Chapter 11 Chemical Equilibrium 11 1 The Equilibrium

Chapter 11 Chemical Equilibrium 11. 1 The Equilibrium Condition 11. 2 The Equilibrium Constant 11. 3 Equilibrium Expressions Involving Pressures 11. 4 The Concept of Activity 11. 5 Heterogeneous Equilibria 11. 6 Applications of the Equilibrium Constant 11. 7 Solving Equilibrium Problems 11. 8 Le Chatelier's Principle 11. 9 Equilibria Involving Real Gases
The Equilibrium Condition (General) Thermal equilibrium indicates two systems in thermal contact with each do not exchange energy by heat. If two bricks are in thermal equilibrium their temperatures are the same. Chemical equilibrium indicates no unbalanced potentials (or driving force). A system in equilibrium experiences no change over time, even infinite time. The opposite of equilibrium systems are non-equilibrium systems that are off balance and change with time. Example 1 atm O 2 + 2 atm H 2 at 298 K
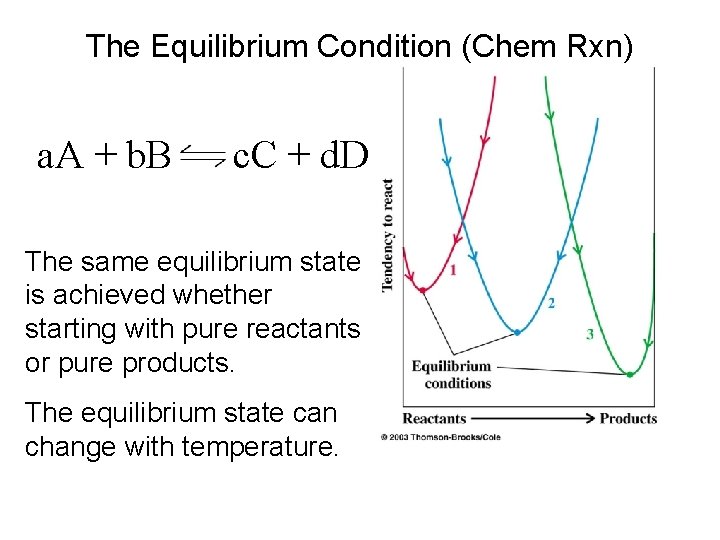
The Equilibrium Condition (Chem Rxn) a. A + b. B c. C + d. D The same equilibrium state is achieved whether starting with pure reactants or pure products. The equilibrium state can change with temperature.
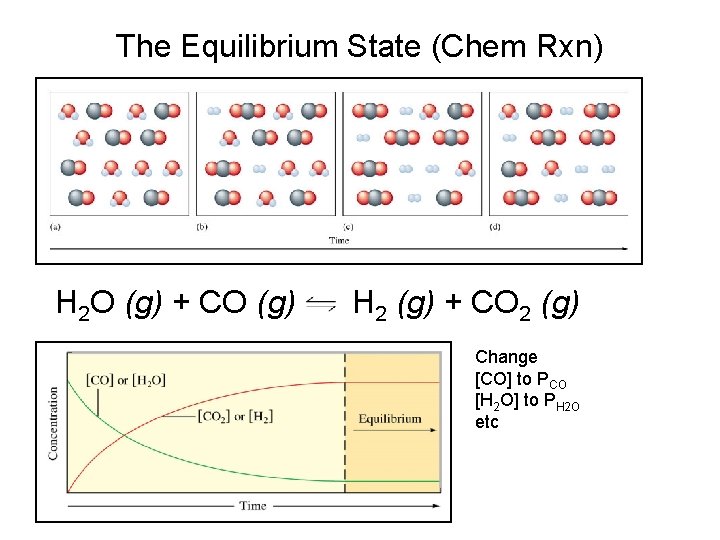
The Equilibrium State (Chem Rxn) H 2 O (g) + CO (g) H 2 (g) + CO 2 (g) Change [CO] to PCO [H 2 O] to PH 2 O etc
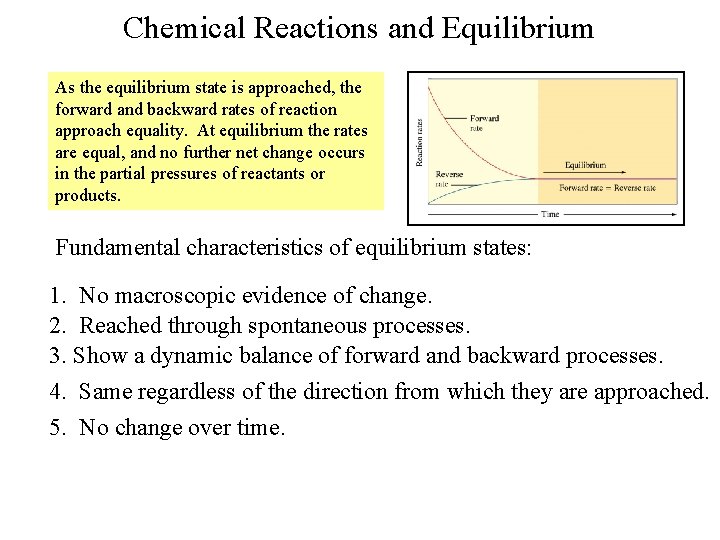
Chemical Reactions and Equilibrium As the equilibrium state is approached, the forward and backward rates of reaction approach equality. At equilibrium the rates are equal, and no further net change occurs in the partial pressures of reactants or products. Fundamental characteristics of equilibrium states: 1. No macroscopic evidence of change. 2. Reached through spontaneous processes. 3. Show a dynamic balance of forward and backward processes. 4. Same regardless of the direction from which they are approached. 5. No change over time.
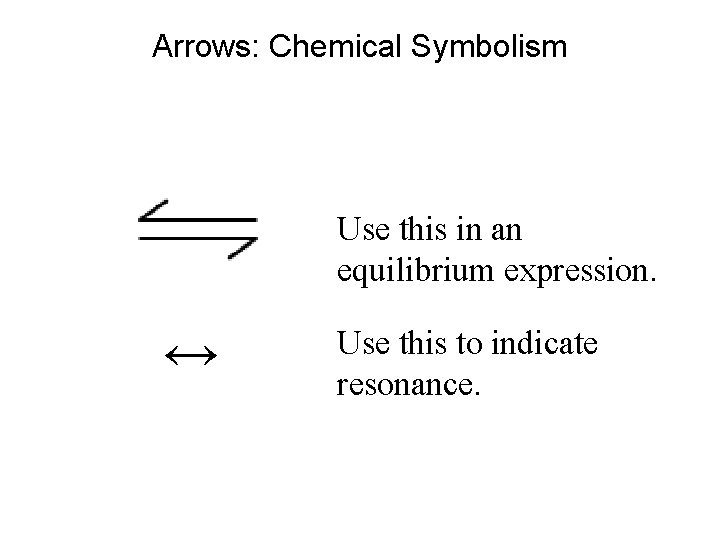
Arrows: Chemical Symbolism Use this in an equilibrium expression. ↔ Use this to indicate resonance.
