Chapter 11 Antibiotics Mechanism of antibiotic n n

Chapter 11 Antibiotics

抗生素的作用机制 Mechanism of antibiotic n n n n n 1. 干扰细菌细胞壁合成 2. 损伤细菌细胞膜 3. 抑制细菌蛋白质合成 4. 抑制细菌核酸合成 5. 增强吞噬细胞的功能 1. To interfere with the synthesis of bacterial cell wall 2. To damage the cell membrane of bacteria 3. To inhibit protein synthesis of bacteria 4. To inhibit nucleic acid synthesis of bacteria 5. To enhance the function of phagocytes

To be classified according to chemical structure: 1,β-内酰胺类, β-lactams n 2,四环类, tetracycline antibiotics n 3,氨基糖苷类, Aminoglycoside antibiotics n 4,大环内酯类, Macrolide antibiotics n 5,其他类, other types n
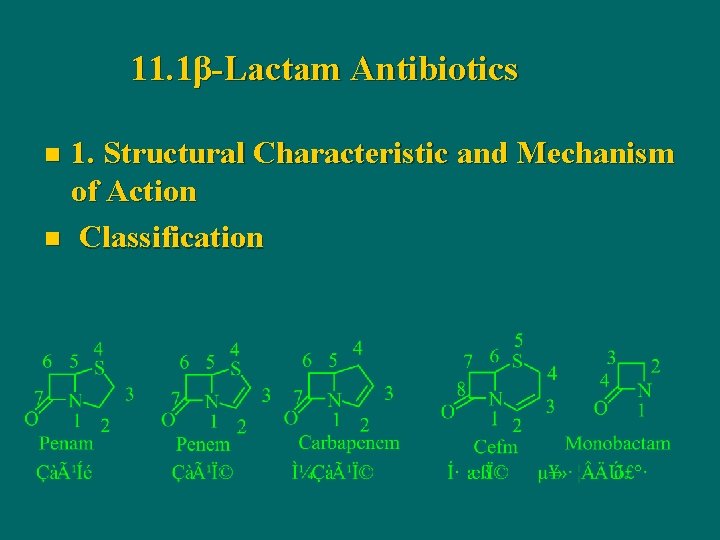
