Chapter 11 Chemical Reactions Augustine Section 11 1




















- Slides: 51

Chapter 11: Chemical Reactions Augustine

Section 11. 1 – Describing Chemical Reactions In a chemical reaction, the reactants are written on the left and the products on the right. The arrow that separates them is called yield. Reactants Products

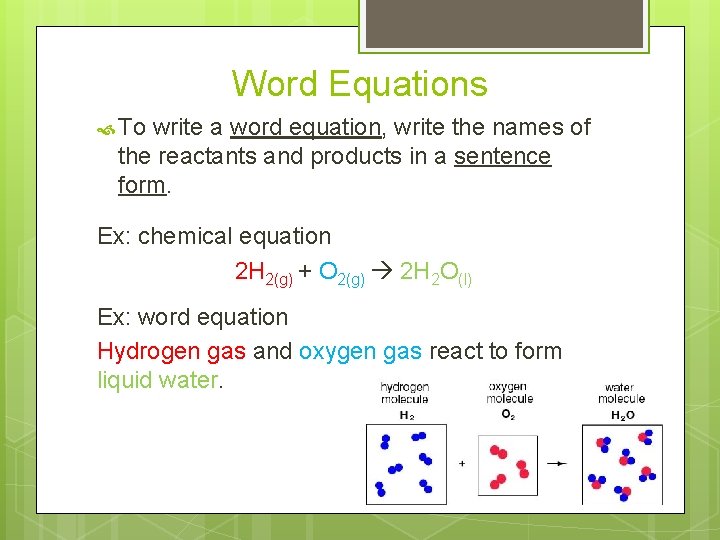
Symbols in Equations Symbol " D (s) (l) (g) (aq) Pt " " Meaning yields reversible reaction solid liquid gas aqueous catalyst heat

Catalyst A catalyst is a substance that speeds up a reaction but is not used up in the reaction. A catalyst is neither a reactant nor a product, so its formula is written above the arrow in a chemical equation.

Word Equations To write a word equation, write the names of the reactants and products in a sentence form. Ex: chemical equation 2 H 2(g) + O 2(g) 2 H 2 O(l) Ex: word equation Hydrogen gas and oxygen gas react to form liquid water.

Sample Problem #1 Write a sentence that describes this chemical reaction: Na(s) + H 2 O(l) Na. OH(aq) + H 2(g) Solid sodium and liquid water react to form aqueous sodium hydroxide and hydrogen gas.

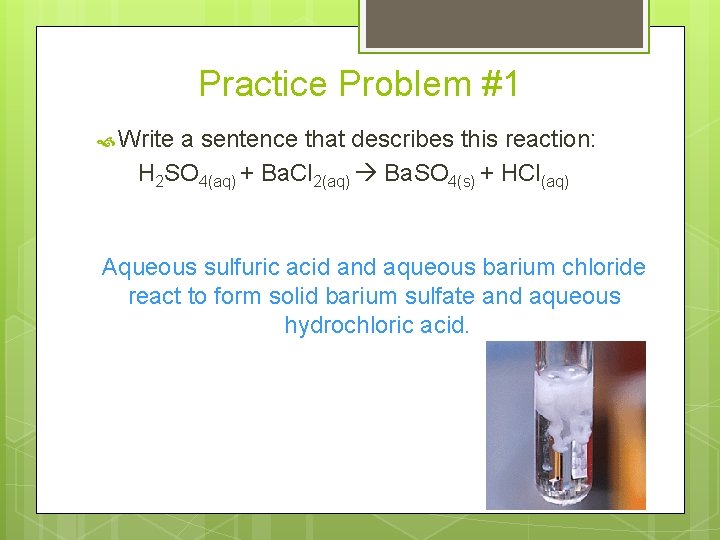
Practice Problem #1 Write a sentence that describes this reaction: H 2 SO 4(aq) + Ba. Cl 2(aq) Ba. SO 4(s) + HCl(aq) Aqueous sulfuric acid and aqueous barium chloride react to form solid barium sulfate and aqueous hydrochloric acid.

Sample Problem #2 Write the chemical equation for the following reaction: Hydrochloric acid and solid sodium hydrogen carbonate react to produce aqueous sodium chloride, water, and carbon dioxide. HCl(aq) + Na. HCO 3(s) Na. Cl(aq) + H 2 O(l) + CO 2(g) Hint: Acids will always be aqueous unless otherwise stated.

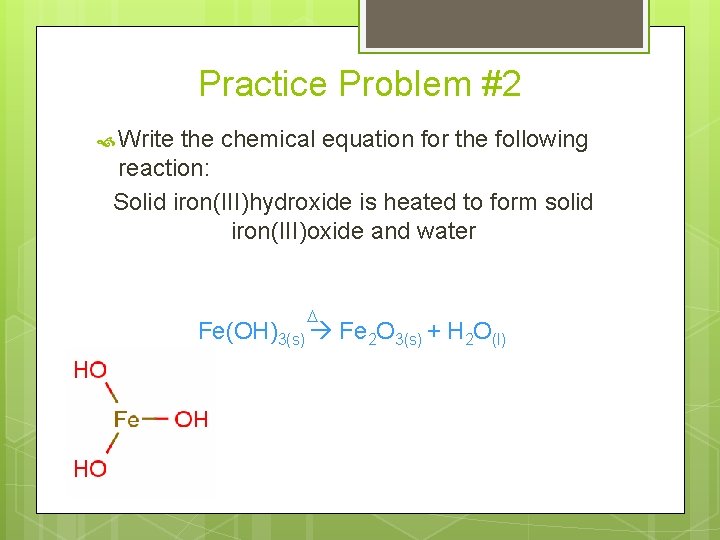
Practice Problem #2 Write the chemical equation for the following reaction: Solid iron(III)hydroxide is heated to form solid iron(III)oxide and water D Fe(OH)3(s) Fe 2 O 3(s) + H 2 O(l)

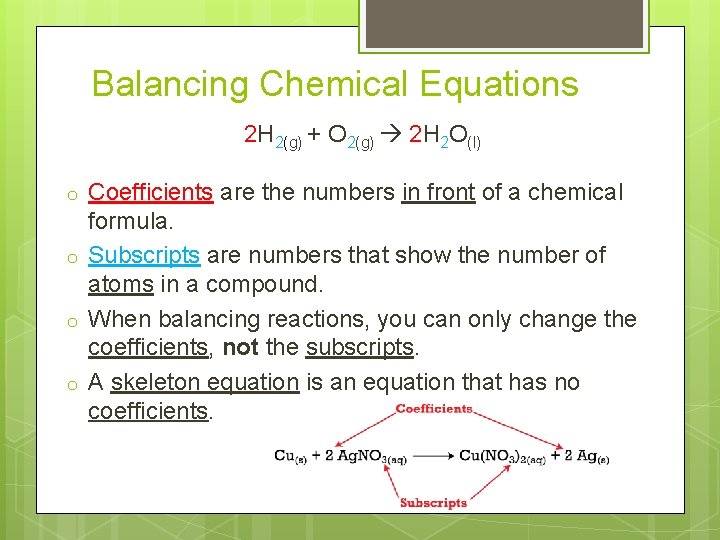
Balancing Chemical Equations 2 H 2(g) + O 2(g) 2 H 2 O(l) o o Coefficients are the numbers in front of a chemical formula. Subscripts are numbers that show the number of atoms in a compound. When balancing reactions, you can only change the coefficients, not the subscripts. A skeleton equation is an equation that has no coefficients.