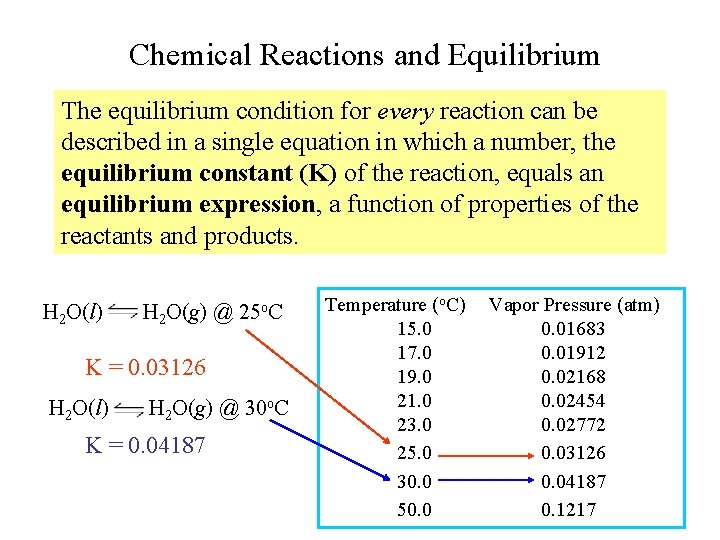
Chemical Reactions and Equilibrium The equilibrium condition for every reaction can be described in a single equation in which a number, the equilibrium constant (K) of the reaction, equals an equilibrium expression, a function of properties of the reactants and products. H 2 O(l) H 2 O(g) @ 25 o. C K = 0. 03126 H 2 O(l) H 2 O(g) @ 30 o. C K = 0. 04187 Temperature (o. C) 15. 0 17. 0 19. 0 21. 0 23. 0 25. 0 30. 0 50. 0 Vapor Pressure (atm) 0. 01683 0. 01912 0. 02168 0. 02454 0. 02772 0. 03126 0. 04187 0. 1217
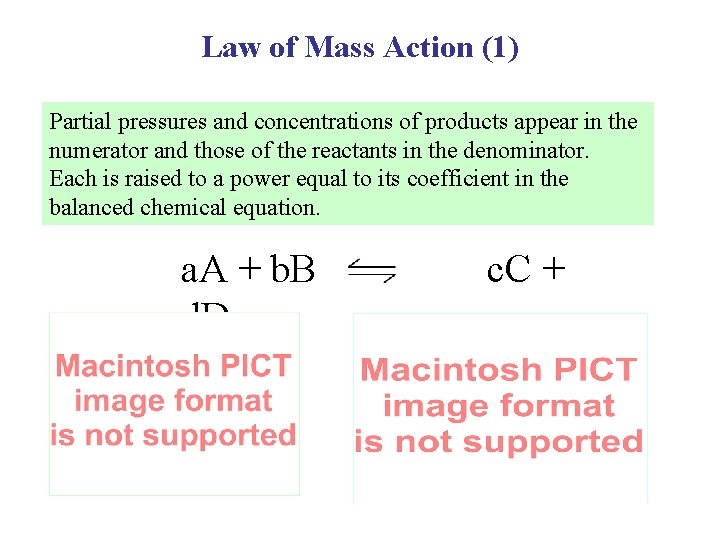
Law of Mass Action (1) Partial pressures and concentrations of products appear in the numerator and those of the reactants in the denominator. Each is raised to a power equal to its coefficient in the balanced chemical equation. a. A + b. B d. D c. C +
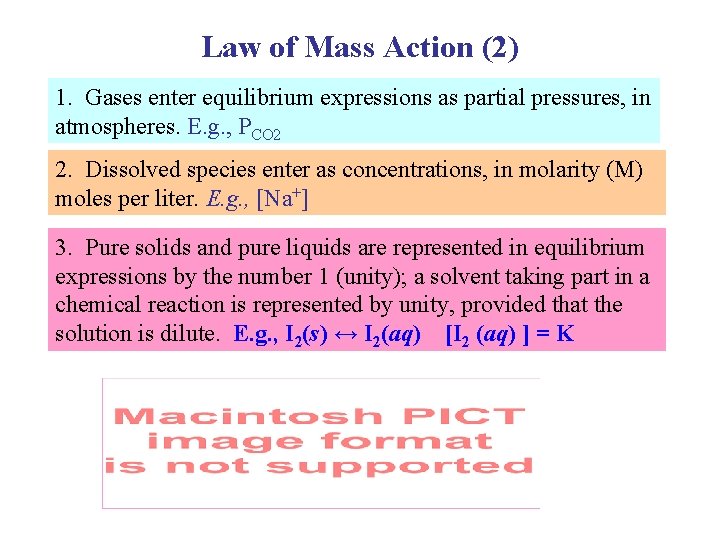
Law of Mass Action (2) 1. Gases enter equilibrium expressions as partial pressures, in atmospheres. E. g. , PCO 2 2. Dissolved species enter as concentrations, in molarity (M) moles per liter. E. g. , [Na+] 3. Pure solids and pure liquids are represented in equilibrium expressions by the number 1 (unity); a solvent taking part in a chemical reaction is represented by unity, provided that the solution is dilute. E. g. , I 2(s) ↔ I 2(aq) [I 2 (aq) ] = K
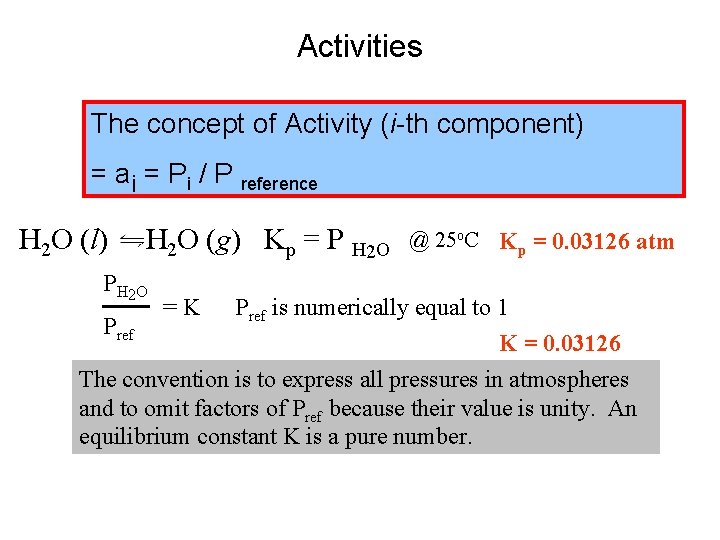
Activities The concept of Activity (i-th component) = ai = Pi / P reference H 2 O (l) H 2 O (g) Kp = P H 2 O PH 2 O =K @ 25 o. C Kp = 0. 03126 atm Pref is numerically equal to 1 Pref K = 0. 03126 The convention is to express all pressures in atmospheres and to omit factors of Pref because their value is unity. An equilibrium constant K is a pure number.