11. 1β-Lactam Antibiotics 1. Structural Characteristic and Mechanism of Action n Classification n
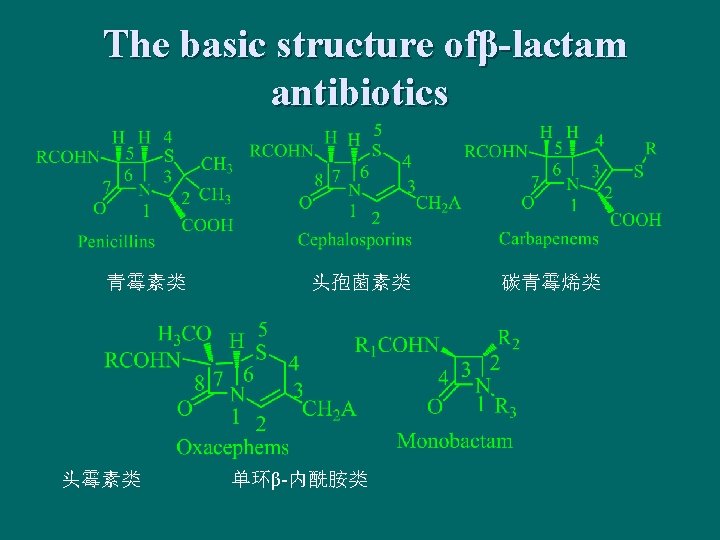
The basic structure ofβ-lactam antibiotics 青霉素类 头孢菌素类 单环β-内酰胺类 碳青霉烯类


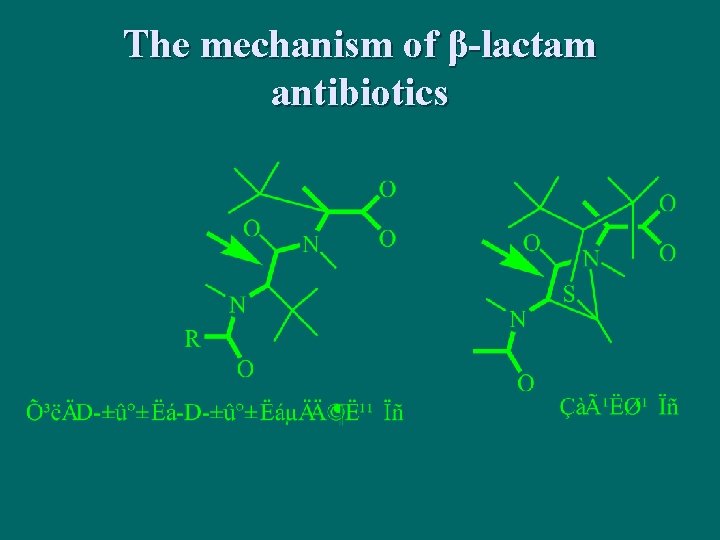
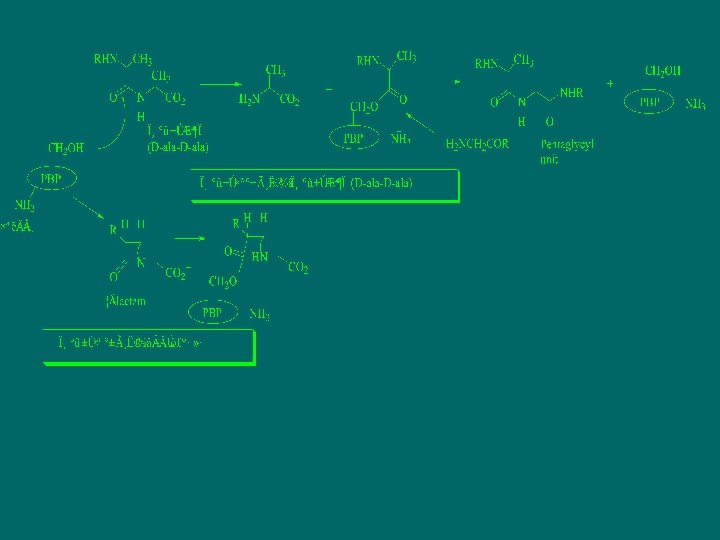
The mechanism of β-lactam antibiotics
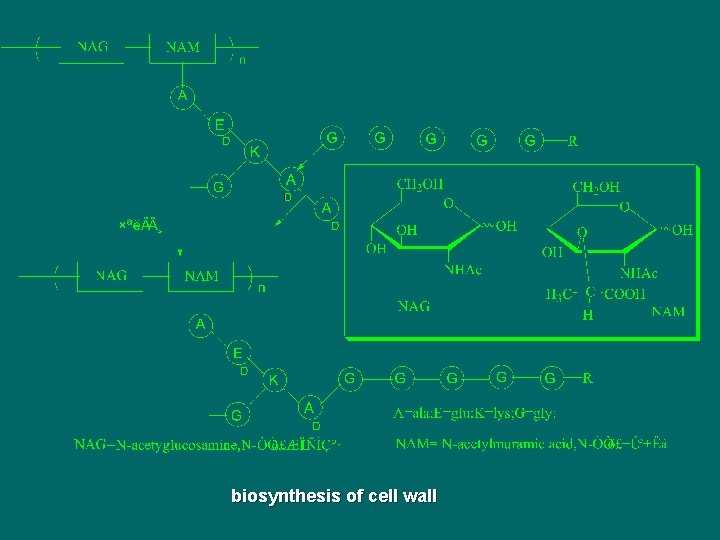
biosynthesis of cell wall
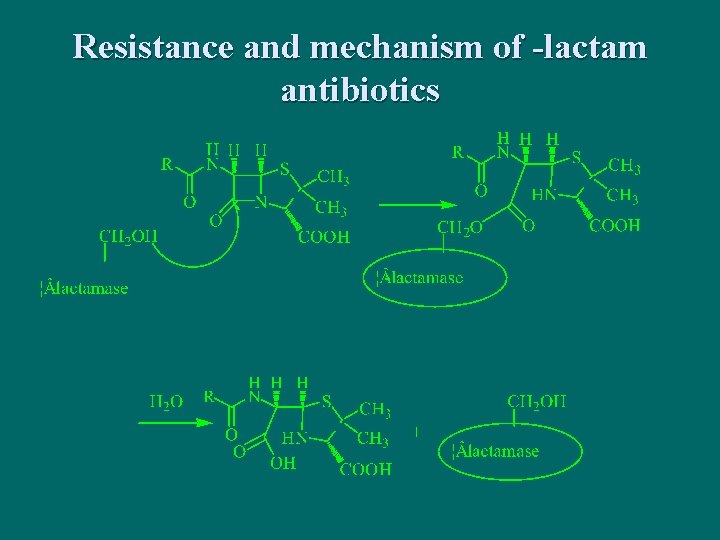
Resistance and mechanism of -lactam antibiotics
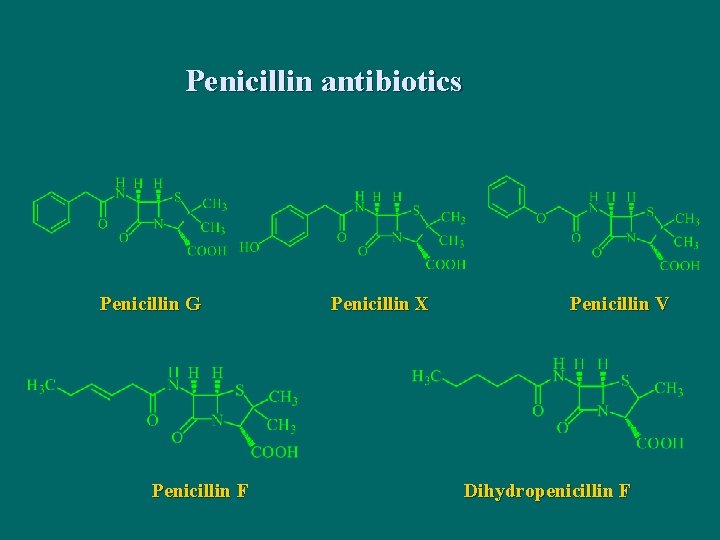
Penicillin antibiotics Penicillin G Penicillin F Penicillin X Penicillin V Dihydropenicillin F
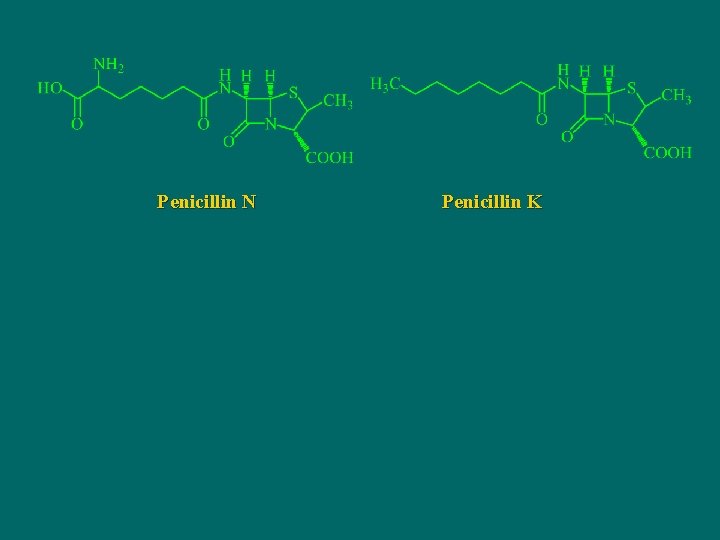