Balancing Chemical Equations To balance a chemical equation, you add coefficients to the substances so that the reactant and product side of the equation contain equals numbers and types of atoms. Coefficients are added so that the equation follows the law of conservation of mass.

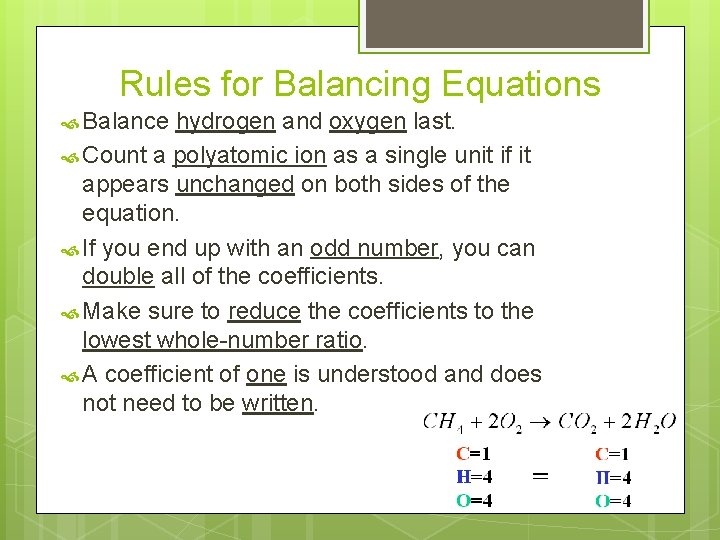
Rules for Balancing Equations Balance hydrogen and oxygen last. Count a polyatomic ion as a single unit if it appears unchanged on both sides of the equation. If you end up with an odd number, you can double all of the coefficients. Make sure to reduce the coefficients to the lowest whole-number ratio. A coefficient of one is understood and does not need to be written.

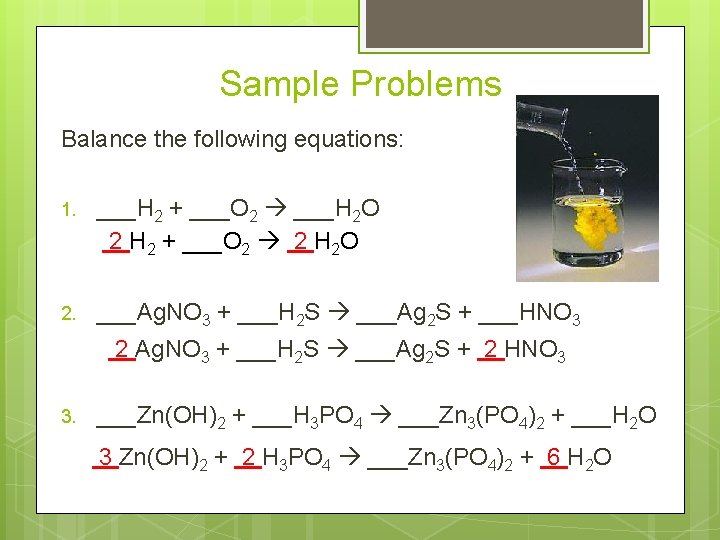
Sample Problems Balance the following equations: 1. ___H 2 + ___O 2 ___H 2 O 2 H 2 + ___O 2 2 H 2 O 2. ___Ag. NO 3 + ___H 2 S ___Ag 2 S + ___HNO 3 2 Ag. NO 3 + ___H 2 S ___Ag 2 S + 2 HNO 3 3. ___Zn(OH)2 + ___H 3 PO 4 ___Zn 3(PO 4)2 + ___H 2 O 3 Zn(OH)2 + 2 H 3 PO 4 ___Zn 3(PO 4)2 + 6 H 2 O

Practice Problems 1. ___Fe. Cl 3 + ___Na. OH ___Fe(OH)3 + ___Na. Cl ___Fe. Cl 3 + 3 Na. OH ___Fe(OH)3 + 3 Na. Cl 2. ___CS 2 + ___Cl 2 ___CCl 4 + ___S 2 Cl 2 ___CS 2 + 3 Cl 2 ___CCl 4 + ___S 2 Cl 2 3. ___C 2 H 6 + ___O 2 ___CO 2 + ___H 2 O 2 C 2 H 6 + 7 O 2 4 CO 2 + 6 H 2 O

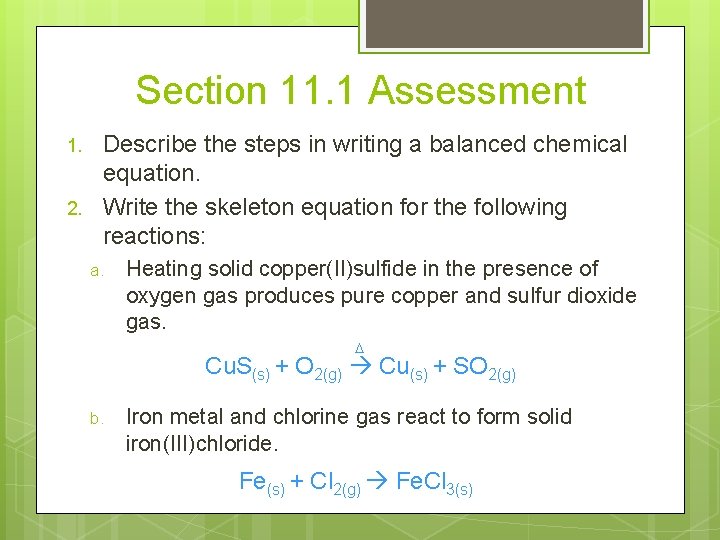
Section 11. 1 Assessment 1. 2. Describe the steps in writing a balanced chemical equation. Write the skeleton equation for the following reactions: a. Heating solid copper(II)sulfide in the presence of oxygen gas produces pure copper and sulfur dioxide gas. D Cu. S(s) + O 2(g) Cu(s) + SO 2(g) b. Iron metal and chlorine gas react to form solid iron(III)chloride. Fe(s) + Cl 2(g) Fe. Cl 3(s)