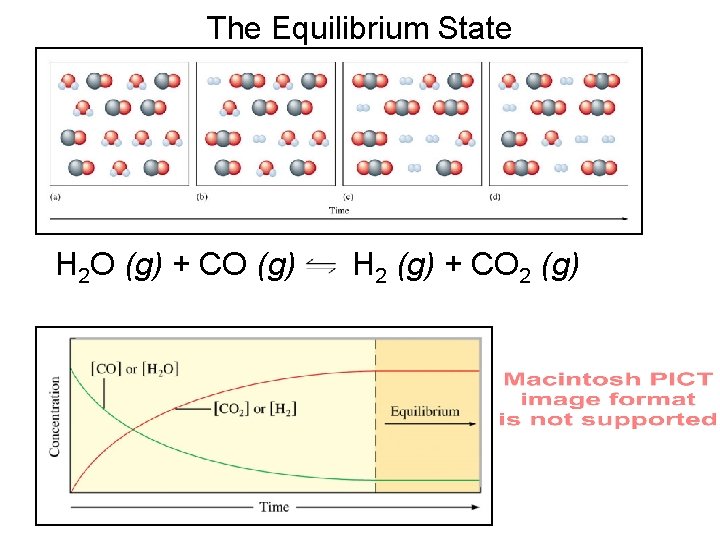
The Equilibrium State H 2 O (g) + CO (g) H 2 (g) + CO 2 (g)
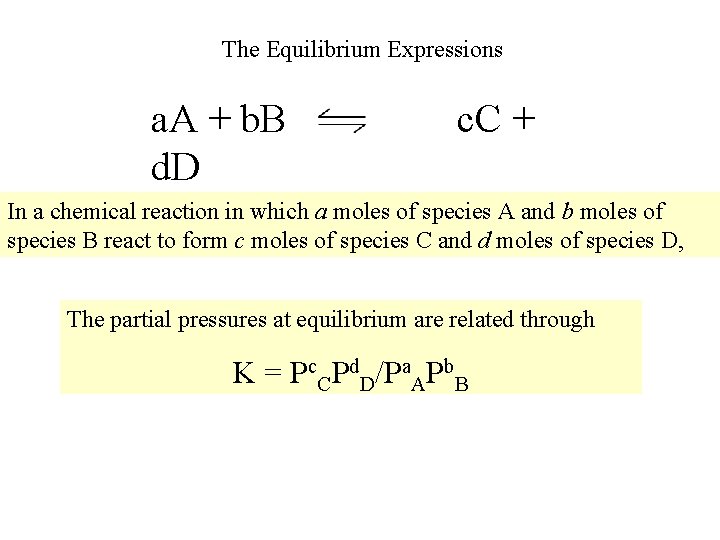
The Equilibrium Expressions a. A + b. B d. D c. C + In a chemical reaction in which a moles of species A and b moles of species B react to form c moles of species C and d moles of species D, The partial pressures at equilibrium are related through K = Pc. CPd. D/Pa. APb. B
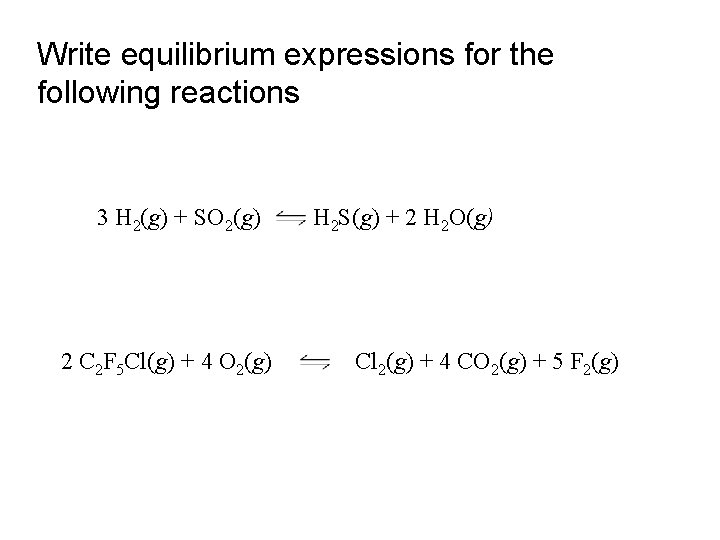
Write equilibrium expressions for the following reactions 3 H 2(g) + SO 2(g) 2 C 2 F 5 Cl(g) + 4 O 2(g) H 2 S(g) + 2 H 2 O(g) Cl 2(g) + 4 CO 2(g) + 5 F 2(g)
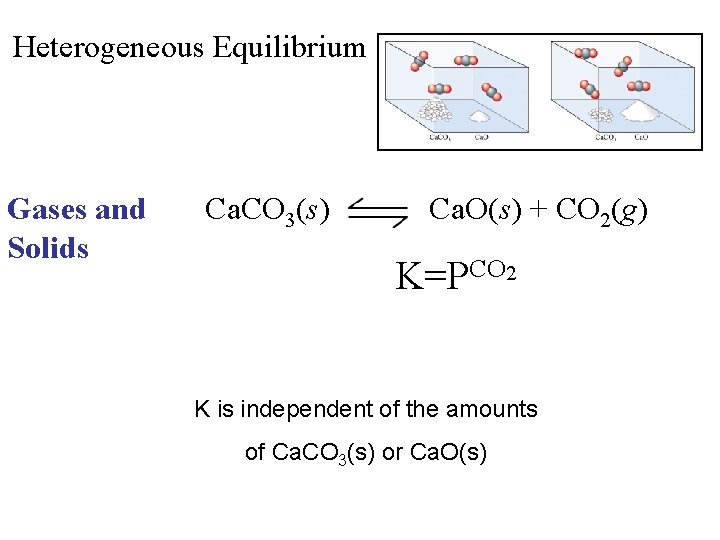
Heterogeneous Equilibrium Gases and Solids Ca. CO 3(s) Ca. O(s) + CO 2(g) K=PCO 2 K is independent of the amounts of Ca. CO 3(s) or Ca. O(s)

Heterogeneous Equilibrium Liquids H 2 O(l) H 2 O(g) K=PH 2 O Solutions I 2(s) I 2(aq) K=[I 2]
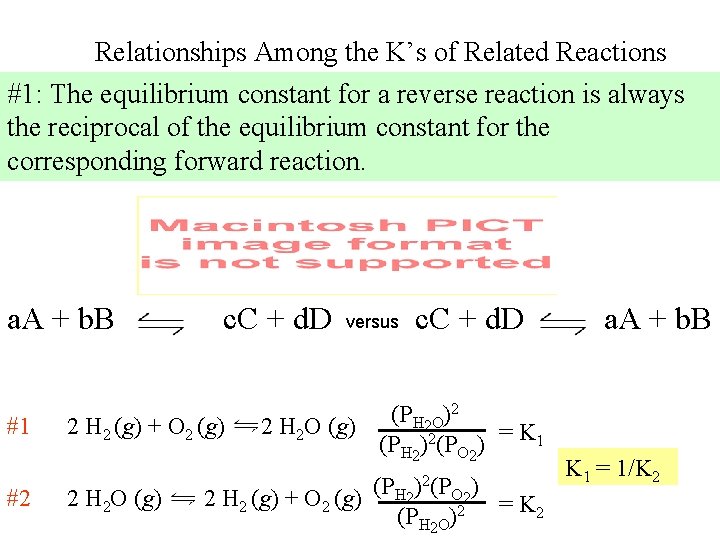
Relationships Among the K’s of Related Reactions #1: The equilibrium constant for a reverse reaction is always the reciprocal of the equilibrium constant for the corresponding forward reaction. a. A + b. B #1 #2 c. C + d. D 2 H 2 (g) + O 2 (g) 2 H 2 O (g) versus c. C + d. D 2 (P ) H O 2 2 H 2 O (g) = K 1 (PH 2)2(PO 2) 2 H 2 (g) + O 2 (g) (PH 2)2(PO 2) (PH 2 O)2 = K 2 a. A + b. B K 1 = 1/K 2
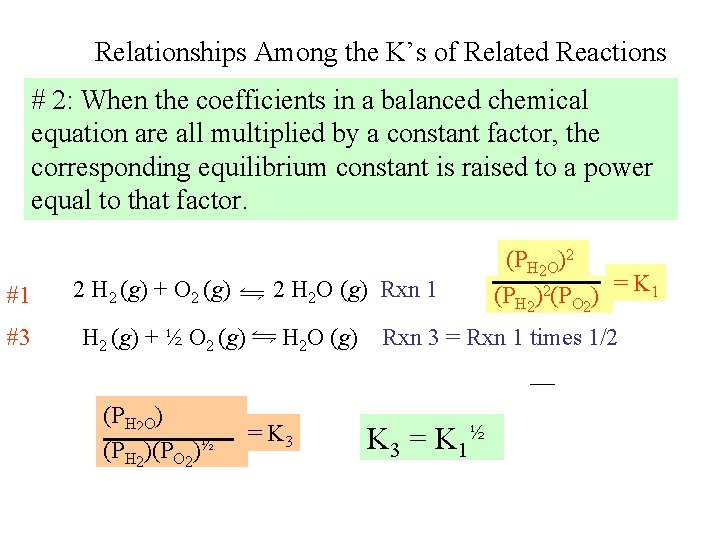
Relationships Among the K’s of Related Reactions # 2: When the coefficients in a balanced chemical equation are all multiplied by a constant factor, the corresponding equilibrium constant is raised to a power equal to that factor. #1 #3 2 H 2 (g) + O 2 (g) H 2 (g) + ½ O 2 (g) (PH 2 O) (PH 2)(PO 2)½ 2 H 2 O (g) Rxn 1 H 2 O (g) = K 3 (PH 2 O)2 = K 1 (PH 2)2(PO 2) Rxn 3 = Rxn 1 times 1/2 K 3 = K 1½