Penicillin N Penicillin K
![Benzylpenicillin (2 S,5 R,6 R)-3,3 -二甲基-6 -(2 -苯乙酰氨基)-7 -氧代-4 -硫杂-1氮杂双环[3. 2. 0]庚烷-2 -甲酸, 青霉素G(Penicillin Benzylpenicillin (2 S,5 R,6 R)-3,3 -二甲基-6 -(2 -苯乙酰氨基)-7 -氧代-4 -硫杂-1氮杂双环[3. 2. 0]庚烷-2 -甲酸, 青霉素G(Penicillin](http://slidetodoc.com/presentation_image_h2/d3e7130fccffc667168d50e30fb3ab6d/image-14.jpg)
Benzylpenicillin (2 S,5 R,6 R)-3,3 -二甲基-6 -(2 -苯乙酰氨基)-7 -氧代-4 -硫杂-1氮杂双环[3. 2. 0]庚烷-2 -甲酸, 青霉素G(Penicillin G) (2 S,5 S,6 R)-3,3 -Dimethyl-6 -(2 -Benzylacetamido)-7 -oxo-4 thia-1 -azabicyclo[3. 2. 0] heptane-2 -carboxylic acid。
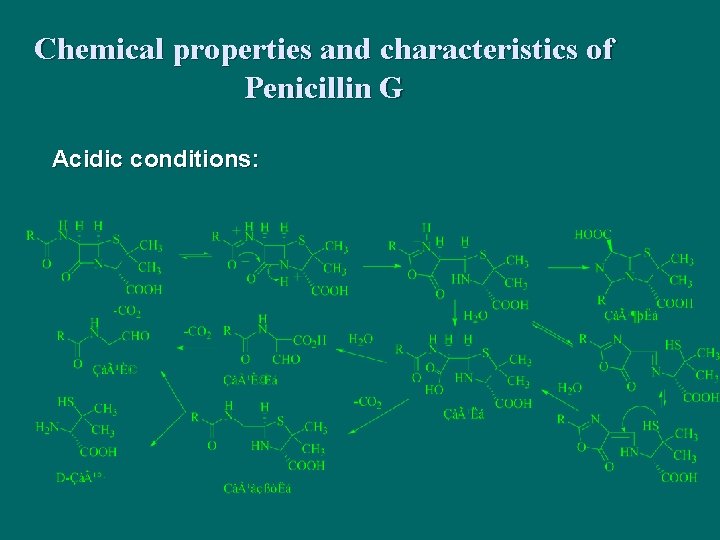
Chemical properties and characteristics of Penicillin G Acidic conditions:
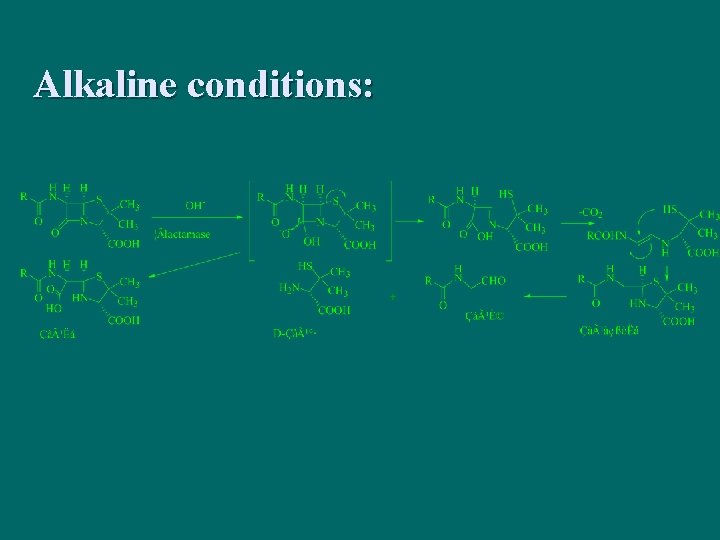
Alkaline conditions:
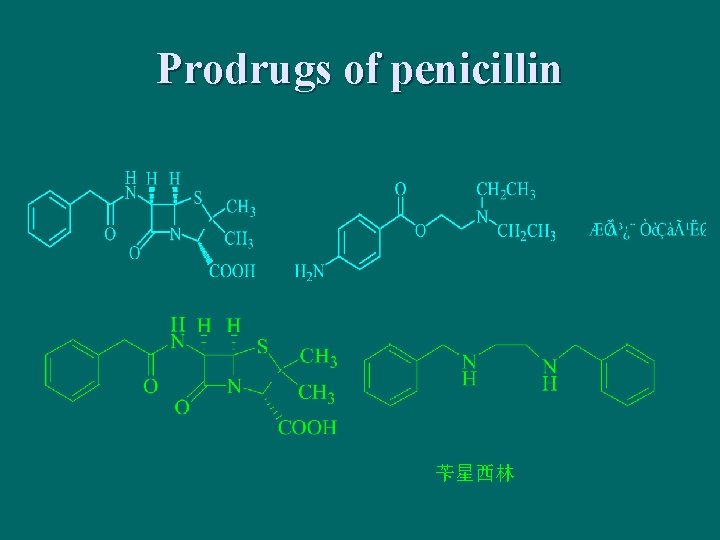
Prodrugs of penicillin 苄星西林

Semi-synthetic penicillin Shortcomings of Penicillin : non acidresistent, non enzyme-resistent, narrow anti -bacterial spectrum and allergic reactions n Semi-synthetic penicillins: n Acid-resistant penicillin n Enzyme-resistant penicillin n Broad-spectrum penicillin n Compounds of Penicillin and β-lactamase inhibitor n

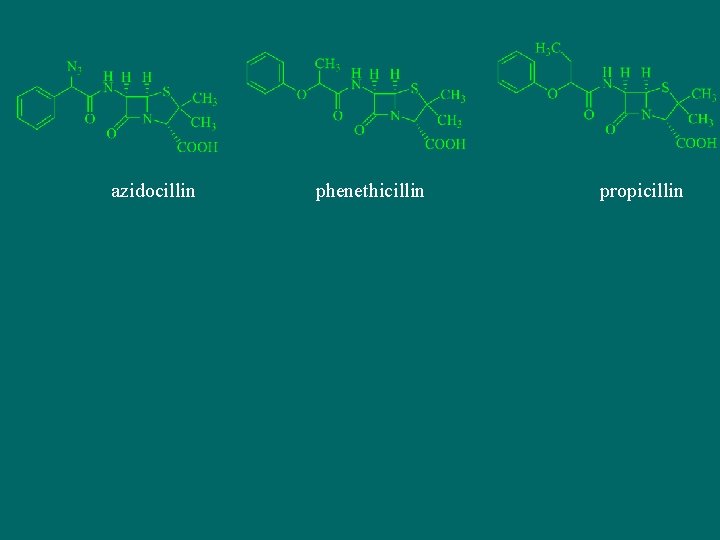
azidocillin phenethicillin propicillin
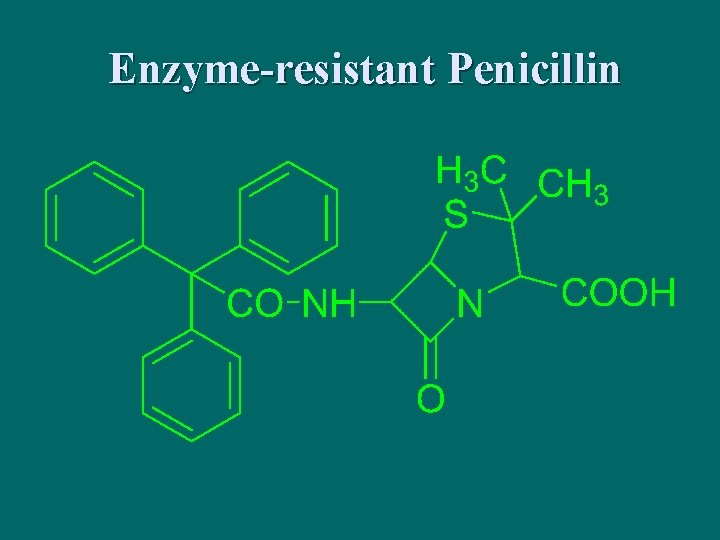
Enzyme-resistant Penicillin
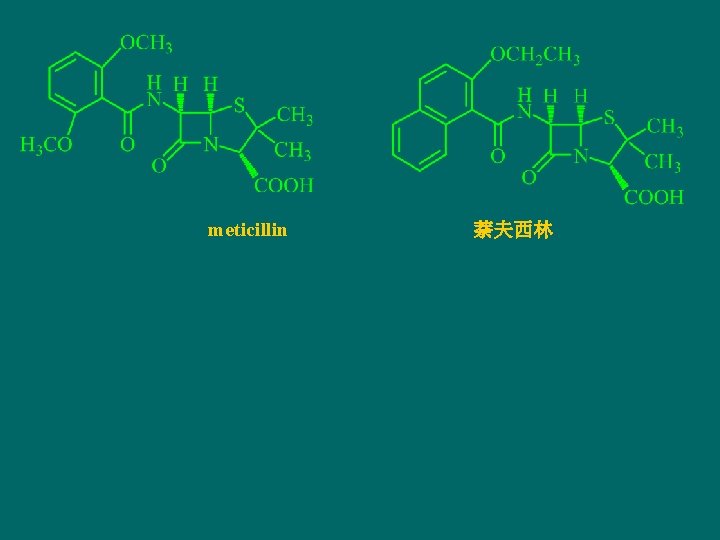
meticillin 萘夫西林
