Chapter 11 Bovine Clinical Procedures Objectives Set up

Chapter 11 Bovine Clinical Procedures


Objectives • Set up and prepare the patient for each procedure – perform the procedure (when appropriate) – assist the clinician in performing diagnostic sampling and medication procedures. • Insert and maintain an intravenous catheter – monitor the catheter for complications. • rationale and indications for each of the clinical procedures described. • Set up materials and equipment – prepare the patient as needed for the procedure. • Perform or assist in necropsy and sample collection procedures – maintain a safe environment during these procedures.

Reading Assignment • Chapter 11: Bovine Clinical Procedures • Key terms – Balling gun – Drenching – Foot rot – Frick speculum – Hemal processes – Hematoma – Hoof block – Injection site lesion – Mastitis – Ororumen – Rumenocentesis – Suburethral diverticulum – Titillating
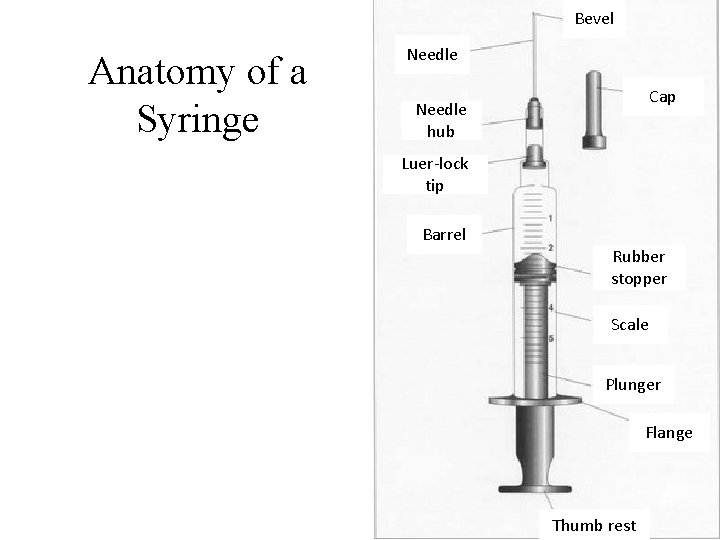
Bevel Anatomy of a Syringe Needle Cap Needle hub Luer-lock tip Barrel Rubber stopper Scale Plunger Flange Thumb rest