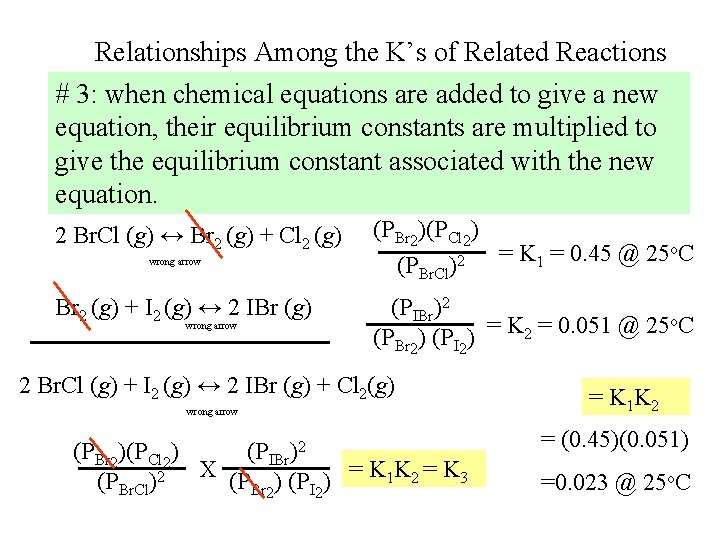
Relationships Among the K’s of Related Reactions # 3: when chemical equations are added to give a new equation, their equilibrium constants are multiplied to give the equilibrium constant associated with the new equation. 2 Br. Cl (g) ↔ Br 2 (g) + Cl 2 (g) (PBr 2)(PCl 2) (PBr. Cl wrong arrow Br 2 (g) + I 2 (g) ↔ 2 IBr (g) wrong arrow )2 = K 1 = 0. 45 @ 25 o. C (PIBr)2 = K 2 = 0. 051 @ 25 o. C (PBr 2) (PI 2) 2 Br. Cl (g) + I 2 (g) ↔ 2 IBr (g) + Cl 2(g) wrong arrow (PBr 2)(PCl 2) (PIBr)2 (PBr. Cl)2 X (PBr 2) (PI 2) = K 1 K 2 = K 3 = K 1 K 2 = (0. 45)(0. 051) =0. 023 @ 25 o. C
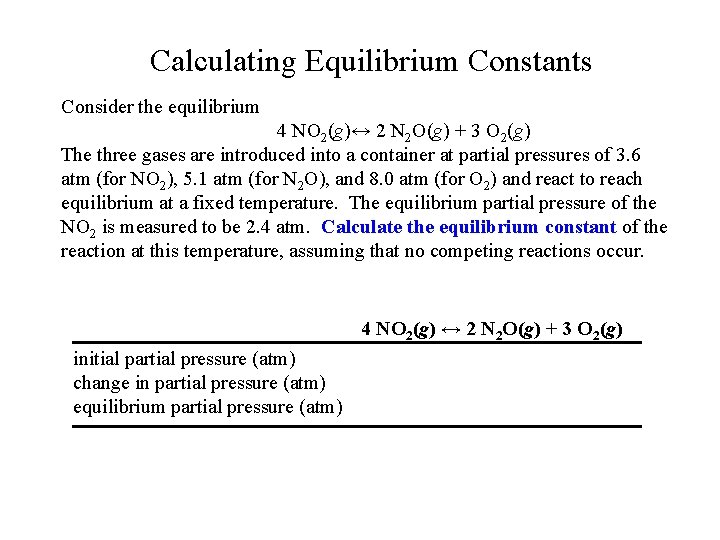
Calculating Equilibrium Constants Consider the equilibrium 4 NO 2(g)↔ 2 N 2 O(g) + 3 O 2(g) The three gases are introduced into a container at partial pressures of 3. 6 atm (for NO 2), 5. 1 atm (for N 2 O), and 8. 0 atm (for O 2) and react to reach equilibrium at a fixed temperature. The equilibrium partial pressure of the NO 2 is measured to be 2. 4 atm. Calculate the equilibrium constant of the reaction at this temperature, assuming that no competing reactions occur. 4 NO 2(g) ↔ 2 N 2 O(g) + 3 O 2(g) initial partial pressure (atm) change in partial pressure (atm) equilibrium partial pressure (atm)
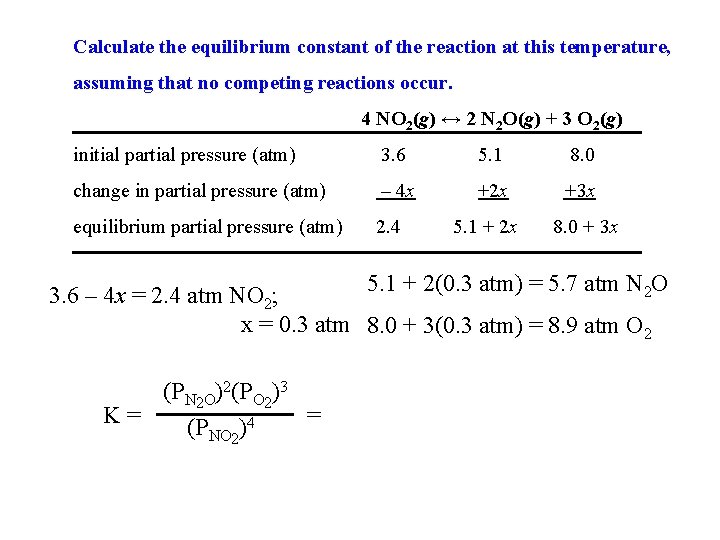
Calculate the equilibrium constant of the reaction at this temperature, assuming that no competing reactions occur. 4 NO 2(g) ↔ 2 N 2 O(g) + 3 O 2(g) initial partial pressure (atm) 3. 6 5. 1 8. 0 change in partial pressure (atm) – 4 x +2 x +3 x equilibrium partial pressure (atm) 2. 4 5. 1 + 2 x 8. 0 + 3 x 5. 1 + 2(0. 3 atm) = 5. 7 atm N 2 O 3. 6 – 4 x = 2. 4 atm NO 2; x = 0. 3 atm 8. 0 + 3(0. 3 atm) = 8. 9 atm O 2 (PN 2 O)2(PO 2)3 K= = (PNO 2)4