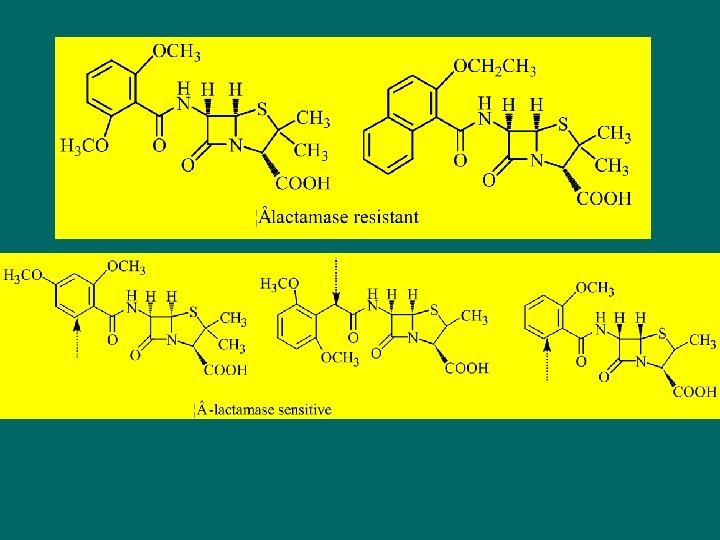
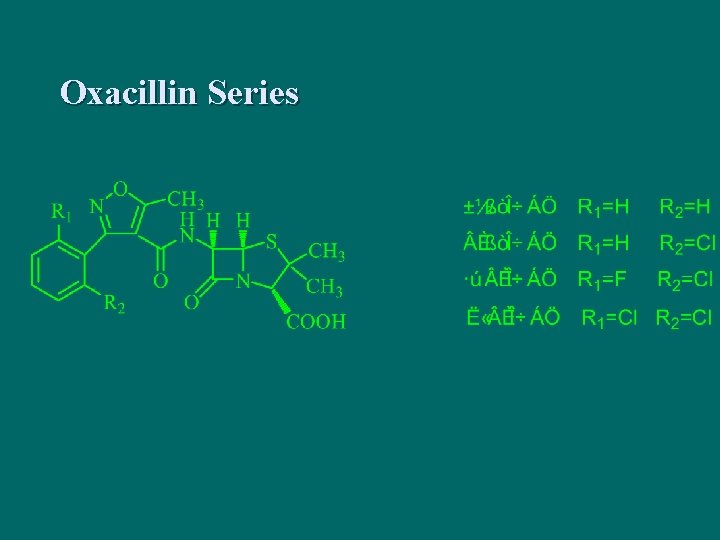
Oxacillin Series
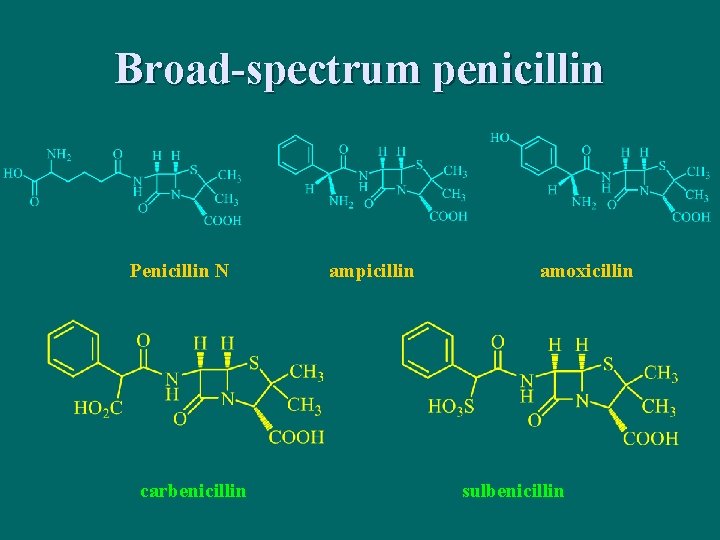
Broad-spectrum penicillin Penicillin N carbenicillin ampicillin amoxicillin sulbenicillin
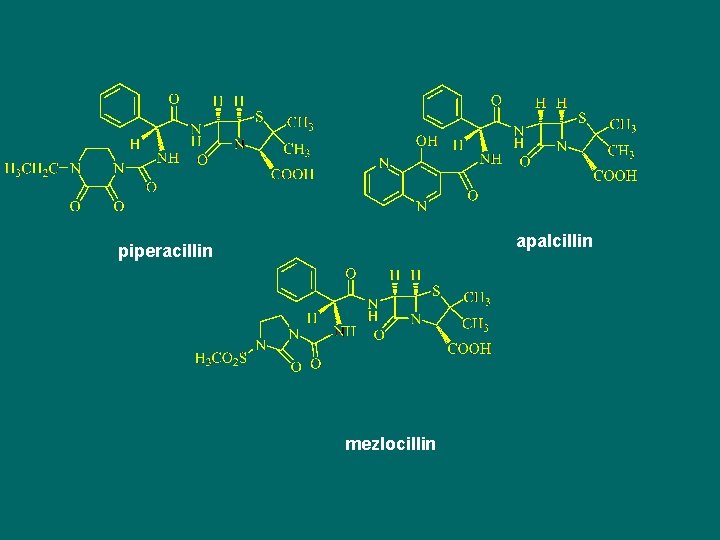
apalcillin piperacillin mezlocillin
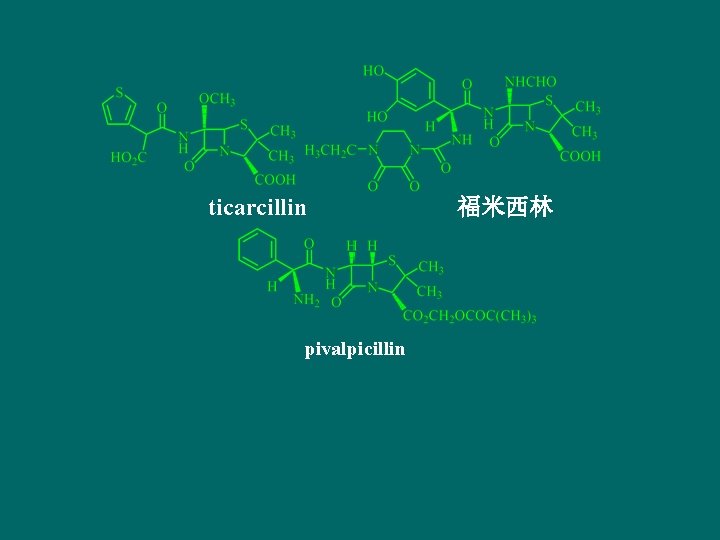
ticarcillin pivalpicillin 福米西林
![Amoxicillin (2 S,5 R,6 R)-3,3 -二甲基-6 -[(R)-(-)-2 -氨基-2 -(4羟基苯基)乙酰氨基]-7 -氧代-4 -硫杂-1 -氮杂双环 [3. 2. Amoxicillin (2 S,5 R,6 R)-3,3 -二甲基-6 -[(R)-(-)-2 -氨基-2 -(4羟基苯基)乙酰氨基]-7 -氧代-4 -硫杂-1 -氮杂双环 [3. 2.](http://slidetodoc.com/presentation_image_h2/d3e7130fccffc667168d50e30fb3ab6d/image-30.jpg)