Section 11. 1 Assessment c. Solid aluminum carbonate decomposes to form solid aluminum oxide and carbon dioxide gas. Al 2(CO 3)3(s) Al 2 O 3(s) + CO 2(g) d. Solid magnesium reacts with aqueous silver(I)nitrate to form solid silver and aqueous magnesium nitrate. Mg(s) + Ag. NO 3(aq) Ag(s) + Mg(NO 3)2(aq)

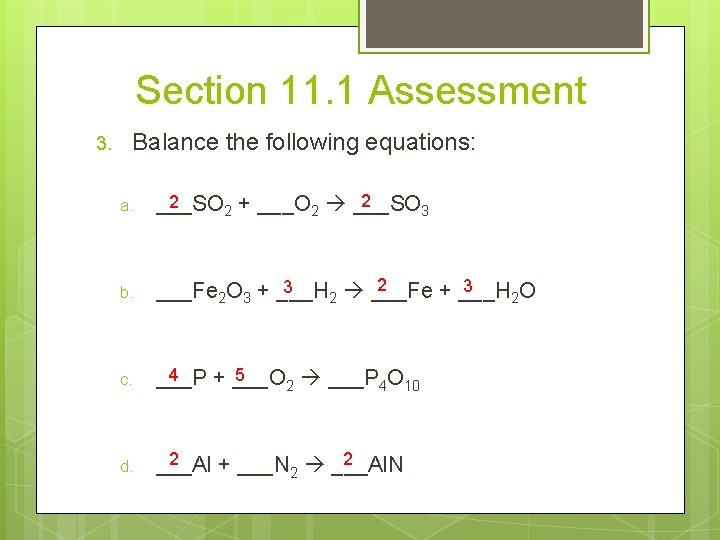
Section 11. 1 Assessment 3. Balance the following equations: a. 2 2 ___SO 2 + ___O 2 ___SO 3 b. 2 3 3 ___Fe 2 O 3 + ___H 2 ___Fe + ___H 2 O c. 4 5 ___P + ___O 2 ___P 4 O 10 d. 2 2 ___Al + ___N 2 ___Al. N

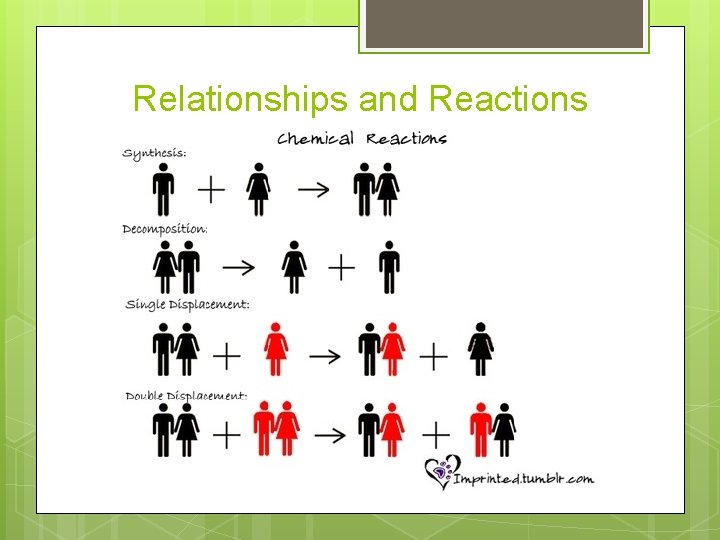
Section 11. 2 – Types of Chemical Reactions The five general types of reactions are synthesis, decomposition, single displacement, double displacement, and combustion.

Synthesis Reactions In a synthesis reaction, two or more substances react to form one product. Generic Reaction: A + B Actual Example: 2 Mg + O 2 2 Mg. O

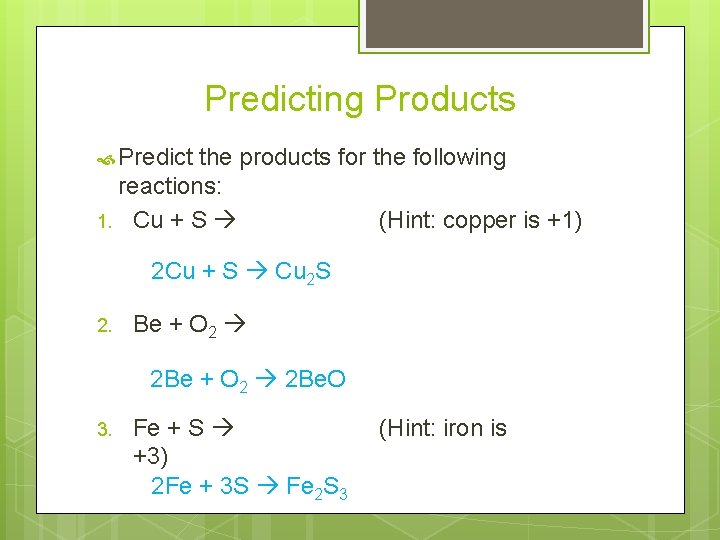
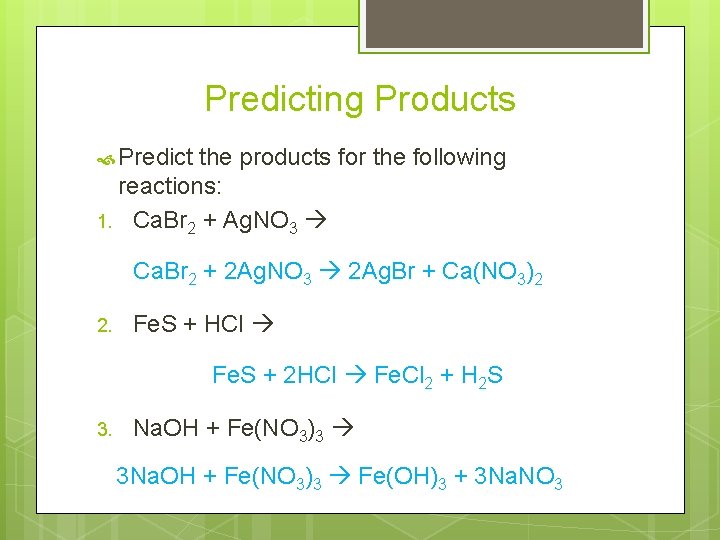
Predicting Products Predict the products for the following reactions: 1. Cu + S (Hint: copper is +1) 2 Cu + S Cu 2 S 2. Be + O 2 2 Be. O 3. Fe + S +3) 2 Fe + 3 S Fe 2 S 3 (Hint: iron is