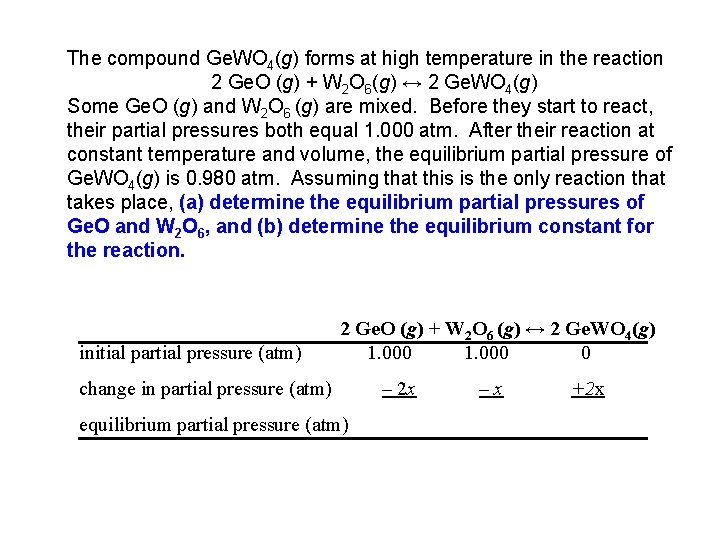
The compound Ge. WO 4(g) forms at high temperature in the reaction 2 Ge. O (g) + W 2 O 6(g) ↔ 2 Ge. WO 4(g) Some Ge. O (g) and W 2 O 6 (g) are mixed. Before they start to react, their partial pressures both equal 1. 000 atm. After their reaction at constant temperature and volume, the equilibrium partial pressure of Ge. WO 4(g) is 0. 980 atm. Assuming that this is the only reaction that takes place, (a) determine the equilibrium partial pressures of Ge. O and W 2 O 6, and (b) determine the equilibrium constant for the reaction. initial partial pressure (atm) 2 Ge. O (g) + W 2 O 6 (g) ↔ 2 Ge. WO 4(g) 1. 000 0 change in partial pressure (atm) equilibrium partial pressure (atm) – 2 x –x +2 x
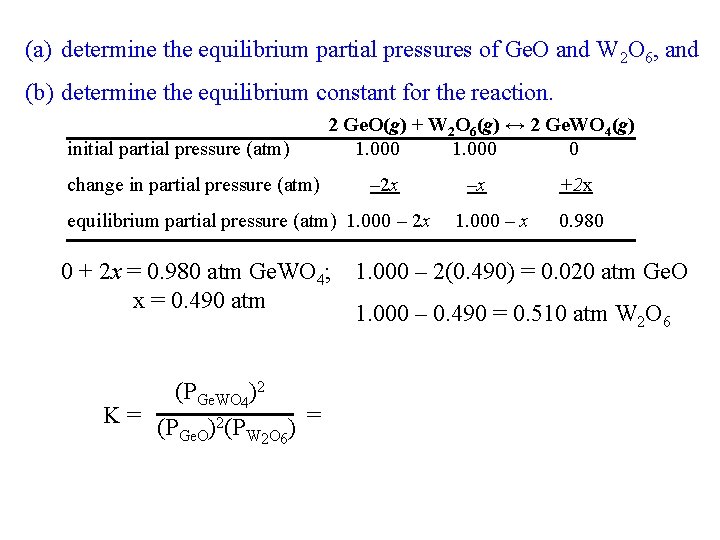
(a) determine the equilibrium partial pressures of Ge. O and W 2 O 6, and (b) determine the equilibrium constant for the reaction. initial partial pressure (atm) change in partial pressure (atm) 2 Ge. O(g) + W 2 O 6(g) ↔ 2 Ge. WO 4(g) 1. 000 0 – 2 x equilibrium partial pressure (atm) 1. 000 – 2 x –x 1. 000 – x +2 x 0. 980 0 + 2 x = 0. 980 atm Ge. WO 4; 1. 000 – 2(0. 490) = 0. 020 atm Ge. O x = 0. 490 atm 1. 000 – 0. 490 = 0. 510 atm W 2 O 6 (PGe. WO 4)2 K = (P )2(P = ) Ge. O W 2 O 6

• Skip Solving quadratic equations • Will utilize approximation method – Systems that have small equilibrium constants. – Assume “x” (the change in concentration) is small (less than 5%) of the initial concentration.