Amoxicillin (2 S,5 R,6 R)-3,3 -二甲基-6 -[(R)-(-)-2 -氨基-2 -(4羟基苯基)乙酰氨基]-7 -氧代-4 -硫杂-1 -氮杂双环 [3. 2. 0]庚烷-2 -甲酸三水合物, 又名羟氨苄青霉素 (2 S, 5 S, 6 R)-3,3 -Dimethyl-6 -[(R)-(-)-2 -amino-2 -(4 -hydroxyphenyl)acetamido]-7 -oxo-4 -thia-1 azabicyclo [3. 2. 0]heptane-2 -carboxylic acid trihydrate

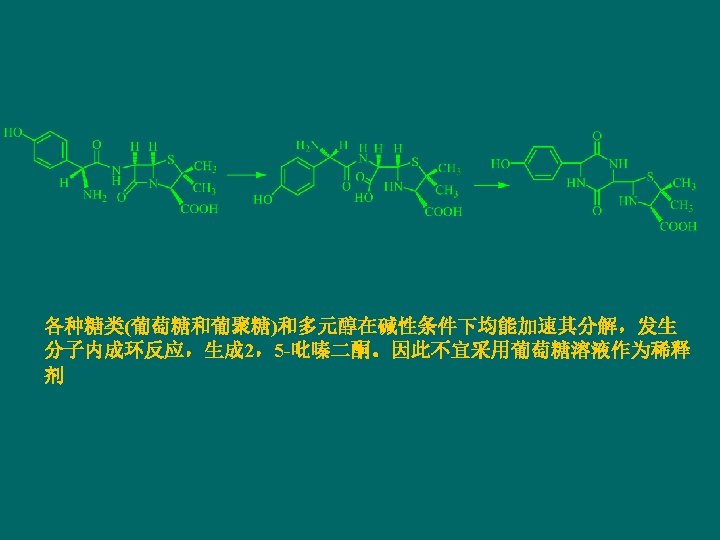
Chemical stability
The structure-activity relationships of penicillins n The side-chain at 6 is important to the antimicrobial spectrum. To change its polarity, make it go through cell membrane easily can expand its antibacterial spectrum. n An extra three-dimensional obstacle group at the appropriate place will increase the resistance to