Venous Blood Sampling • For state/ diagnostic labs – TB, Brucella, Trichomoniasis • Disease control
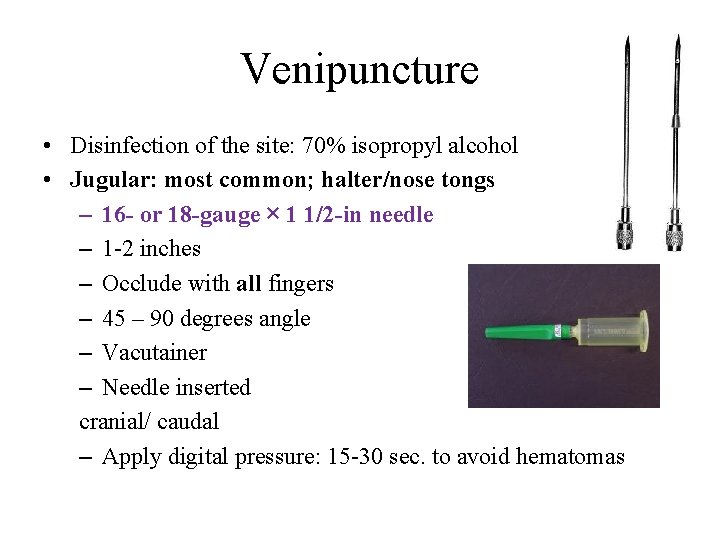
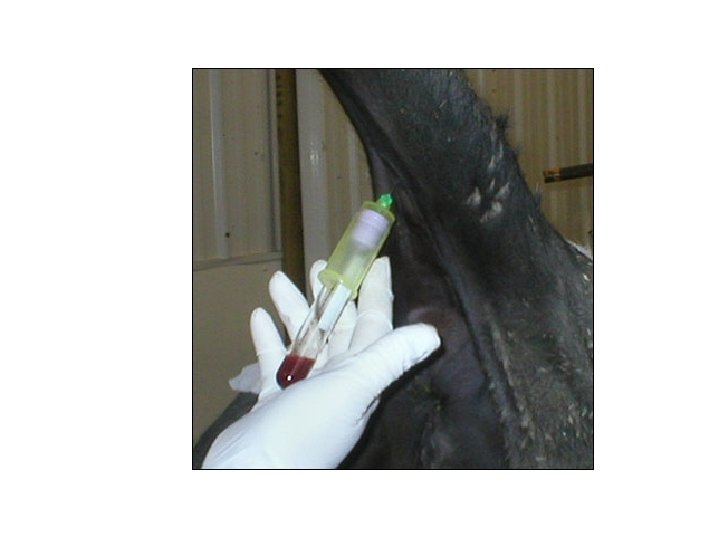
Venipuncture • Disinfection of the site: 70% isopropyl alcohol • Jugular: most common; halter/nose tongs – 16 - or 18 -gauge × 1 1/2 -in needle – 1 -2 inches – Occlude with all fingers – 45 – 90 degrees angle – Vacutainer – Needle inserted cranial/ caudal – Apply digital pressure: 15 -30 sec. to avoid hematomas
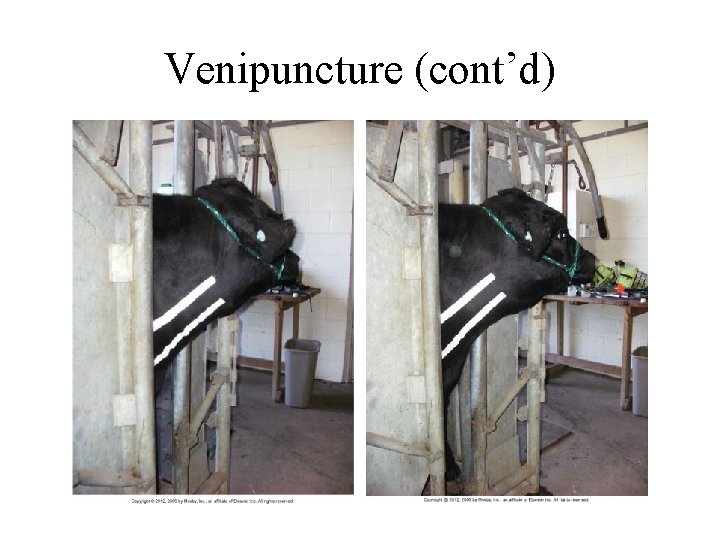
Venipuncture (cont’d)
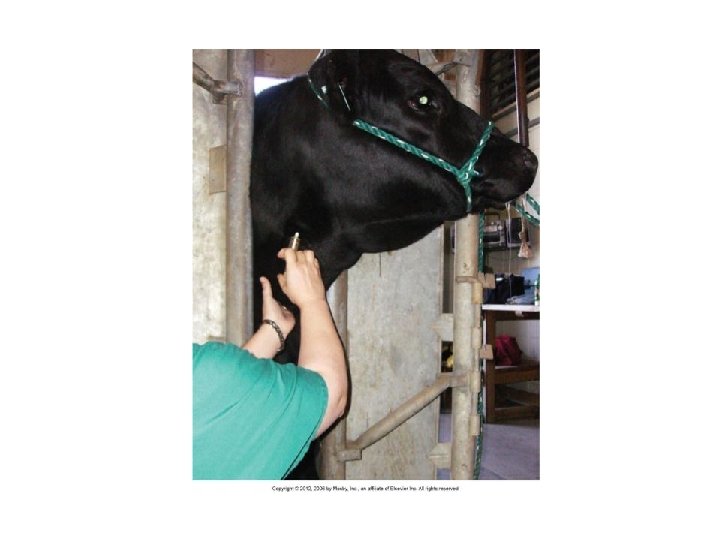
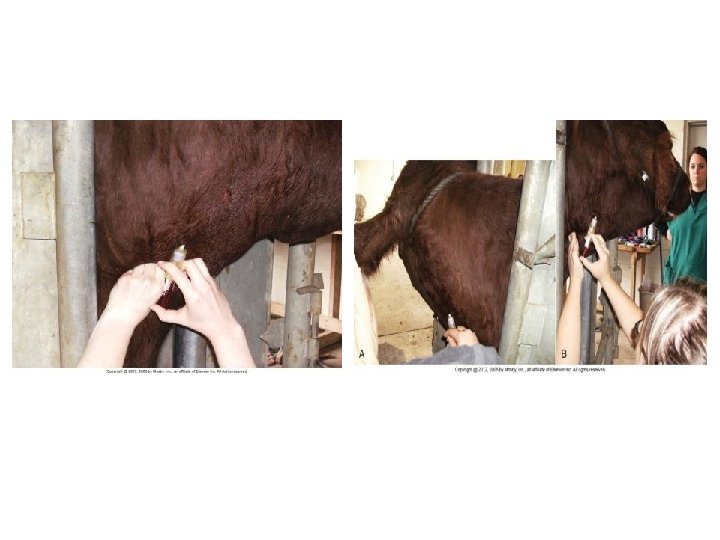
Venipuncture • Tail veins – Animal is more tolerant of tail than jugular – Proximal 1/3 of tail – 18 -, 19 -, 20 -gauge × 1 1/2 -in needle – 45 – 90 degrees angle – Hemal processes on coccygeal vertebrae protect coccygeal artery and vein • Go in between vertebrae – Digital pressure after collection 15 sec. (hematoma)
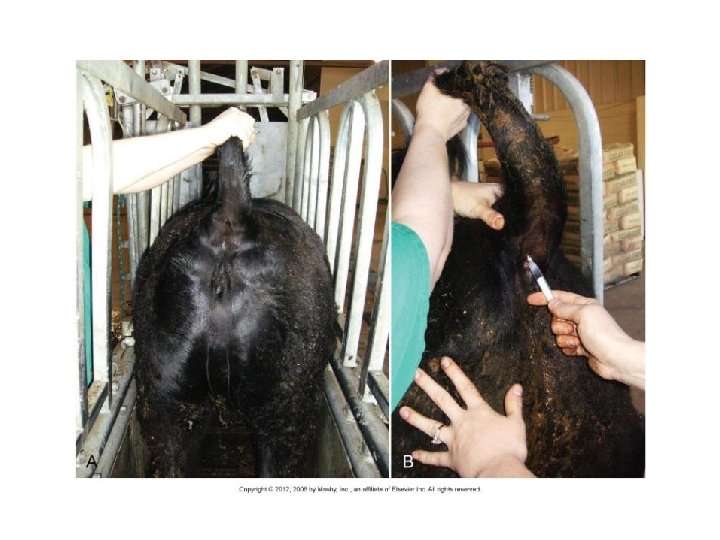
Venipuncture • Milk veins/ subcutaneous abdominal vein – Avoid – Hematomas!!
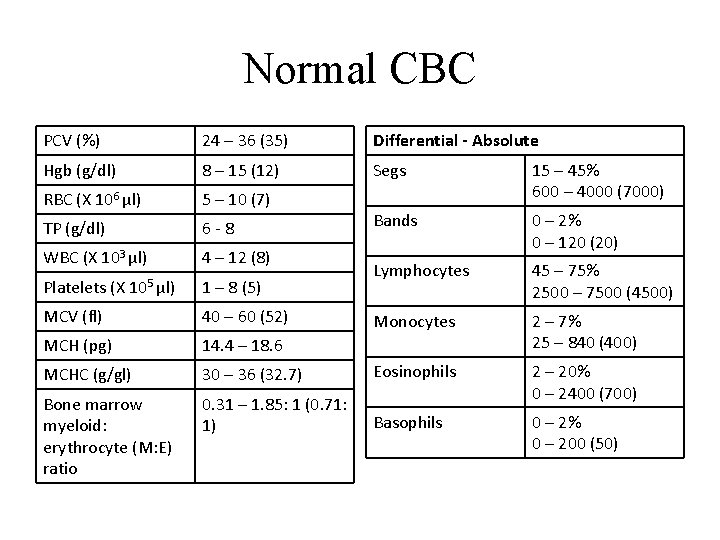
