Chapter 11 Chemical Reactions Activities and resources Writing





















- Slides: 29

Chapter 11 Chemical Reactions Activities and resources • Writing Chemical equations • Balancing Equations • Classifying reactions • Predicting the products • Reactions in aqueous solutions (complete and net ionic equations) • Predicting the formation of a precipitate Lab –chemical reactions Workshops TB & Workbooks Animations and games for balancing equations and types of reactions: http: //funbasedlearning. com/che mistry/chem. Balancer/ques 5. h tm http: //www. youtube. com/watch? v=i. HHvx 1 VC_8&NR=1 http: //www. youtube. com/watch? v=t. E 4668 aarck&feature=related

11. 1 Describing Chemical Reactions > Writing Chemical Equations Word Equations To write a word equation, write the names of the reactants to the left of the arrow separated by plus signs; write the names of the products to the right of the arrow, also separated by plus signs. Reactant + Reactant Product + Product Slide 2 of 37 © Copyright Pearson Prentice Hall End Show

11. 1 Describing Chemical Reactions > Writing Chemical Equations Methane + Oxygen Carbon dioxide + Water (the reaction between methane and oxygen can be described as a combustion reaction. ) Slide 3 of 37 © Copyright Pearson Prentice Hall End Show

11. 1 Describing Chemical Reactions > Writing Chemical Equations iron + oxygen iron(III) oxide (the reaction between Fe and O 2 to form Fe 2 O 3 can be described as a combination or synthesis reaction) Slide 4 of 37 © Copyright Pearson Prentice Hall End Show

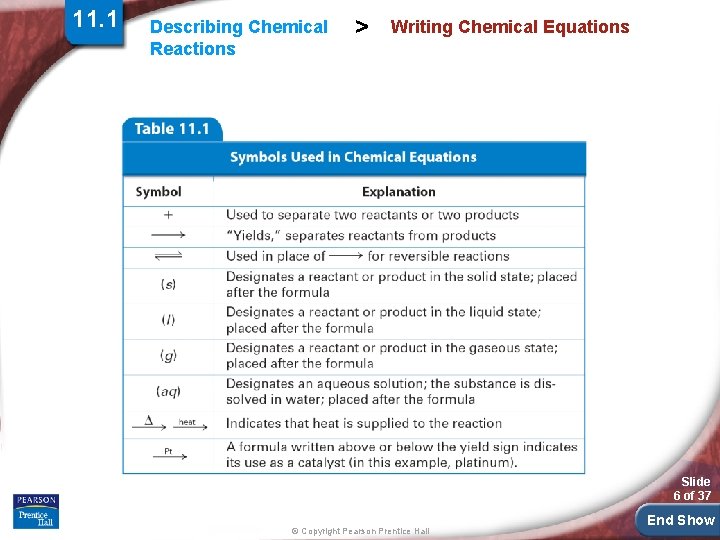
11. 1 Describing Chemical Reactions > Writing Chemical Equations A chemical equation is a representation of a chemical reaction; the formulas of the reactants (on the left) are connected by an arrow with the formulas of the products (on the right). Slide 5 of 37 © Copyright Pearson Prentice Hall End Show

11. 1 Describing Chemical Reactions > Writing Chemical Equations Slide 6 of 37 © Copyright Pearson Prentice Hall End Show

11. 1 Describing Chemical Reactions > Writing Chemical Equations A skeleton equation is a chemical equation that does not indicate the relative amounts of the reactants and products. (i. e. the equation is not yet balanced) Here is the equation for rusting: Fe + O 2 Fe 2 O 3 How would you balance this equation? Slide 7 of 37 © Copyright Pearson Prentice Hall End Show

11. 1 Describing Chemical Reactions > Writing Chemical Equations A catalyst is a substance that speeds up the reaction but is not used up in the reaction. Without Catalyst With Catalyst Slide 8 of 37 © Copyright Pearson Prentice Hall End Show

Slide 9 of 37 © Copyright Pearson Prentice Hall End Show

Slide 10 of 37 © Copyright Pearson Prentice Hall End Show

Slide 11 of 37 © Copyright Pearson Prentice Hall End Show

Practice Problems for Conceptual Problem 11. 1 Slide 12 of 37 © Copyright Pearson Prentice Hall End Show