A vessel holds pure CO (g) at a pressure of 1. 282 atm and a temperature of 354 K. A quantity of nickel is added, and the partial pressure of CO (g) drops to an equilibrium value of 0. 709 atm because of the reaction Ni (s) + 4 CO (g) ↔ Ni(CO)4 (g) Compute the equilibrium constant for this reaction at 354 K. Ni (s) + 4 CO (g) ↔ Ni(CO)4 (g) Construct an “ICE” table initial partial pressure (atm) change in partial pressure (atm) equilibrium partial pressure (atm) At equil. Pco= P CO (atm) P Ni(CO)4 (atm) 1. 282 -4 x 0. 709 0 +1 x x x = PNi(CO)4 =

Equilibrium Calculations At a particular temperature, K = 2. 0 x 10 -6 mol/L for the reaction 2 CO 2 (g) 2 CO (g) + O 2 (g) If 2. 0 mol CO 2 is initially placed into a 5. 0 -L vessel, calculate the equilibrium concentrations of all species. 2 CO 2 (g) initial partial pressure (mol/L) 0. 4 change in partial pressure (mol/L) – 2 x equilibrium partial pressure (mol/L) 0. 4 -2 x 2 CO (g) + O 2 (g) 0 +2 x 2 x 0 +1 x 1 x
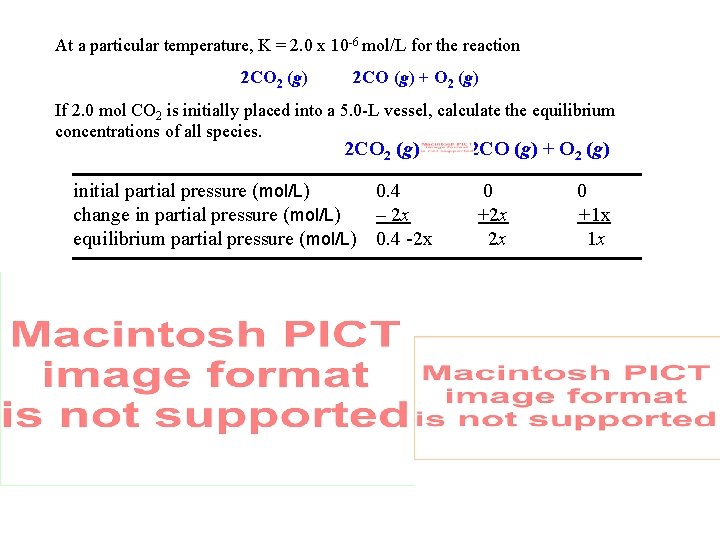
At a particular temperature, K = 2. 0 x 10 -6 mol/L for the reaction 2 CO 2 (g) 2 CO (g) + O 2 (g) If 2. 0 mol CO 2 is initially placed into a 5. 0 -L vessel, calculate the equilibrium concentrations of all species. 2 CO 2 (g) initial partial pressure (mol/L) 0. 4 change in partial pressure (mol/L) – 2 x equilibrium partial pressure (mol/L) 0. 4 -2 x 2 CO (g) + O 2 (g) 0 +2 x 2 x 0 +1 x 1 x
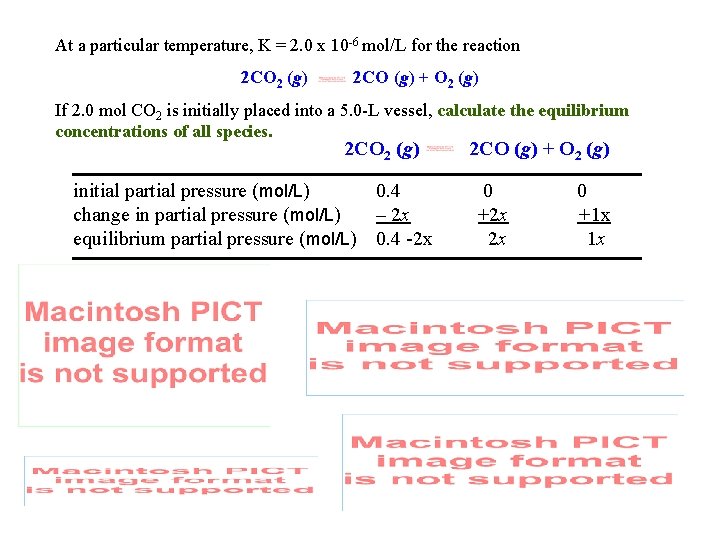
At a particular temperature, K = 2. 0 x 10 -6 mol/L for the reaction 2 CO 2 (g) 2 CO (g) + O 2 (g) If 2. 0 mol CO 2 is initially placed into a 5. 0 -L vessel, calculate the equilibrium concentrations of all species. 2 CO 2 (g) initial partial pressure (mol/L) 0. 4 change in partial pressure (mol/L) – 2 x equilibrium partial pressure (mol/L) 0. 4 -2 x 2 CO (g) + O 2 (g) 0 +2 x 2 x 0 +1 x 1 x
Non-Equilibrium Conditions: The Reaction Quotient (1) wrong arrow K (the Equilibrium Constant) uses equilibrium partial pressures Q (the reaction quotient) uses prevailing partial pressures, not necessarily at equilibrium
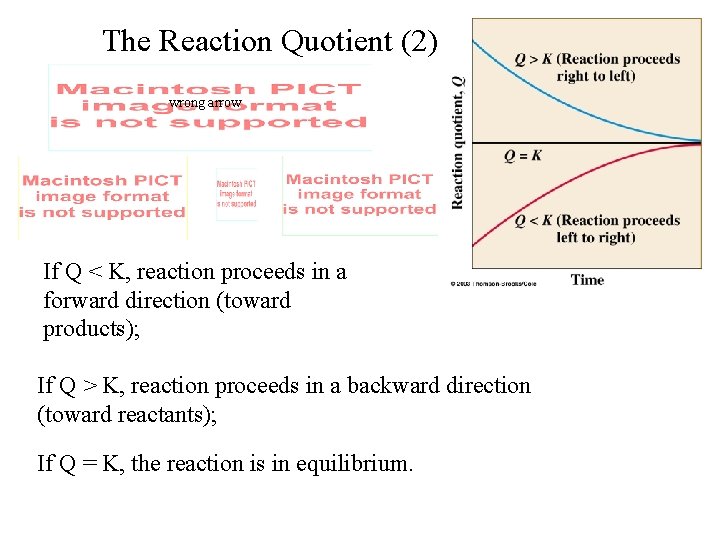
The Reaction Quotient (2) wrong arrow If Q < K, reaction proceeds in a forward direction (toward products); If Q > K, reaction proceeds in a backward direction (toward reactants); If Q = K, the reaction is in equilibrium.
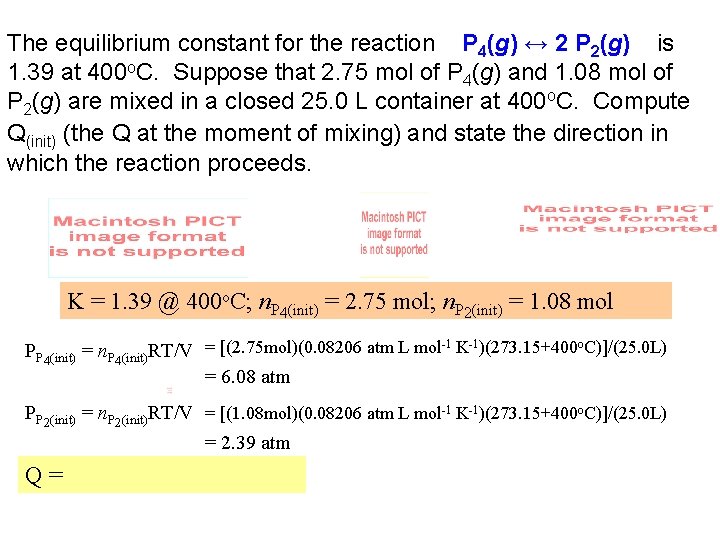
The equilibrium constant for the reaction P 4(g) ↔ 2 P 2(g) is 1. 39 at 400 o. C. Suppose that 2. 75 mol of P 4(g) and 1. 08 mol of P 2(g) are mixed in a closed 25. 0 L container at 400 o. C. Compute Q(init) (the Q at the moment of mixing) and state the direction in which the reaction proceeds. K = 1. 39 @ 400 o. C; n. P 4(init) = 2. 75 mol; n. P 2(init) = 1. 08 mol PP 4(init) = n. P 4(init)RT/V = [(2. 75 mol)(0. 08206 atm L mol-1 K-1)(273. 15+400 o. C)]/(25. 0 L) = 6. 08 atm PP 2(init) = n. P 2(init)RT/V = [(1. 08 mol)(0. 08206 atm L mol-1 K-1)(273. 15+400 o. C)]/(25. 0 L) = 2. 39 atm Q=

Henri Louis Le Châtelier (1850 -1936) Highlights – 1884 Le Chatelier's Principle: A system in equilibrium that is subjected to a stress reacts in a way that counteracts the stress – If a chemical system at equilibrium experiences a change in concentration, temperature or total pressure the equilibrium will shift in order to minimize that change. – Industrial chemist involved with industrial efficiency and labor-management relations Moments in a Life – Le Chatelier was named "chevalier" (knight) of the Légion d'honneur in 1887, decoration established by Napoléon Bonaparte in 1802.

