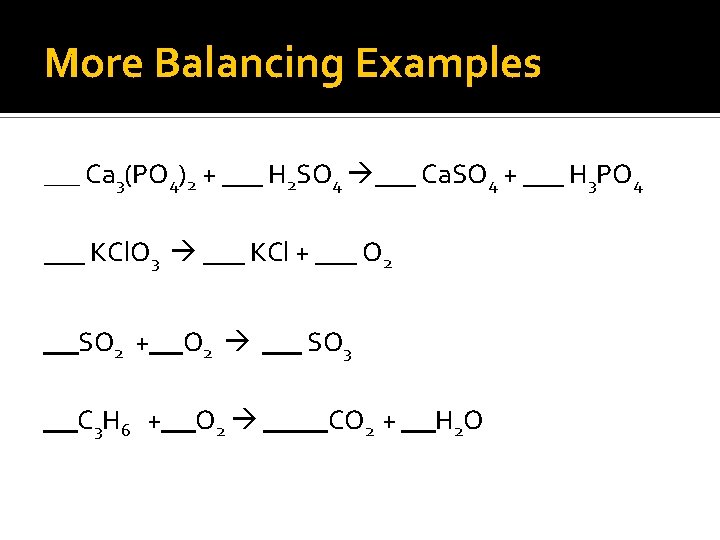
More Balancing Examples ___ Ca 3(PO 4)2 + ___ H 2 SO 4 ___ Ca. SO 4 + ___ H 3 PO 4 ___ KCl. O 3 ___ KCl + ___ O 2 SO 2 + C 3 H 6 + O 2 SO 3 CO 2 + H 2 O

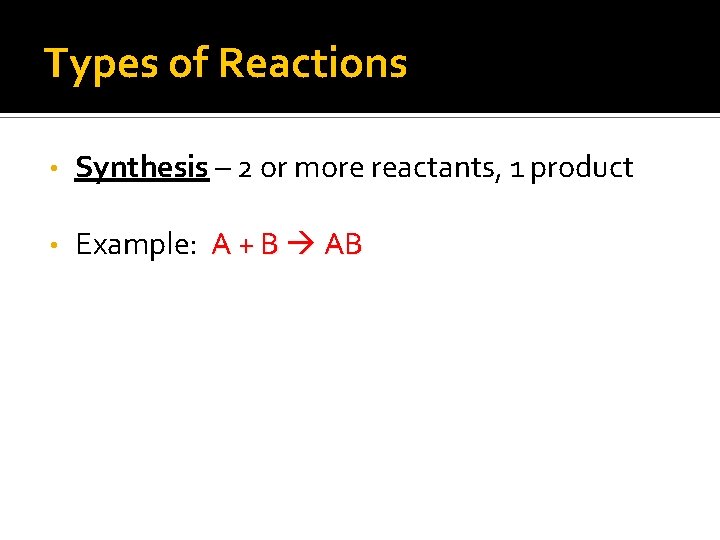
Types of Reactions • Synthesis – 2 or more reactants, 1 product • Example: A + B AB

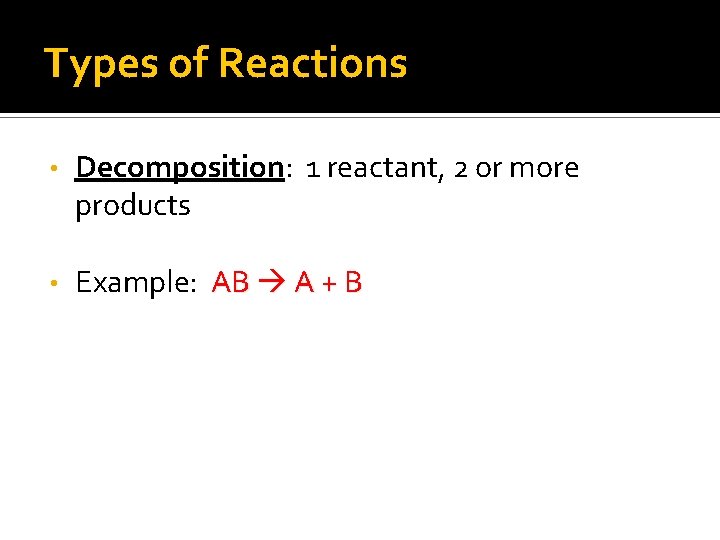
Types of Reactions • Decomposition: 1 reactant, 2 or more products • Example: AB A + B