Normal CBC PCV (%) 24 – 36 (35) Differential - Absolute Hgb (g/dl) 8 – 15 (12) Segs RBC (X 106 µl) 5 – 10 (7) 15 – 45% 600 – 4000 (7000) TP (g/dl) 6 -8 Bands WBC (X 103 µl) 4 – 12 (8) 0 – 2% 0 – 120 (20) Lymphocytes 45 – 75% 2500 – 7500 (4500) Monocytes 2 – 7% 25 – 840 (400) Platelets (X 105 µl) 1 – 8 (5) MCV (fl) 40 – 60 (52) MCH (pg) 14. 4 – 18. 6 MCHC (g/gl) 30 – 36 (32. 7) Eosinophils Bone marrow myeloid: erythrocyte (M: E) ratio 0. 31 – 1. 85: 1 (0. 71: 1) 2 – 20% 0 – 2400 (700) Basophils 0 – 2% 0 – 200 (50)
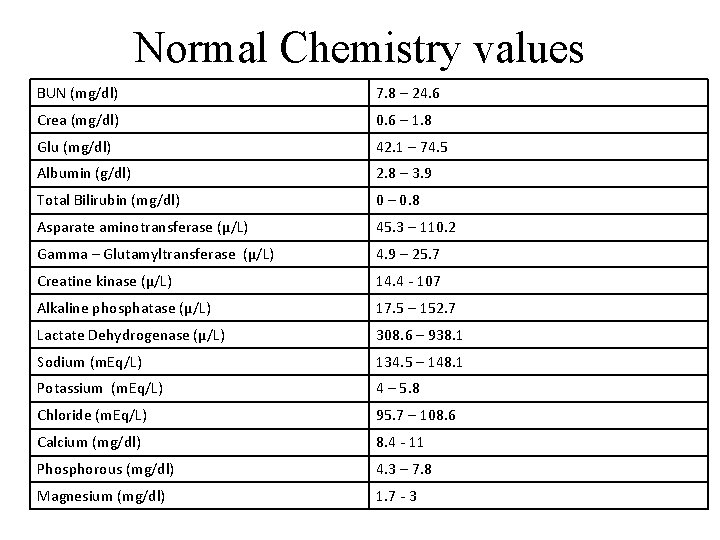
Normal Chemistry values BUN (mg/dl) 7. 8 – 24. 6 Crea (mg/dl) 0. 6 – 1. 8 Glu (mg/dl) 42. 1 – 74. 5 Albumin (g/dl) 2. 8 – 3. 9 Total Bilirubin (mg/dl) 0 – 0. 8 Asparate aminotransferase (µ/L) 45. 3 – 110. 2 Gamma – Glutamyltransferase (µ/L) 4. 9 – 25. 7 Creatine kinase (µ/L) 14. 4 - 107 Alkaline phosphatase (µ/L) 17. 5 – 152. 7 Lactate Dehydrogenase (µ/L) 308. 6 – 938. 1 Sodium (m. Eq/L) 134. 5 – 148. 1 Potassium (m. Eq/L) 4 – 5. 8 Chloride (m. Eq/L) 95. 7 – 108. 6 Calcium (mg/dl) 8. 4 - 11 Phosphorous (mg/dl) 4. 3 – 7. 8 Magnesium (mg/dl) 1. 7 - 3
Arterial Blood Sampling • Arteries – Brachial arteries – Femoral arteries – Auricular arteries – Anesthetized patients