Effects of External Stresses on Equilibria: Le Châtelier’s Principle A system in equilibrium that is subjected to a stress reacts in a way that counteracts the stress. Le Châtelier’s Principle provides a way to predict the response of an equilibrium system to an external perturbation, such as… 1. Effects of Adding or Removing Reactants or Products 2. Effects of Changing the Volume (or Pressure) of the System 3. Effects of Changing the Temperature
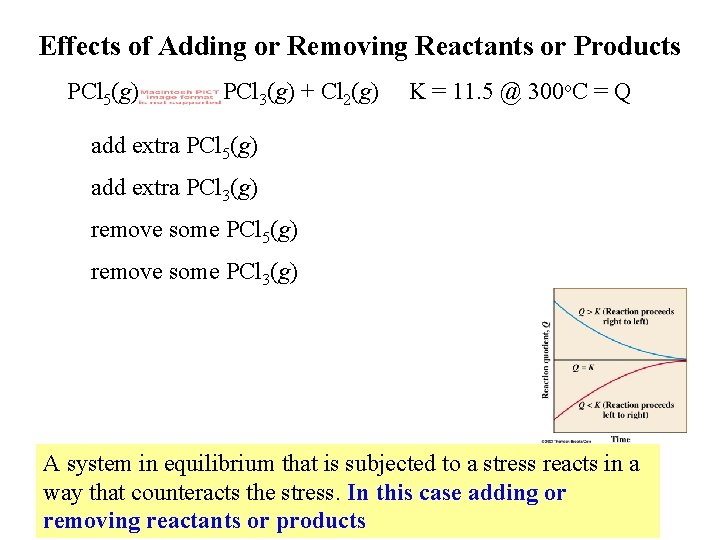
Effects of Adding or Removing Reactants or Products PCl 5(g) PCl 3(g) + Cl 2(g) K = 11. 5 @ 300 o. C = Q add extra PCl 5(g) add extra PCl 3(g) remove some PCl 5(g) remove some PCl 3(g) A system in equilibrium that is subjected to a stress reacts in a way that counteracts the stress. In this case adding or removing reactants or products
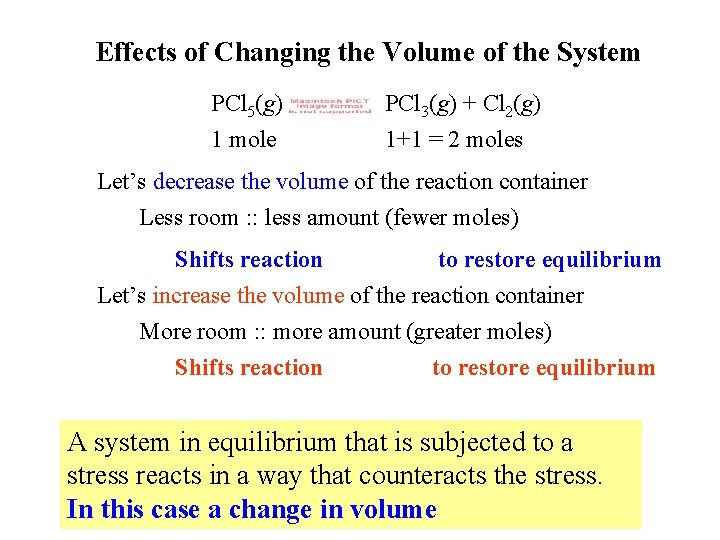
Effects of Changing the Volume of the System PCl 5(g) 1 mole PCl 3(g) + Cl 2(g) 1+1 = 2 moles Let’s decrease the volume of the reaction container Less room : : less amount (fewer moles) Shifts reaction to restore equilibrium Let’s increase the volume of the reaction container More room : : more amount (greater moles) Shifts reaction to restore equilibrium A system in equilibrium that is subjected to a stress reacts in a way that counteracts the stress. In this case a change in volume
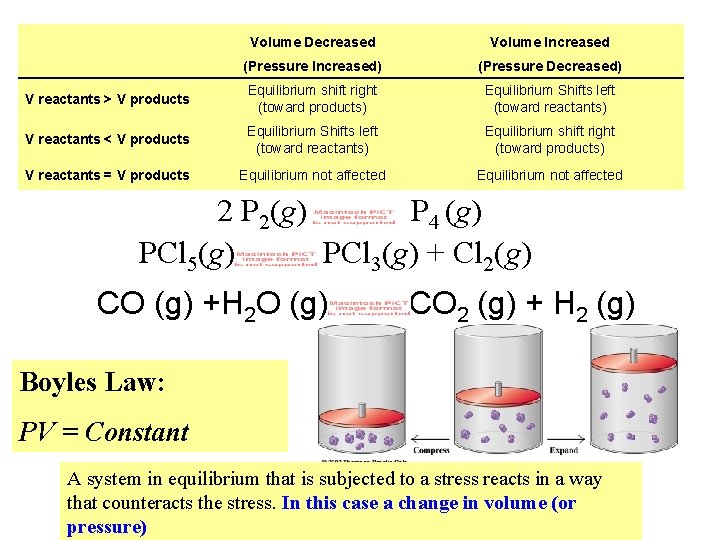
Volume Decreased Volume Increased (Pressure Increased) (Pressure Decreased) V reactants > V products Equilibrium shift right (toward products) Equilibrium Shifts left (toward reactants) V reactants < V products Equilibrium Shifts left (toward reactants) Equilibrium shift right (toward products) V reactants = V products Equilibrium not affected 2 P 2(g) P 4 (g) PCl 5(g) PCl 3(g) + Cl 2(g) CO (g) +H 2 O (g) CO 2 (g) + H 2 (g) Boyles Law: PV = Constant A system in equilibrium that is subjected to a stress reacts in a way that counteracts the stress. In this case a change in volume (or pressure)
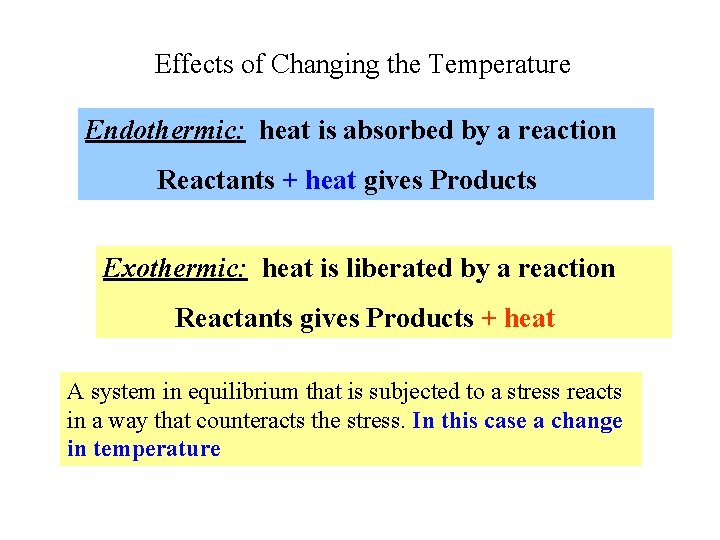