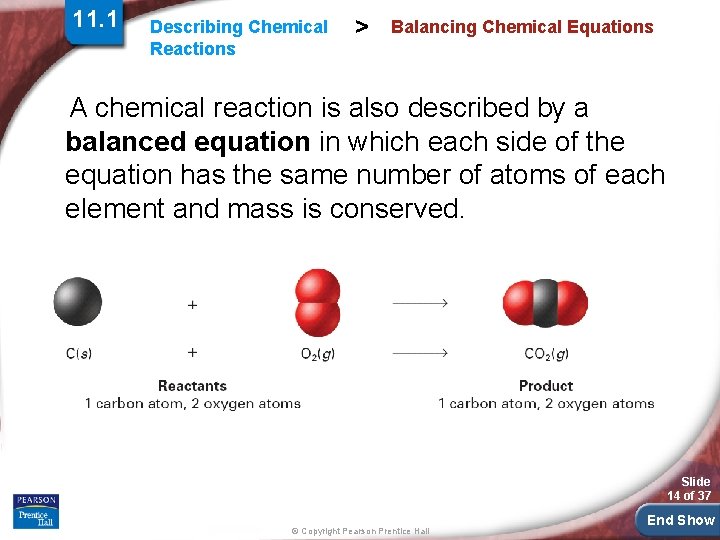
11. 1 Describing Chemical Reactions > Balancing Chemical Equations What are the steps in writing a balanced chemical equation? To write a balanced chemical equation, first write the skeleton equation. Then use coefficients to balance the equation so that it obeys the law of conservation Slide of mass. 13 of 37 © Copyright Pearson Prentice Hall End Show

11. 1 Describing Chemical Reactions > Balancing Chemical Equations A chemical reaction is also described by a balanced equation in which each side of the equation has the same number of atoms of each element and mass is conserved. Slide 14 of 37 © Copyright Pearson Prentice Hall End Show

Slide 15 of 37 © Copyright Pearson Prentice Hall End Show

Slide 16 of 37 © Copyright Pearson Prentice Hall End Show

Slide 17 of 37 © Copyright Pearson Prentice Hall End Show

Slide 18 of 37 © Copyright Pearson Prentice Hall End Show

Go to following website for balancing chemical equation game: http: //funbasedlearning. com/chemistry/chem. Balancer/ques 5. htm Complete worksheet (class handout) and turn in for grading Slide 19 of 37 © Copyright Pearson Prentice Hall End Show

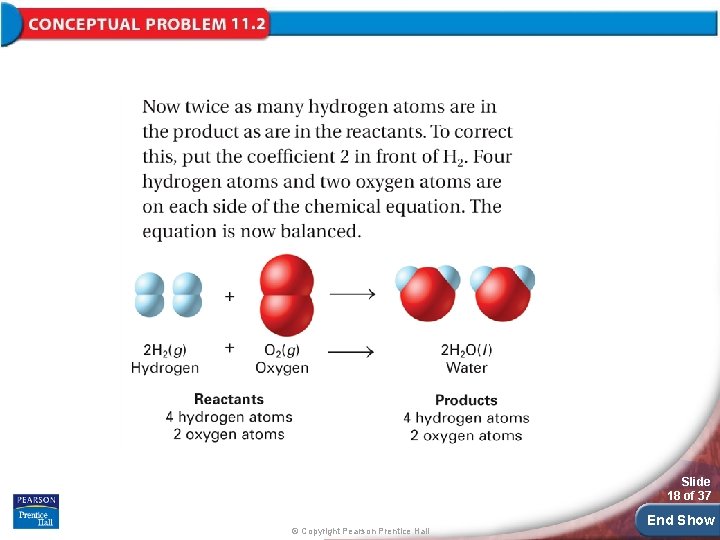
Practice Problems for Conceptual Problem 11. 2 Slide 20 of 37 © Copyright Pearson Prentice Hall End Show

Slide 21 of 37 © Copyright Pearson Prentice Hall End Show

Slide 22 of 37 © Copyright Pearson Prentice Hall End Show

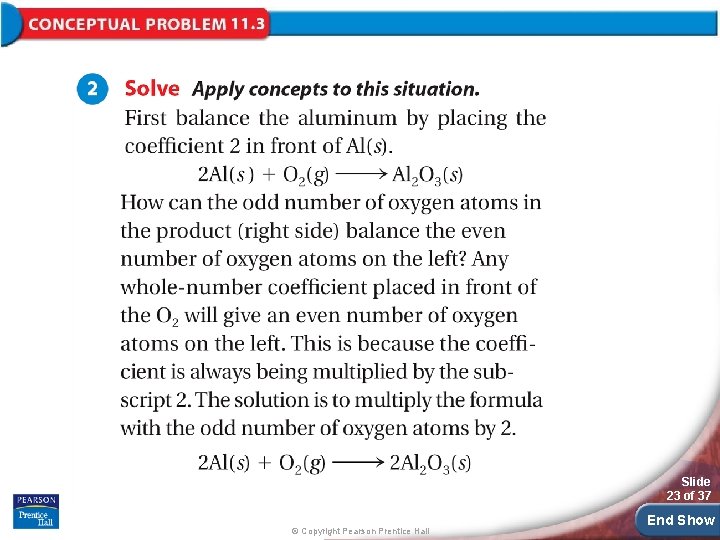
Slide 23 of 37 © Copyright Pearson Prentice Hall End Show