Abdominocentesis • • • Ventral abdomen Adults, slightly right of ventral midline Clip and prep Needle or cannula 1 1/2 to 3 inches, 18 - to 20 -gauge Avoid the subcutaneous abdominal veins
Rumen Fluid Collection • Ororumen/ orogastric • Rumenocentesis: lower left abdominal wall – 14 gauge – Xiphoid process and left of ventral midline – Clip and prep – Aspiration – Rumen fluid: green, sweet Fermented odor, motile protozoa, 6. 5 – 7. 5
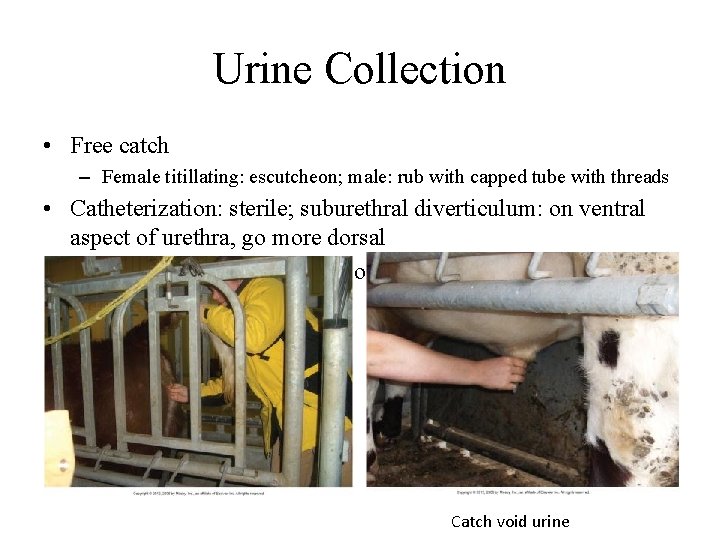
Urine Collection • Free catch – Female titillating: escutcheon; male: rub with capped tube with threads • Catheterization: sterile; suburethral diverticulum: on ventral aspect of urethra, go more dorsal • Cystocentesis in calves: not done often Catch void urine