Decomposition Reactions A decomposition reaction occurs when a single reactant breaks down into two or more products. Generic Reaction: AB A + B Actual Example: 2 Hg. O 2 Hg + O 2

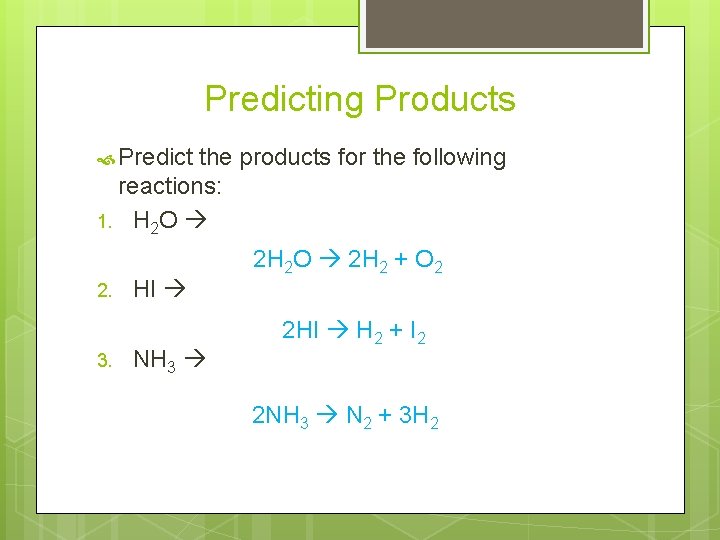
Predicting Products Predict the products for the following reactions: 1. H 2 O 2. 3. HI NH 3 2 H 2 O 2 H 2 + O 2 2 HI H 2 + I 2 2 NH 3 N 2 + 3 H 2

Single Displacement Reactions A single displacement reaction occurs when one element replaces a second element in a compound. Generic Reaction: A + BC B + AC Actual Example: Zn + Cu(NO 3)2 Cu + Zn(NO 3)2

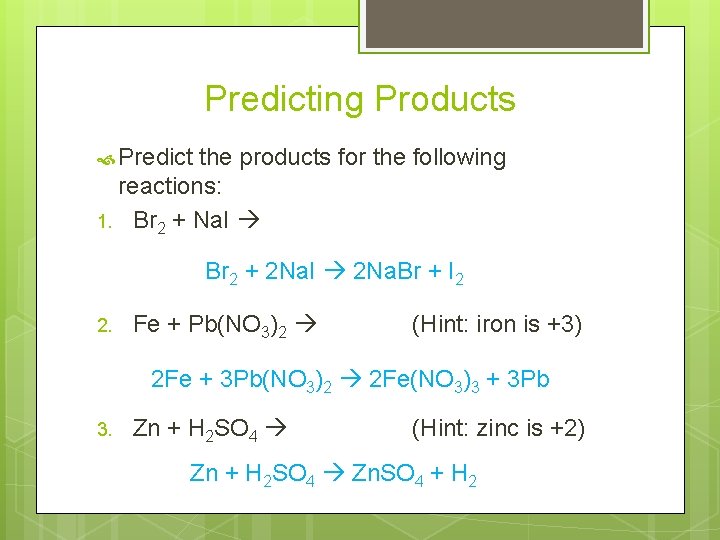
Predicting Products Predict the products for the following reactions: 1. Br 2 + Na. I Br 2 + 2 Na. I 2 Na. Br + I 2 2. Fe + Pb(NO 3)2 (Hint: iron is +3) 2 Fe + 3 Pb(NO 3)2 2 Fe(NO 3)3 + 3 Pb 3. Zn + H 2 SO 4 (Hint: zinc is +2) Zn + H 2 SO 4 Zn. SO 4 + H 2

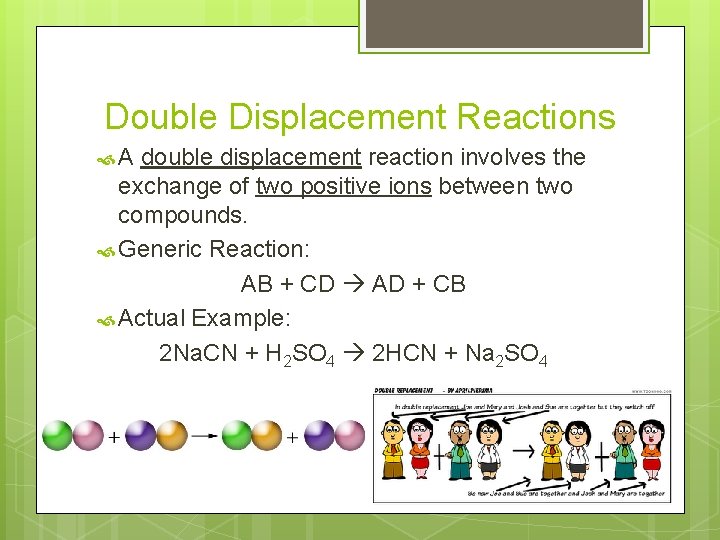
Double Displacement Reactions A double displacement reaction involves the exchange of two positive ions between two compounds. Generic Reaction: AB + CD AD + CB Actual Example: 2 Na. CN + H 2 SO 4 2 HCN + Na 2 SO 4

Predicting Products Predict the products for the following reactions: 1. Ca. Br 2 + Ag. NO 3 Ca. Br 2 + 2 Ag. NO 3 2 Ag. Br + Ca(NO 3)2 2. Fe. S + HCl Fe. S + 2 HCl Fe. Cl 2 + H 2 S 3. Na. OH + Fe(NO 3)3 3 Na. OH + Fe(NO 3)3 Fe(OH)3 + 3 Na. NO 3

Relationships and Reactions

Combustion Reactions A combustion reaction occurs when a substance burns in oxygen and produces a lot of heat and light. Generic Reaction: Cx. Hy + O 2 CO 2 + H 2 O Actual Example: 2 C 8 H 18 + 25 O 2 16 CO 2 + 18 H 2 O