Effects of Changing the Temperature Endothermic: heat is absorbed by a reaction Reactants + heat gives Products Exothermic: heat is liberated by a reaction Reactants gives Products + heat A system in equilibrium that is subjected to a stress reacts in a way that counteracts the stress. In this case a change in temperature
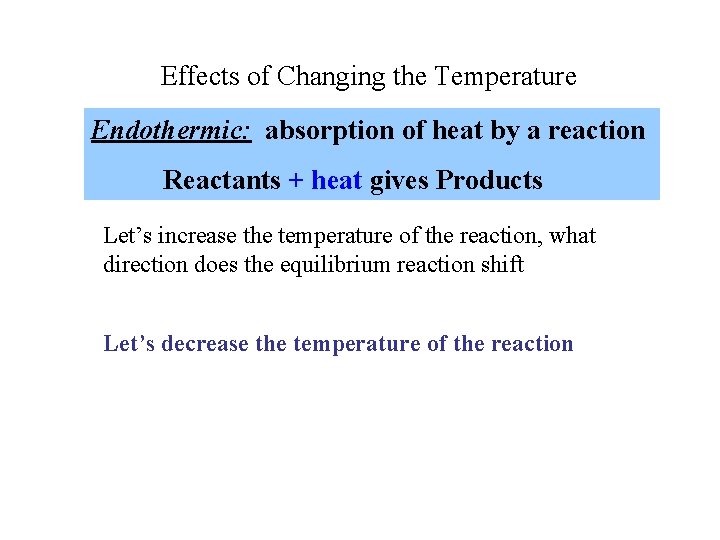
Effects of Changing the Temperature Endothermic: absorption of heat by a reaction Reactants + heat gives Products Let’s increase the temperature of the reaction, what direction does the equilibrium reaction shift Let’s decrease the temperature of the reaction
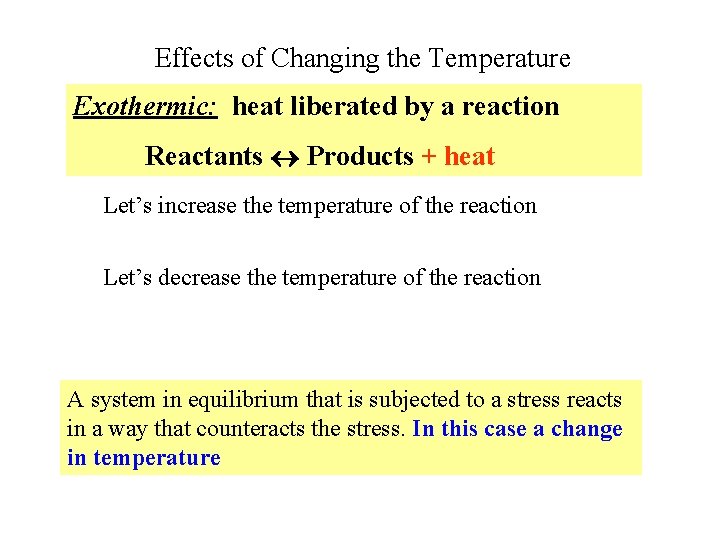
Effects of Changing the Temperature Exothermic: heat liberated by a reaction Reactants Products + heat Let’s increase the temperature of the reaction Let’s decrease the temperature of the reaction A system in equilibrium that is subjected to a stress reacts in a way that counteracts the stress. In this case a change in temperature
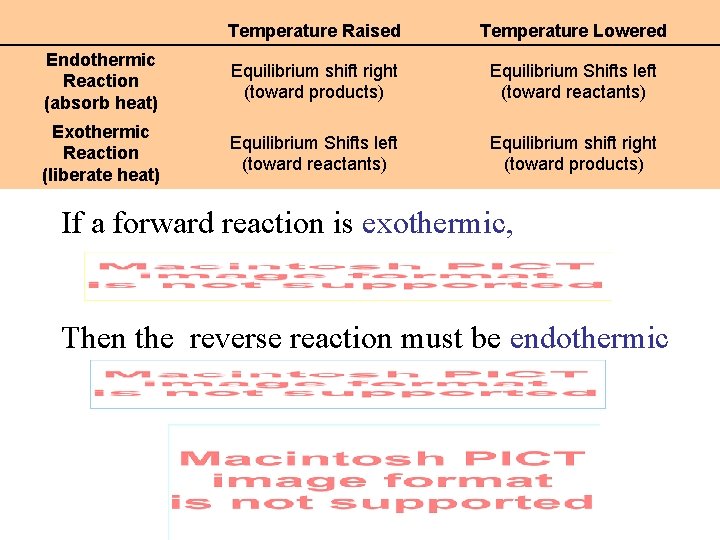
Temperature Raised Temperature Lowered Endothermic Reaction (absorb heat) Equilibrium shift right (toward products) Equilibrium Shifts left (toward reactants) Exothermic Reaction (liberate heat) Equilibrium Shifts left (toward reactants) Equilibrium shift right (toward products) If a forward reaction is exothermic, Then the reverse reaction must be endothermic
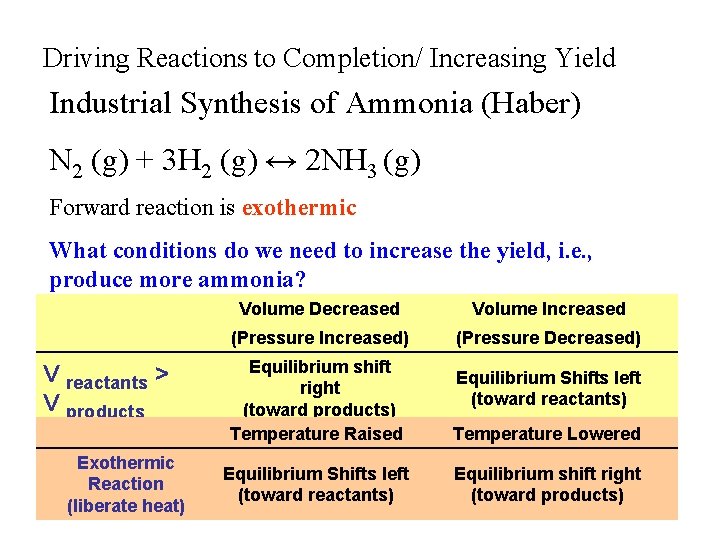
Driving Reactions to Completion/ Increasing Yield Industrial Synthesis of Ammonia (Haber) N 2 (g) + 3 H 2 (g) ↔ 2 NH 3 (g) Forward reaction is exothermic What conditions do we need to increase the yield, i. e. , produce more ammonia? Volume Decreased Volume Increased (Pressure Increased) (Pressure Decreased) V reactants > V products Equilibrium shift right (toward products) Temperature Raised Exothermic Reaction (liberate heat) Equilibrium Shifts left (toward reactants) Temperature Lowered Equilibrium Shifts left Equilibrium shift right (toward reactants) (toward products)
- Slides: 40