Fecal Collection • Gloved hand – Ground – Rectum: lubricate
Cerebrospinal Fluid Sampling • Alantooccipital space (cisterna magna) – Lateral recumbency nose flexed down • Lumbosacral space • Heavy sedation or general anesthesia • 18 - to 20 -gauge 3 1/2 -in length – Not as deep as in horses Lumbosacral space

Milk Collection • • Palpate the udder and teats. Wash teats with a sanitizing solution. Dry teats thoroughly with individual paper towels. Strip the teat.

Black cup CMT results

• Foul smell and necrotic odor • Arcanobacterium pyogenes (another)anaerobe • Watery milk, swollen udder • Coliforms • Watery and red, sick cow • Staph aureus: poor prognosis • Subclinical mastitis: contagious organisms: Staphylococcus aureus, Streptococcus agalactia, Mycoplasma bovis • Clinical mastitis: • Environmental organisms: Strep. nonag. Group: 50% • Coliform organisms (Eschericia coli, Klebsiella, Enterobacter sp. , etc. ) 90% of the time

Oral Medication • Balling gun: poor technique: laceration/ abscess: pharynx, epiglottis, oral cavity. Don’t use in horses or young animals • Drenching • Gastric intubation Nose should not be higher than top of head
Oral Medication • Drenching – Liquid medication – Go slowly – If animal coughs let them settle before inserting any other medication Nose should not be higher than top of head
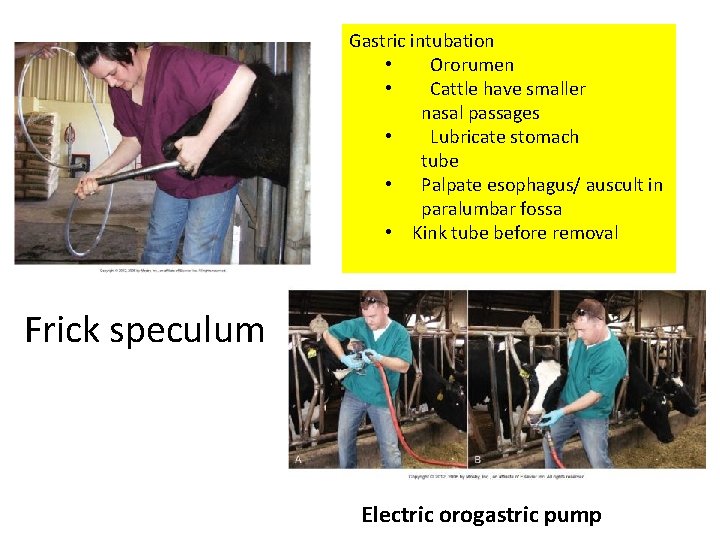
Gastric intubation • Ororumen • Cattle have smaller nasal passages • Lubricate stomach tube • Palpate esophagus/ auscult in paralumbar fossa • Kink tube before removal Frick speculum Electric orogastric pump


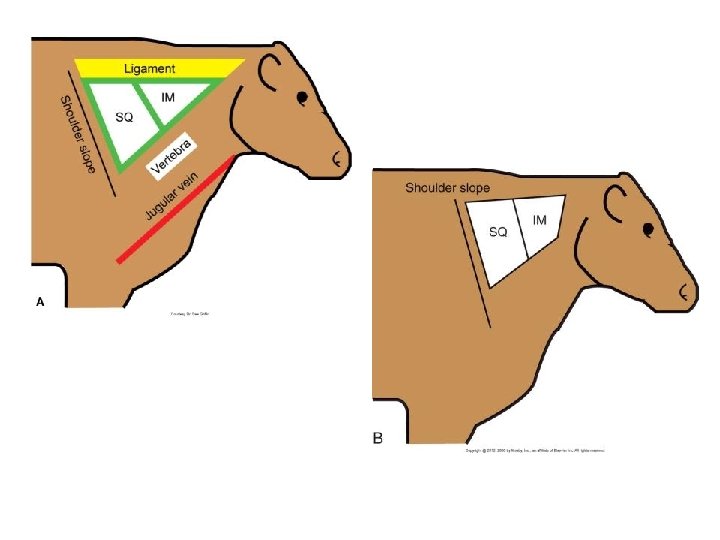
Injections • Use SQ products whenever possible. • Use sharp, single-use, sterile needles from 16 - to 20 -gauge, 1 - to 1 1/2 -inch length. • No more than 10 ml per IM injection site. • Keep IM sites separated by at least 4 inches. • Preferred IM injection site is in front of the shoulder. • Avoid injecting through wet or dirty skin. • Do not use chemical disinfectants in syringes. • Replace needles when they are bent or burred, contaminated with dirt or feces, and after every 10 to 15 head. • Never mix products. • Keep records. • CLEAN WITH 70% alcohol