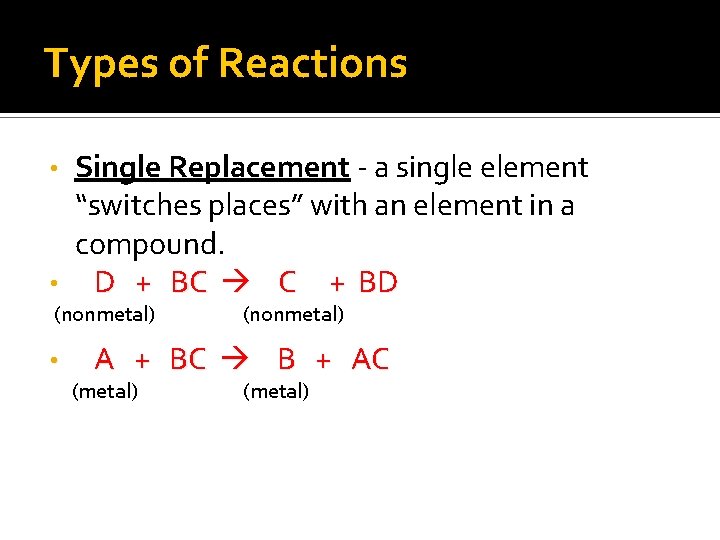
Types of Reactions Single Replacement - a single element “switches places” with an element in a compound. • D + BC C + BD • (nonmetal) A + BC B + AC (metal)

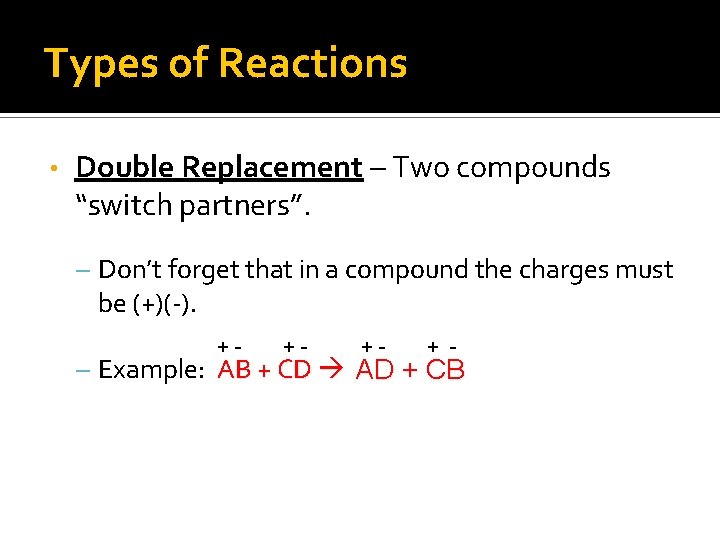
Types of Reactions • Double Replacement – Two compounds “switch partners”. – Don’t forget that in a compound the charges must be (+)(-). +- +- +- + - – Example: AB + CD AD + CB

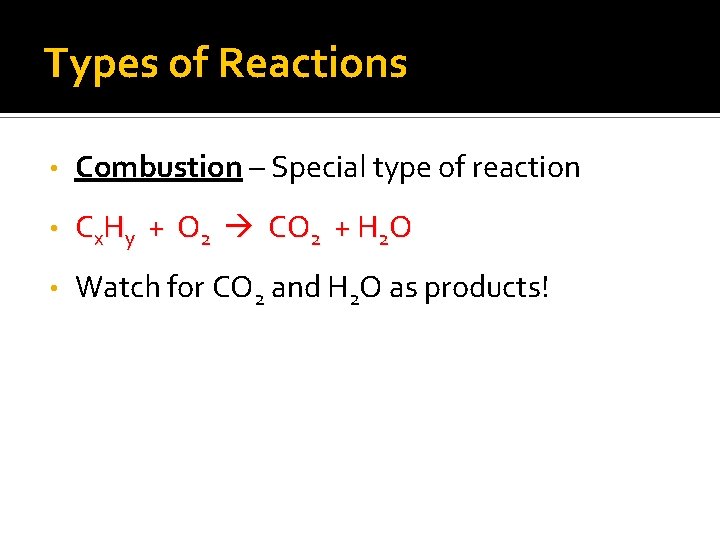
Types of Reactions • Combustion – Special type of reaction • Cx. Hy + O 2 CO 2 + H 2 O • Watch for CO 2 and H 2 O as products!