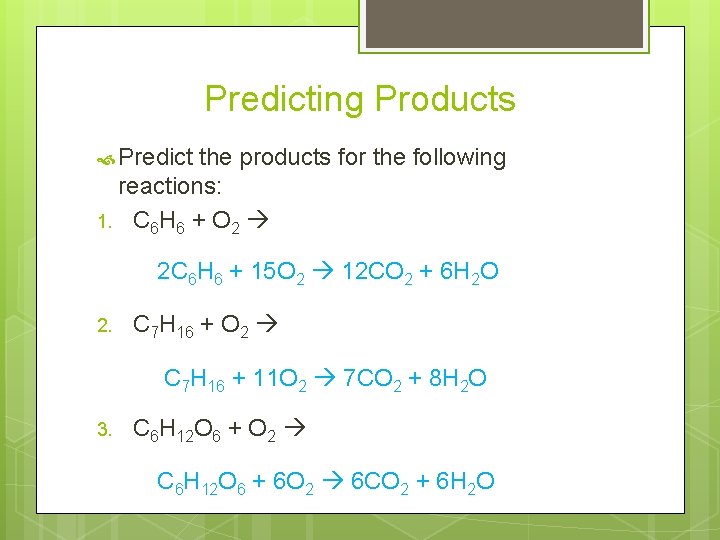
Predicting Products Predict the products for the following reactions: 1. C 6 H 6 + O 2 2 C 6 H 6 + 15 O 2 12 CO 2 + 6 H 2 O 2. C 7 H 16 + O 2 C 7 H 16 + 11 O 2 7 CO 2 + 8 H 2 O 3. C 6 H 12 O 6 + O 2 C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O

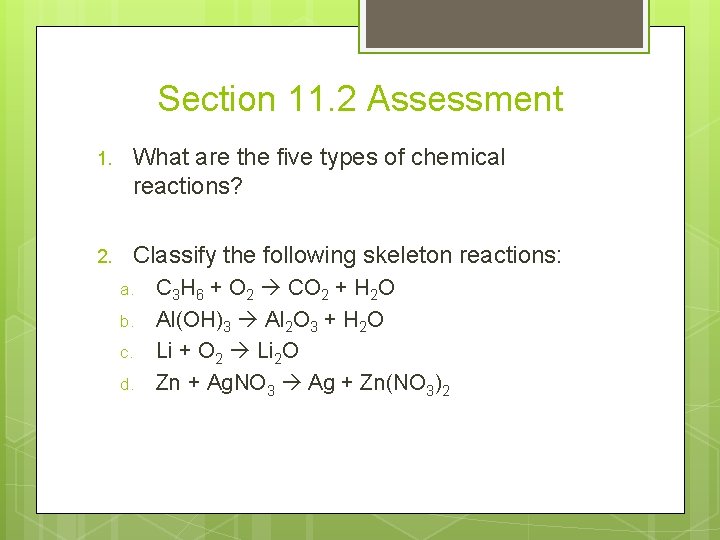
Section 11. 2 Assessment 1. What are the five types of chemical reactions? 2. Classify the following skeleton reactions: a. b. c. d. C 3 H 6 + O 2 CO 2 + H 2 O Al(OH)3 Al 2 O 3 + H 2 O Li + O 2 Li 2 O Zn + Ag. NO 3 Ag + Zn(NO 3)2

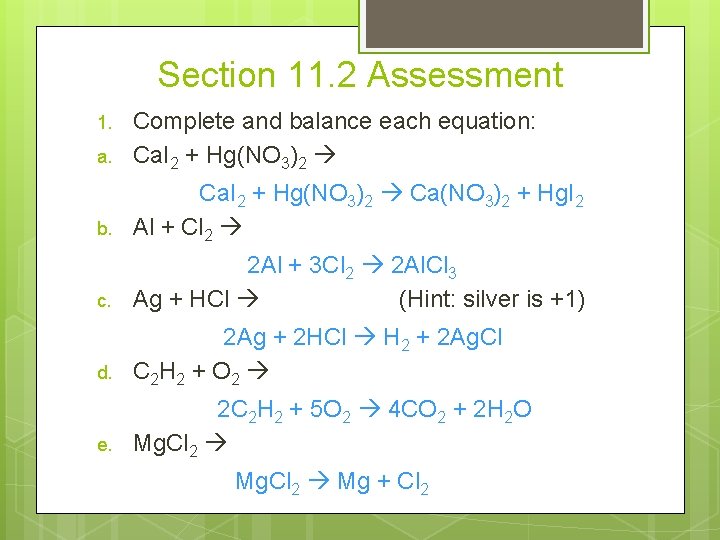
Section 11. 2 Assessment a. Complete and balance each equation: Ca. I 2 + Hg(NO 3)2 b. Ca. I 2 + Hg(NO 3)2 Ca(NO 3)2 + Hg. I 2 Al + Cl 2 c. 2 Al + 3 Cl 2 2 Al. Cl 3 Ag + HCl (Hint: silver is +1) d. 2 Ag + 2 HCl H 2 + 2 Ag. Cl C 2 H 2 + O 2 e. 2 C 2 H 2 + 5 O 2 4 CO 2 + 2 H 2 O Mg. Cl 2 1. Mg. Cl 2 Mg + Cl 2

Section 11. 3 – Reactions in Aqueous Solution A complete ionic equation is an equation that shows dissolved ionic compounds as dissociated free ions. A spectator ion is an ion that is not directly involved in a reaction. Compounds that are aqueous will break into ions, and compounds that are solid will remain bonded.

Aqueous Ions When a compound is aqueous it breaks into its ions. Ex: Na. Cl = Mg. Br 2 = Al 2 O 3 = Li. NO 3 = K 2 CO 3 = Sr(NO 2)2 = Na+ and Cl. Mg+2 and 2 Br 2 Al+3 and 3 O-2 Li+ and NO 32 K+ and CO 3 -2 Sr+2 and 2 NO 2 -

Rules for Writing Complete Ionic Equations 1. 2. 3. Balance the equation. Separate all aqueous substances into ions. Leave any non-aqueous substances or precipitates together.