Injection Sites • Lateral cervical muscles • Gluteal muscles – Avoid • Semitendinosus/semimembranosus muscle – Avoid • Triceps muscle – Avoid • Longissimus muscle – Avoid
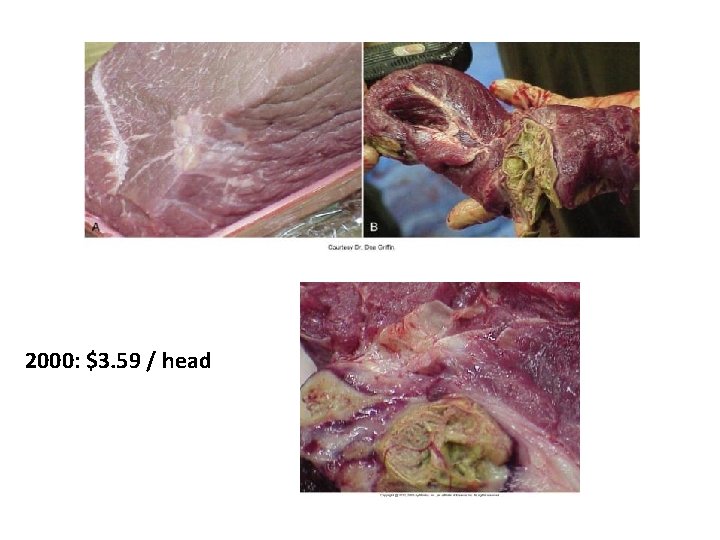
2000: $3. 59 / head
Intravenous Injections • Jugular – Preferred – Bell IV • Cephalic • Caudal auricular vein (ear vein) • 10 - to 14 -gauge for cattle (18 - to 20 -gauge for the ear vein), and 14 - to 18 -gauge for calves. Small individuals may use catheters that are 2 to 3 inches in length. • Procedure is the same as horses.
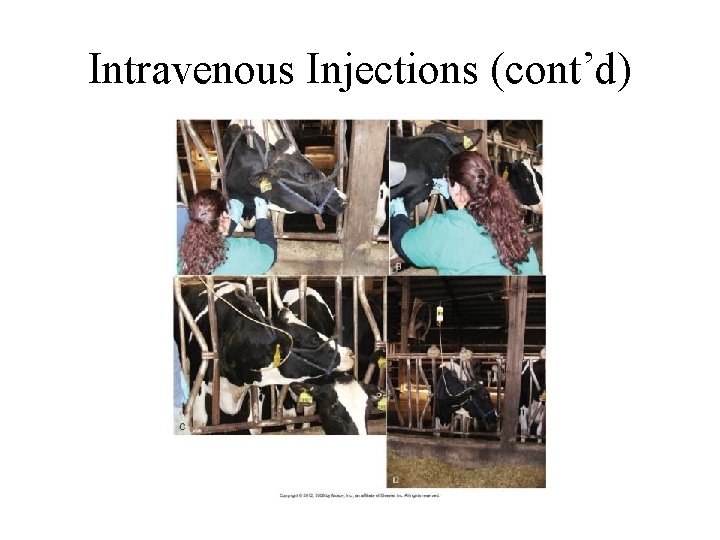
Intravenous Injections (cont’d)
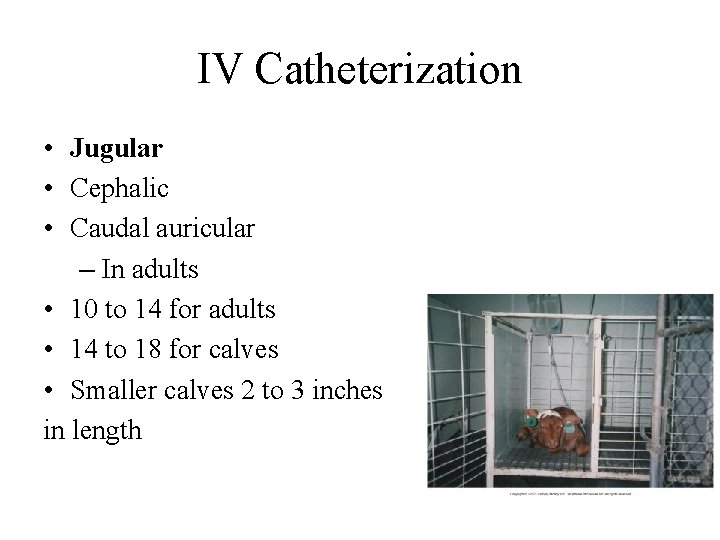
IV Catheterization • Jugular • Cephalic • Caudal auricular – In adults • 10 to 14 for adults • 14 to 18 for calves • Smaller calves 2 to 3 inches in length
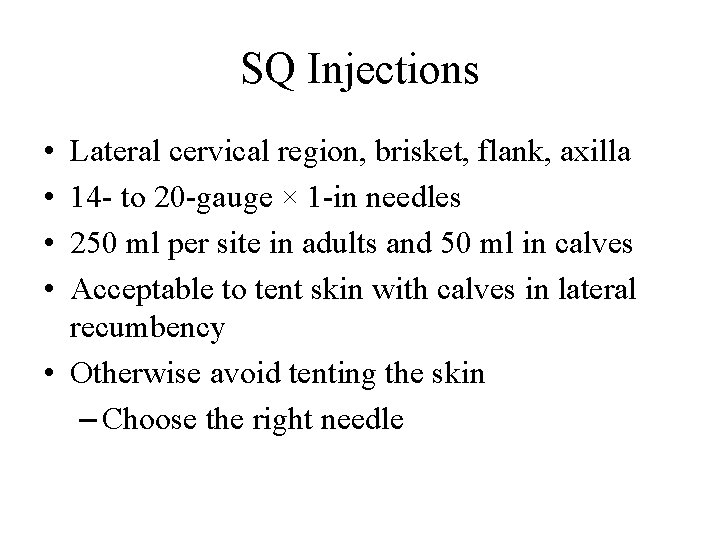
SQ Injections • • Lateral cervical region, brisket, flank, axilla 14 - to 20 -gauge × 1 -in needles 250 ml per site in adults and 50 ml in calves Acceptable to tent skin with calves in lateral recumbency • Otherwise avoid tenting the skin – Choose the right needle
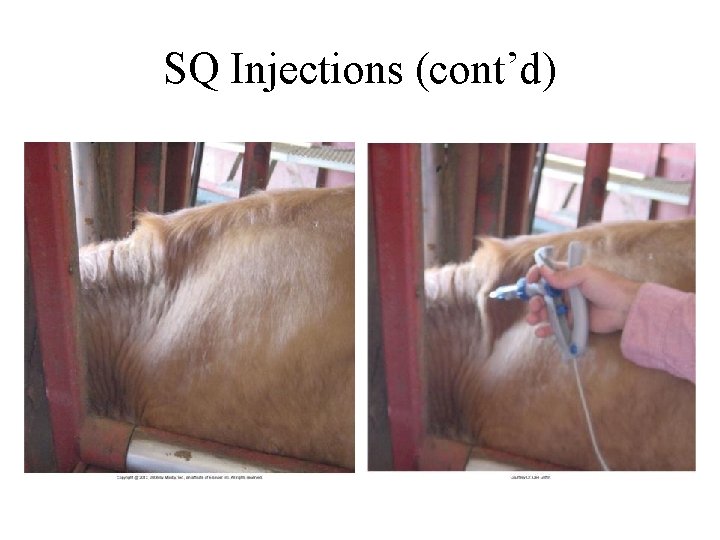
SQ Injections (cont’d)