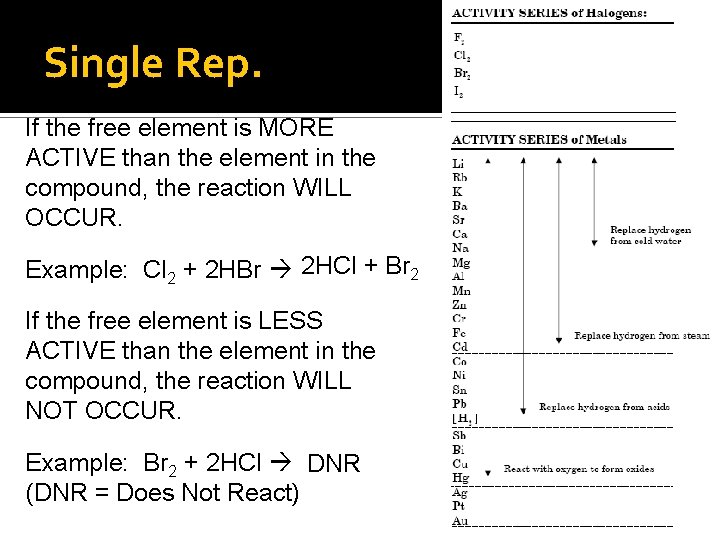
Predicting the Products – Single Replacement • To predict if a single replacement will react, use the ACTIVITY SERIES • The free element MUST BE more reactive than the element in the compound.

Single Rep. If the free element is MORE ACTIVE than the element in the compound, the reaction WILL OCCUR. Example: Cl 2 + 2 HBr 2 HCl + Br 2 If the free element is LESS ACTIVE than the element in the compound, the reaction WILL NOT OCCUR. Example: Br 2 + 2 HCl DNR (DNR = Does Not React)

Note **Note – Don’t EVER bring a subscript across the arrow UNLESS it’s part of a polyatomic ion!!**

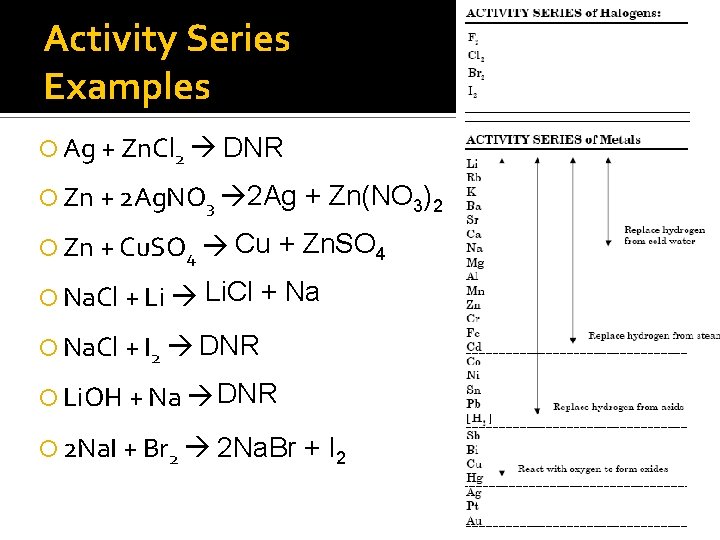
Activity Series Examples Ag + Zn. Cl 2 DNR Zn + 2 Ag. NO 3 2 Ag Zn + Cu. SO 4 Cu Na. Cl + Li Li. Cl + Zn(NO 3)2 + Zn. SO 4 + Na Na. Cl + I 2 DNR Li. OH + Na DNR 2 Na. I + Br 2 2 Na. Br + I 2

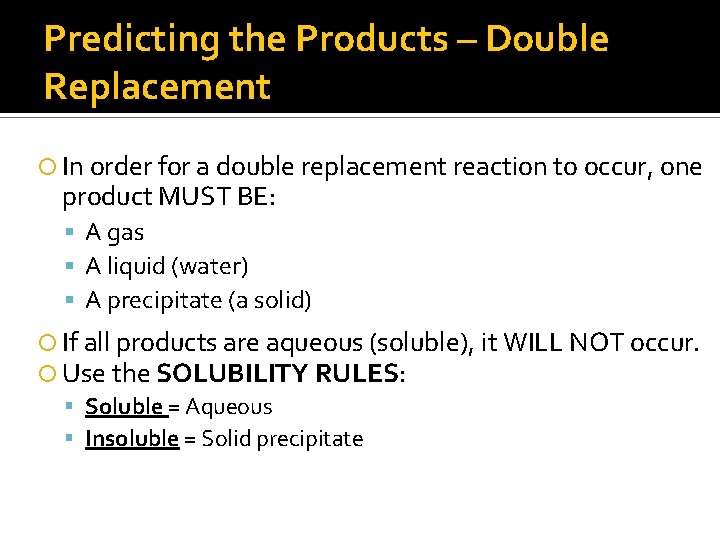
Predicting the Products – Double Replacement In order for a double replacement reaction to occur, one product MUST BE: A gas A liquid (water) A precipitate (a solid) If all products are aqueous (soluble), it WILL NOT occur. Use the SOLUBILITY RULES: Soluble = Aqueous Insoluble = Solid precipitate