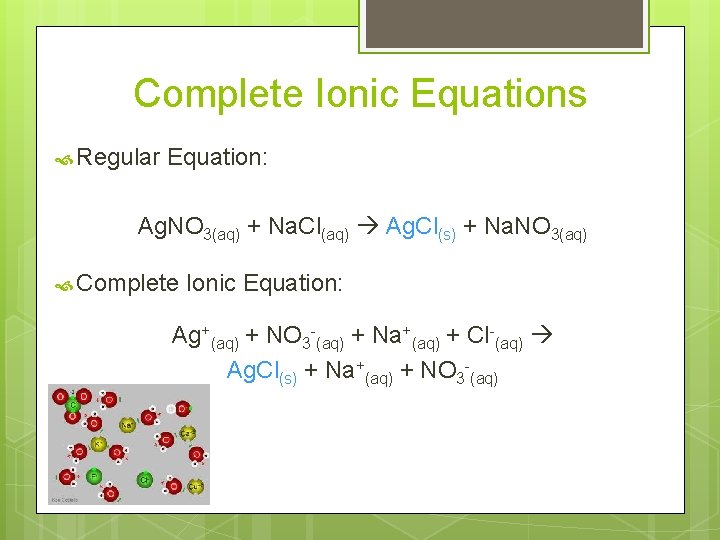
Complete Ionic Equations Regular Equation: Ag. NO 3(aq) + Na. Cl(aq) Ag. Cl(s) + Na. NO 3(aq) Complete Ionic Equation: Ag+(aq) + NO 3 -(aq) + Na+(aq) + Cl-(aq) Ag. Cl(s) + Na+(aq) + NO 3 -(aq)

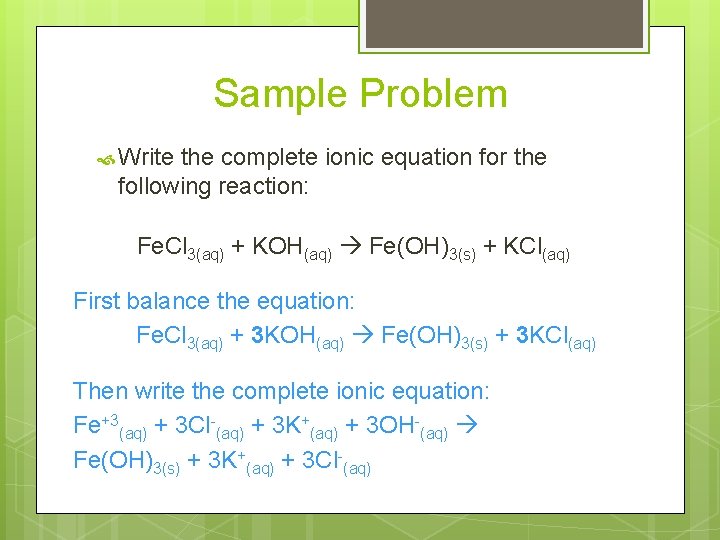
Sample Problem Write the complete ionic equation for the following reaction: Fe. Cl 3(aq) + KOH(aq) Fe(OH)3(s) + KCl(aq) First balance the equation: Fe. Cl 3(aq) + 3 KOH(aq) Fe(OH)3(s) + 3 KCl(aq) Then write the complete ionic equation: Fe+3(aq) + 3 Cl-(aq) + 3 K+(aq) + 3 OH-(aq) Fe(OH)3(s) + 3 K+(aq) + 3 Cl-(aq)

Practice Problems Write the complete ionic equation for the following reaction: Na. OH(aq) + Fe(NO 3)3(aq) Fe(OH)3(s) + Na. NO 3(aq) First balance the equation: 3 Na. OH(aq) + Fe(NO 3)3(aq) Fe(OH)3(s) + 3 Na. NO 3(aq) Then write the complete ionic equation: 3 Na+(aq) + 3 OH-(aq) + Fe+3(aq) + 3 NO 3 -(aq) Fe(OH)3(s) + 3 Na+(aq) + 3 NO 3 -(aq)

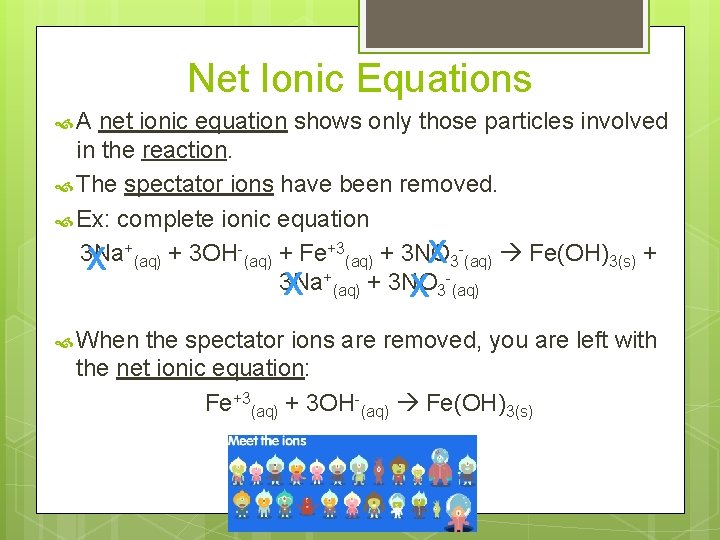
Net Ionic Equations A net ionic equation shows only those particles involved in the reaction. The spectator ions have been removed. Ex: complete ionic equation + +3 3 Na + 3 OH + Fe + 3 NO X (aq) 3 (aq) Fe(OH)3(s) + X 3 Na X +(aq) + 3 NO X 3 (aq) When the spectator ions are removed, you are left with the net ionic equation: Fe+3(aq) + 3 OH-(aq) Fe(OH)3(s)

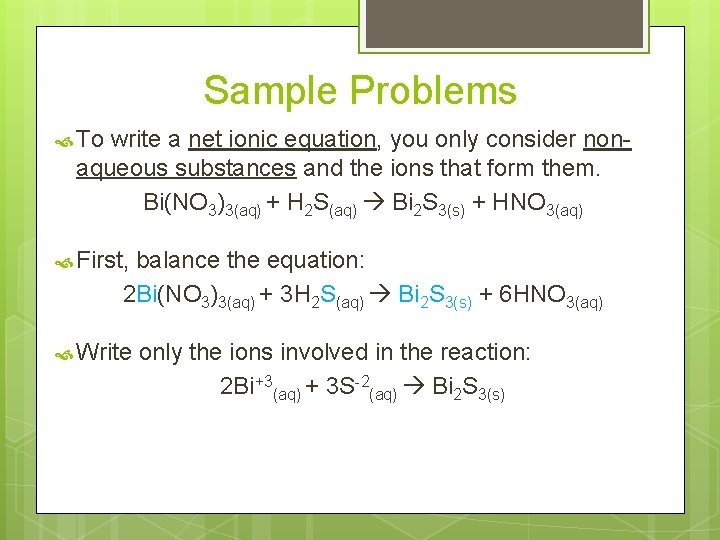
Sample Problems To write a net ionic equation, you only consider nonaqueous substances and the ions that form them. Bi(NO 3)3(aq) + H 2 S(aq) Bi 2 S 3(s) + HNO 3(aq) First, balance the equation: 2 Bi(NO 3)3(aq) + 3 H 2 S(aq) Bi 2 S 3(s) + 6 HNO 3(aq) Write only the ions involved in the reaction: 2 Bi+3(aq) + 3 S-2(aq) Bi 2 S 3(s)