Care of Vaccines and Automatic Dosing Syringes • Rinse the external portion with soap and water. • Rinse with distilled or deionized water near boiling point (hotter than 180° F). – The water should be squirted through the syringe at least three to five times. • • Do not use soap on internal components. Take the automatic dosing syringe completely apart. Store in a dust-free, dry, low-humidity environment. Transport vaccines in a cooler with ice. Do not freeze. Keep cool. Only mix what will be used in 1 hour.

Intradermal Injections • Used for TB testing – Caudal tail fold • For allergies – Lateral cervical or flank area • Clip the hair. • Antiseptics for the skin may or may not be used. – If used, allow to dry • A 25 - or 26 -gauge needle, though a 22 - or 23 -gauge • Pinch the skin, and insert the needle parallel to the pinched skin. • Should create small bleb; if not it should be repeated.

Intramammary Infusion • Antibiotics – Wet (lactating) and dry cow • Disposable plastic syringes • Infusion tips, cannulas, or catheters should never be used on more than one teat • Treatment performed after milking • The procedure should be as clean as possible • Adhere to withdrawal times • CLEAN: cranial first treat caudal teat first

Intranasal Administration • • Vaccines Head restraint is necessary. Clear nasal passages. Nose should be slightly elevated. Inserted without needle. Common to throw head. It is OK if the animal sneezes.
Implants • Hormone placed in ears – Increase weight gain 8% to 15% – Compudose: Estradiol (24 mg), steers, heifers lasts for 200 days • Ralgro: Zeranol (36 mg) , 70 – 100 days
Implants (cont’d) • Synovex-S – Progesterone (100 mg) + estradiol benzoate (20 mg) – Steers over 400 lb – Effective for 70 to 100 days • Synovex-H – Testosterone (200 mg) + estradiol benzoate (20 mg) – Heifers over 400 lb – Effective for 70 to 100 days • Synovex-C – Progesterone (100 mg) + estradiol benzoate (10 mg) – Calves (steers and heifers) 45 days of age to 400 lb – Effective for 70 to 100 days

Implants (cont’d) • Implus-S – Progesterone 200 mg + estradiol benzoate 20 mg – Steers over 400 lb – Effective for 70 to 100 days • Implus-H – Testosterone 200 mg + estradiol benzoate 20 mg – Heifers over 400 lb – Effective for 70 to 100 days
Implants (cont’d) • Finaplix-S – Trenbolone acetate 140 mg – Feeder steers over 650 lb – Effective for 70 to 100 days • Finaplix-H – Trenbolone acetate 200 mg – Feeder heifers over 650 lb – Effective for 70 to 100 days