passivation enzyme, can avoid the attack from βlactamase. It is possible to produce the enzymeresistant antibiotics.

n The carboxyl on thiazolidine ring is the essential activity group of penicillin: 被硫代酸或酰胺取代活性降低,被还 原为醇时失去抗菌活性。对其羧基可利用前药原理进行结 构修饰,以增加口服吸收和改善药物代谢动力学性质。 n The configuration of the three chiral carbons is essential for the activity, but the two methyls on the thiazole ring is not necessary to maintain the activity.
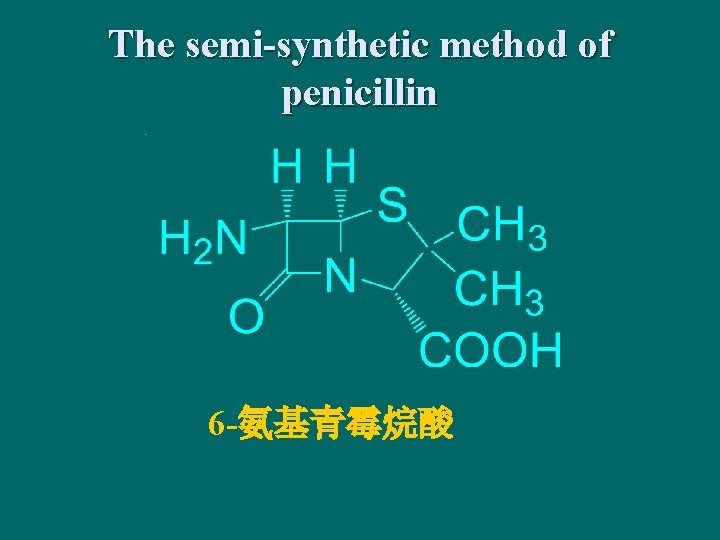
The semi-synthetic method of penicillin 6 -氨基青霉烷酸
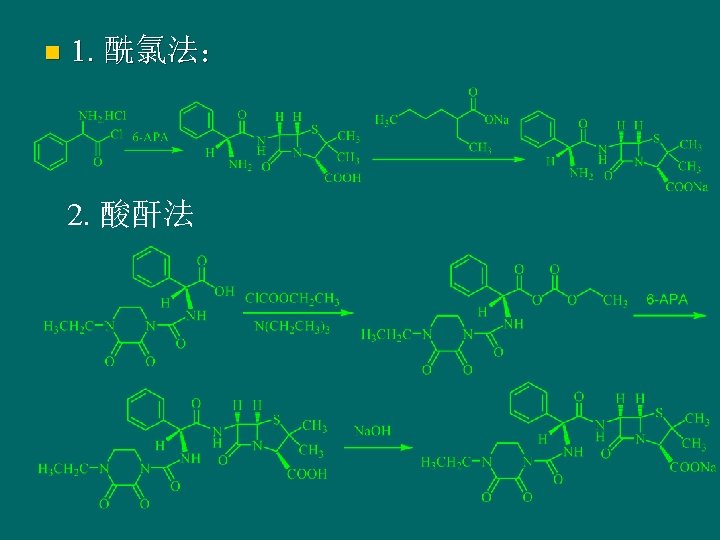
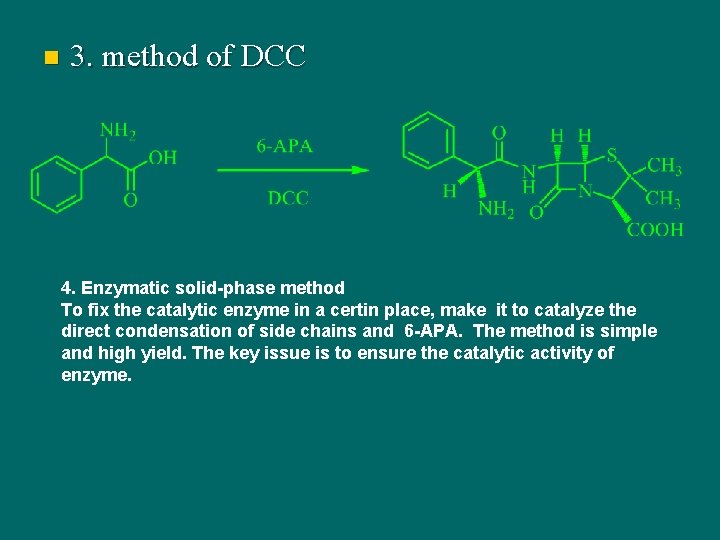