Practice Problems 1. Write the net ionic equation for the following reaction: Pb(NO 3)2(aq) + H 2 SO 4(aq) Pb. SO 4(s) + HNO 3(aq) First balance the equation: Pb(NO 3)2(aq) + H 2 SO 4(aq) Pb. SO 4(s) + 2 HNO 3(aq) Write the net ionic equation: Pb+2(aq) + SO 4 -2(aq) Pb. SO 4(s)

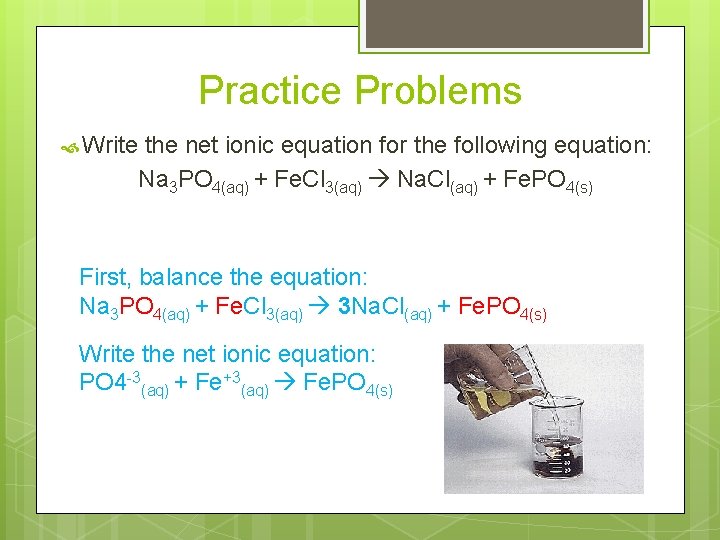
Practice Problems Write the net ionic equation for the following equation: Na 3 PO 4(aq) + Fe. Cl 3(aq) Na. Cl(aq) + Fe. PO 4(s) First, balance the equation: Na 3 PO 4(aq) + Fe. Cl 3(aq) 3 Na. Cl(aq) + Fe. PO 4(s) Write the net ionic equation: PO 4 -3(aq) + Fe+3(aq) Fe. PO 4(s)

Predicting Precipitates You can predict whether a precipitate forms by using solubility rules. If a substance is soluble, then it will dissolve in water and be aqueous. If a substance is insoluble, then it will bond with another ion and will be a solid.

Solubility Rules alkali metals - Group 1 soluble NH 4+ soluble NO 3 - soluble Cl. O 3 SO 4 -2 (except with Pb+2, Ag+, Hg 2+2, Ba+2, Sr+2, and Ca+2) soluble Cl- (exception Ag+, Pb+2, Hg 2+2) CO 3 -2, PO 4 -3, Cr. O 4 -2, S-2, and OH- soluble insoluble

Soluble or Insoluble? If any part of a compound is soluble, then the compound will be soluble. NH 4 Cl Soluble/Aqueous Ba. SO 4 Insoluble/Solid Na 2 SO 4 Soluble/Aqueous Ca(OH)2 Insoluble/Solid K 3 PO 4 Soluble/Aqueous Mg(NO 3)2 Soluble/Aqueous Ag. Cl Insoluble/Solid Ni. Cr. O 4 Insoluble/Solid

Predicting Precipitates Rules for Predicting Precipitates 1. Switch the ions and balance the charges to form the products. 2. Balance the equation. 3. Identify whether the products are solid or aqueous.

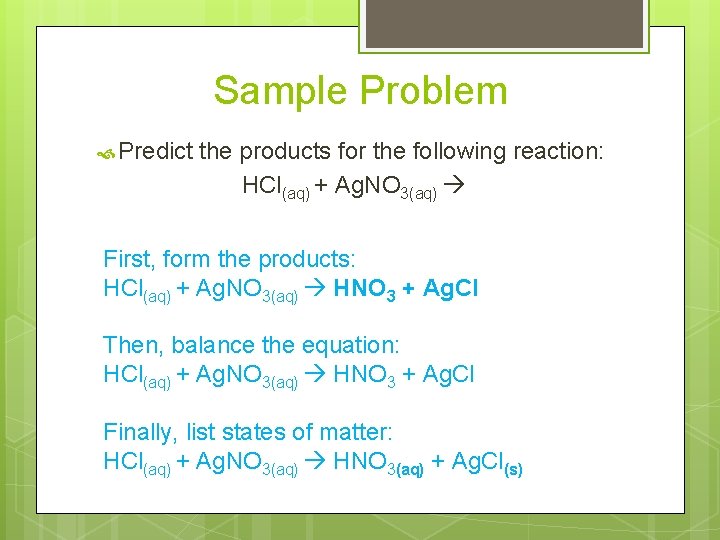
Sample Problem Predict the products for the following reaction: HCl(aq) + Ag. NO 3(aq) First, form the products: HCl(aq) + Ag. NO 3(aq) HNO 3 + Ag. Cl Then, balance the equation: HCl(aq) + Ag. NO 3(aq) HNO 3 + Ag. Cl Finally, list states of matter: HCl(aq) + Ag. NO 3(aq) HNO 3(aq) + Ag. Cl(s)

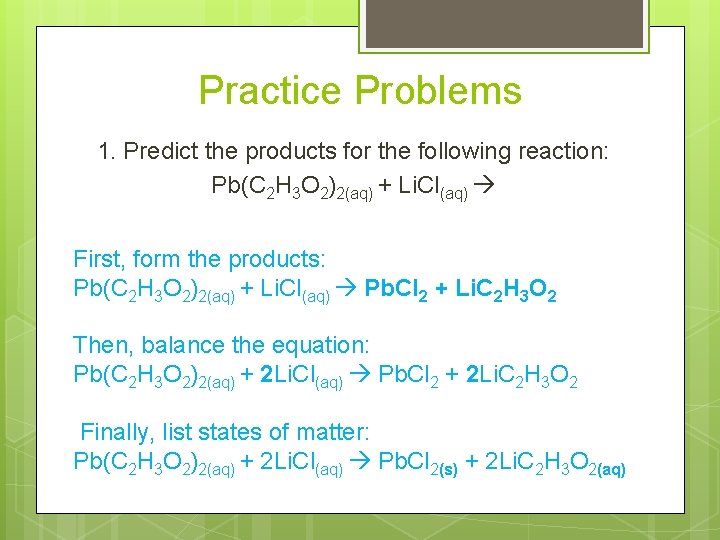
Practice Problems 1. Predict the products for the following reaction: Pb(C 2 H 3 O 2)2(aq) + Li. Cl(aq) First, form the products: Pb(C 2 H 3 O 2)2(aq) + Li. Cl(aq) Pb. Cl 2 + Li. C 2 H 3 O 2 Then, balance the equation: Pb(C 2 H 3 O 2)2(aq) + 2 Li. Cl(aq) Pb. Cl 2 + 2 Li. C 2 H 3 O 2 Finally, list states of matter: Pb(C 2 H 3 O 2)2(aq) + 2 Li. Cl(aq) Pb. Cl 2(s) + 2 Li. C 2 H 3 O 2(aq)