Solubility Rules

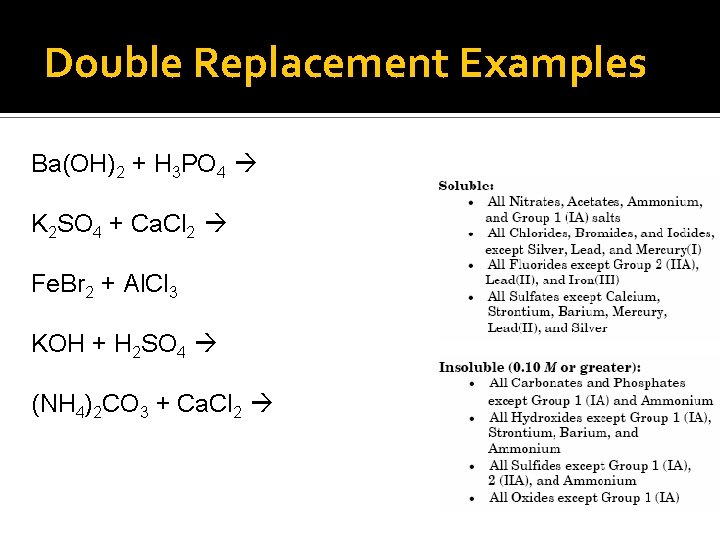
Double Replacement Examples Ba(OH)2 + H 3 PO 4 K 2 SO 4 + Ca. Cl 2 Fe. Br 2 + Al. Cl 3 KOH + H 2 SO 4 (NH 4)2 CO 3 + Ca. Cl 2

Net Ionic Equations Rules: Aqueous ionic compounds can be split into ions. (Don’t forget charges!) Strong acids can be separated into ions. Substances that are solids, liquids, or gases cannot be separated. Spectator ions are removed from the ionic equation, leaving the net ionic equation.

If Aqueous… For all aqueous compounds: Step 1 – Split it up Step 2 – Write charges for each thing Step 3 – Write how many you have as a coefficient

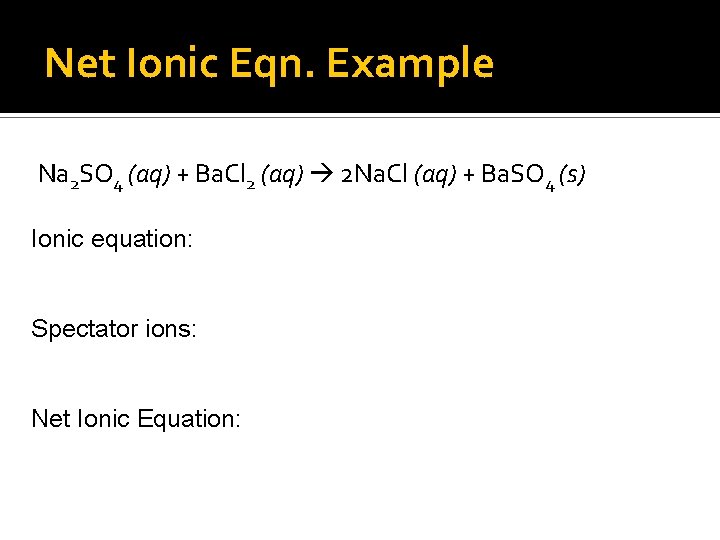
Net Ionic Eqn. Example Na 2 SO 4 (aq) + Ba. Cl 2 (aq) 2 Na. Cl (aq) + Ba. SO 4 (s) Ionic equation: Spectator ions: Net Ionic Equation:

Lab Tests – Burning splint A burning splint can be used to test for: Hydrogen (squeaky “pop” sound) Oxygen (blow out the splint and it will reignite) ▪ Because fire needs O 2 to burn Carbon dioxide (flame will go out) ▪ Because CO 2 smothers it

Lab test - Limewater and CO 2 Clear, colorless limewater will turn a cloudy white if CO 2 is added.