Implants (cont’d) • Revalor-S – Trenbolone acetate 120 mg + estradiol 24 mg – Feeder steers over 650 lb – Effective for 110 to 120 days
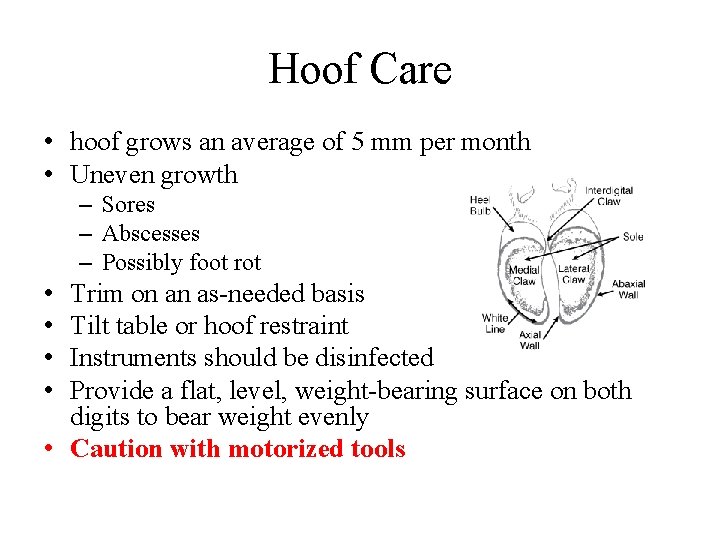
Hoof Care • hoof grows an average of 5 mm per month • Uneven growth – Sores – Abscesses – Possibly foot rot • • Trim on an as-needed basis Tilt table or hoof restraint Instruments should be disinfected Provide a flat, level, weight-bearing surface on both digits to bear weight evenly • Caution with motorized tools

Hoof Care (cont’d) • Excess toe removed • Outer wall trimmed parallel to the coronary band • Inner hoof wall trimmed • Heels seldom need trimming • Inspect the hoof • Hoof blocks

Euthanasia • AVMA Euthanasia Panel 2000 • IV injection of barbituric acid derivatives • IV injection of potassium chloride (KCl) in conjunction with general anesthesia • Penetrating captive bolt • The following euthanasia methods are considered conditionally acceptable – Gunshot - head – Electrocution: head/ brain after unconcious

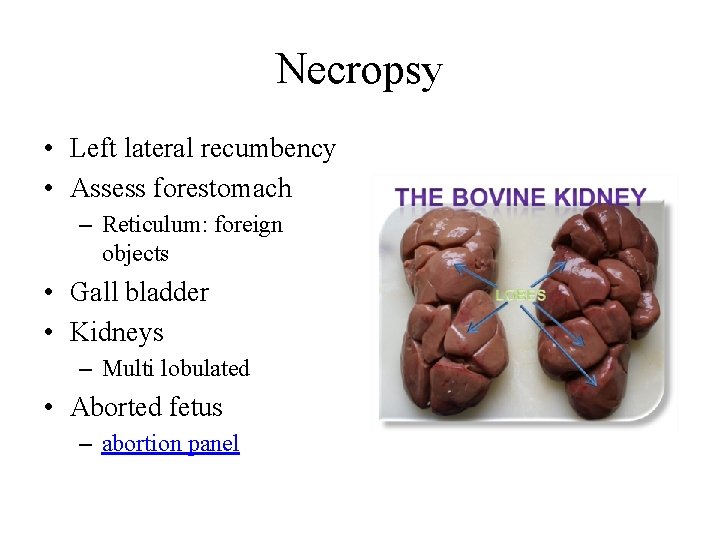
Necropsy • Left lateral recumbency • Assess forestomach – Reticulum: foreign objects • Gall bladder • Kidneys – Multi lobulated • Aborted fetus – abortion panel

References • K Holtgrew-Bohling , Large Animal Clinical Procedures for Veterinary Technicians, 2 nd Edition, Mosby, 2012, ISBN: 97803223077323 • http: //extension. missouri. edu/publications/D isplay. Printer. Friendly. Pub. aspx? P=G 2140 • http: //ucanr. org/repository/cao/landingpage. cfm? article=ca. v 058 n 01 p 54&fulltext=yes • http: //www. cdfa. ca. gov/ahfss/Animal_Healt h/TB_Info 2. html

References • http: //dairyhoofhealth. webs. com/hooflesions. ht m • http: //people. upei. ca/bate/html/notesonurinarys ystem. html
- Slides: 55