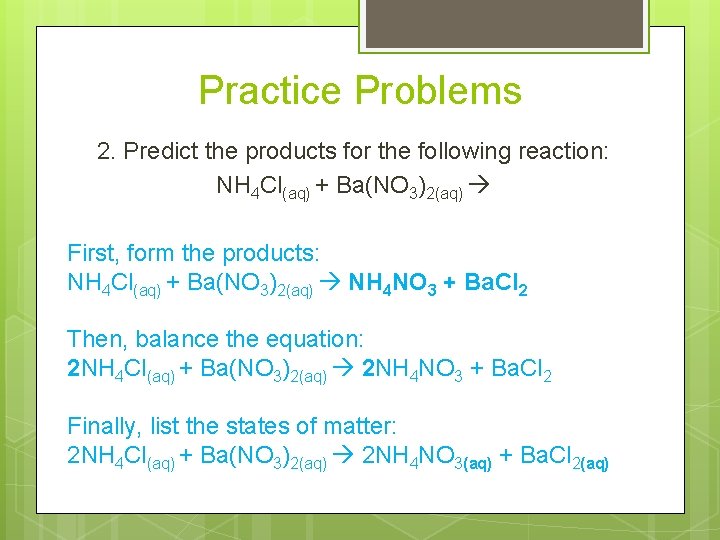
Practice Problems 2. Predict the products for the following reaction: NH 4 Cl(aq) + Ba(NO 3)2(aq) First, form the products: NH 4 Cl(aq) + Ba(NO 3)2(aq) NH 4 NO 3 + Ba. Cl 2 Then, balance the equation: 2 NH 4 Cl(aq) + Ba(NO 3)2(aq) 2 NH 4 NO 3 + Ba. Cl 2 Finally, list the states of matter: 2 NH 4 Cl(aq) + Ba(NO 3)2(aq) 2 NH 4 NO 3(aq) + Ba. Cl 2(aq)

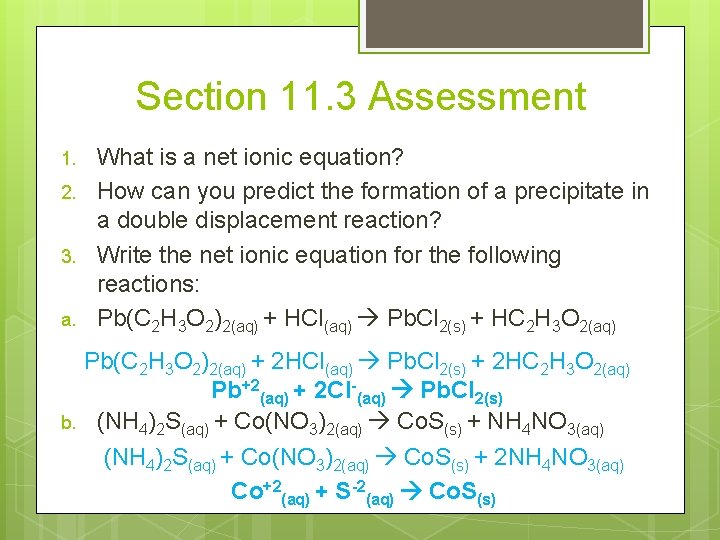
Section 11. 3 Assessment 1. 2. 3. a. b. What is a net ionic equation? How can you predict the formation of a precipitate in a double displacement reaction? Write the net ionic equation for the following reactions: Pb(C 2 H 3 O 2)2(aq) + HCl(aq) Pb. Cl 2(s) + HC 2 H 3 O 2(aq) Pb(C 2 H 3 O 2)2(aq) + 2 HCl(aq) Pb. Cl 2(s) + 2 HC 2 H 3 O 2(aq) Pb+2(aq) + 2 Cl-(aq) Pb. Cl 2(s) (NH 4)2 S(aq) + Co(NO 3)2(aq) Co. S(s) + NH 4 NO 3(aq) (NH 4)2 S(aq) + Co(NO 3)2(aq) Co. S(s) + 2 NH 4 NO 3(aq) Co+2(aq) + S-2(aq) Co. S(s)

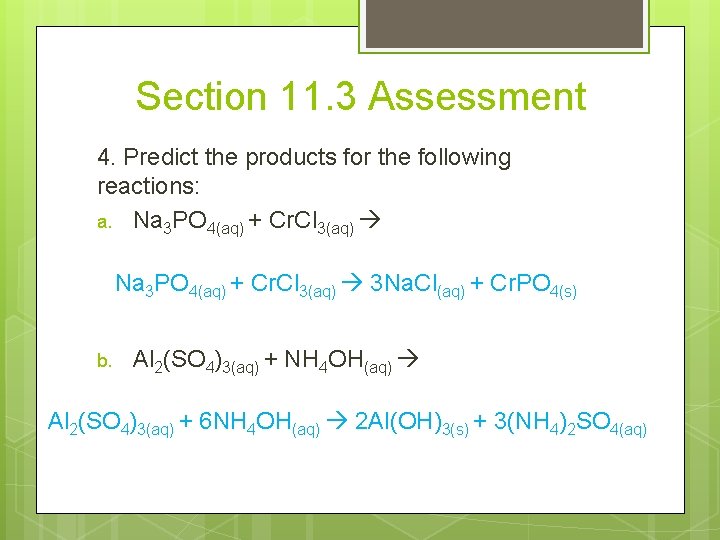
Section 11. 3 Assessment 4. Predict the products for the following reactions: a. Na 3 PO 4(aq) + Cr. Cl 3(aq) 3 Na. Cl(aq) + Cr. PO 4(s) b. Al 2(SO 4)3(aq) + NH 4 OH(aq) Al 2(SO 4)3(aq) + 6 NH 4 OH(aq) 2 Al(OH)3(s) + 3(NH 4)2 SO 4(aq)

THE END!!