Slide 24 of 37 © Copyright Pearson Prentice Hall End Show

Practice Problems for Conceptual Problem 11. 2 Slide 25 of 37 © Copyright Pearson Prentice Hall End Show

11. 1 Section Quiz. Assess students’ understanding of 11. 1. the concepts in Section Continue to: -or- Launch: Section Quiz Slide 26 of 37 © Copyright Pearson Prentice Hall End Show

11. 1 Section Quiz. 1. Propane gas reacts with oxygen to produce water vapor and carbon dioxide. Choose the correct word equation for this reaction. a. propane + carbon dioxide water + oxygen b. propane + oxygen + water carbon dioxide c. propane + oxygen + water + carbon dioxide d. propane + oxygen water + carbon dioxide Slide 27 of 37 © Copyright Pearson Prentice Hall End Show

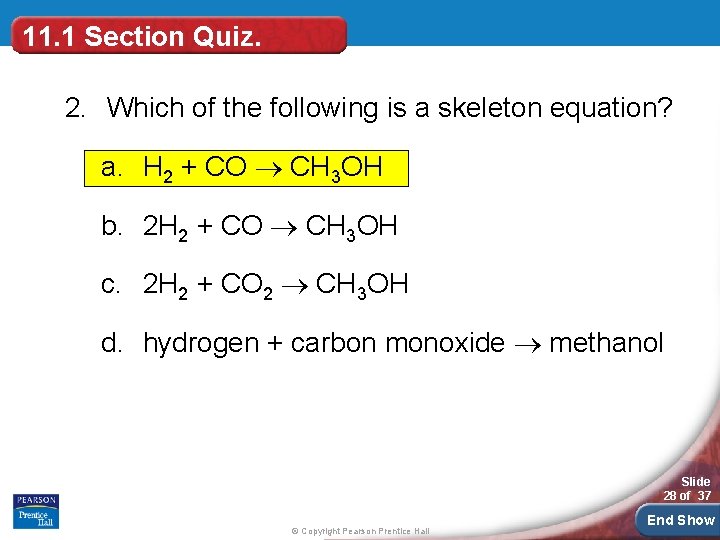
11. 1 Section Quiz. 2. Which of the following is a skeleton equation? a. H 2 + CO CH 3 OH b. 2 H 2 + CO CH 3 OH c. 2 H 2 + CO 2 CH 3 OH d. hydrogen + carbon monoxide methanol Slide 28 of 37 © Copyright Pearson Prentice Hall End Show

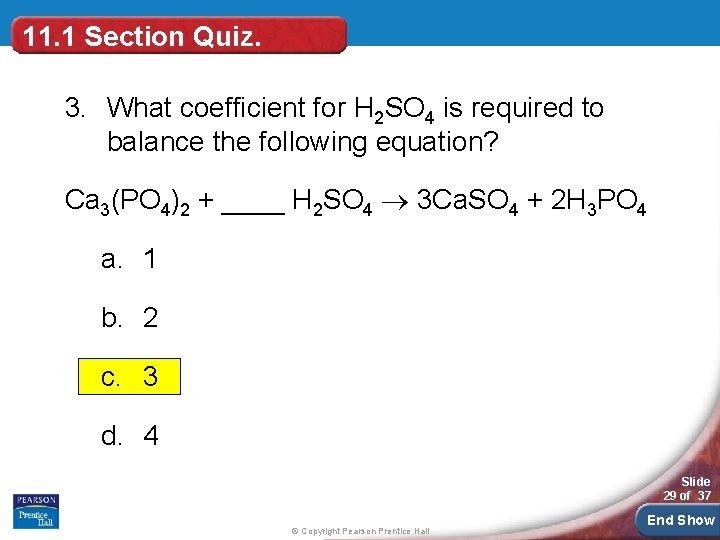
11. 1 Section Quiz. 3. What coefficient for H 2 SO 4 is required to balance the following equation? Ca 3(PO 4)2 + ____ H 2 SO 4 3 Ca. SO 4 + 2 H 3 PO 4 a. 1 b. 2 c. 3 d. 4 Slide 29 of 37 © Copyright Pearson Prentice Hall End Show