n 3. method of DCC 4. Enzymatic solid-phase method To fix the catalytic enzyme in a certin place, make it to catalyze the direct condensation of side chains and 6 -APA. The method is simple and high yield. The key issue is to ensure the catalytic activity of enzyme.

1. 2 Cephalosporins n n Natural cephalosporins Cephalosporin was separated from cephalosporin (Cephalosporium), it was hydrogenated lactam ring of antibiotics thiazine ring, there were three natural cephalosporins : cephalosporin bacteria Su-C, N and P. 头 孢菌素P抗菌活性中等,但耐药性强。头孢菌素N抗菌活 性较低,而头孢菌素C的抗菌谱广、毒性较小。但由于抗 菌活性与其半合成头孢菌素的活性无法比拟,所以在临床 上几乎没有应用。
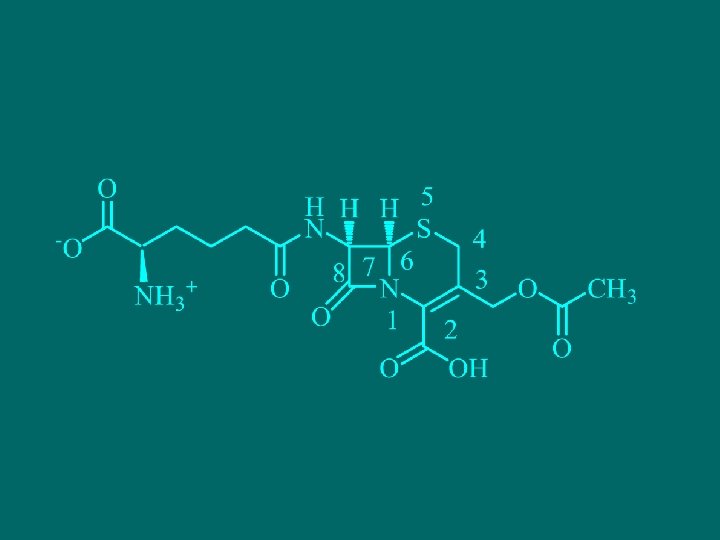
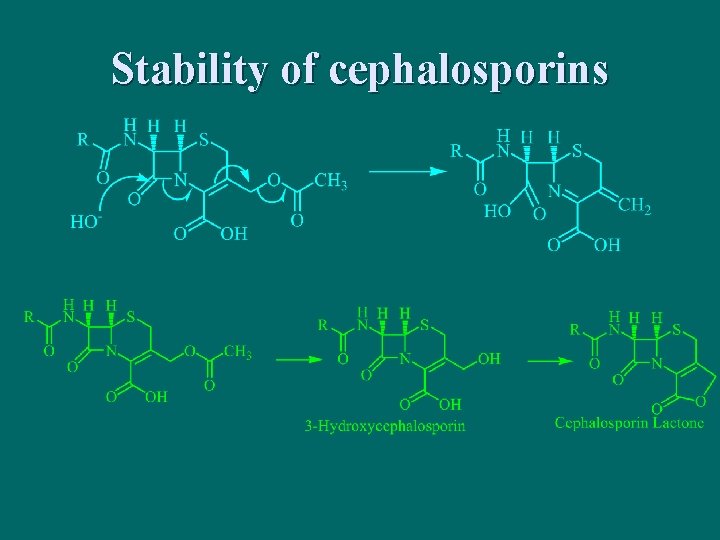
Stability of cephalosporins
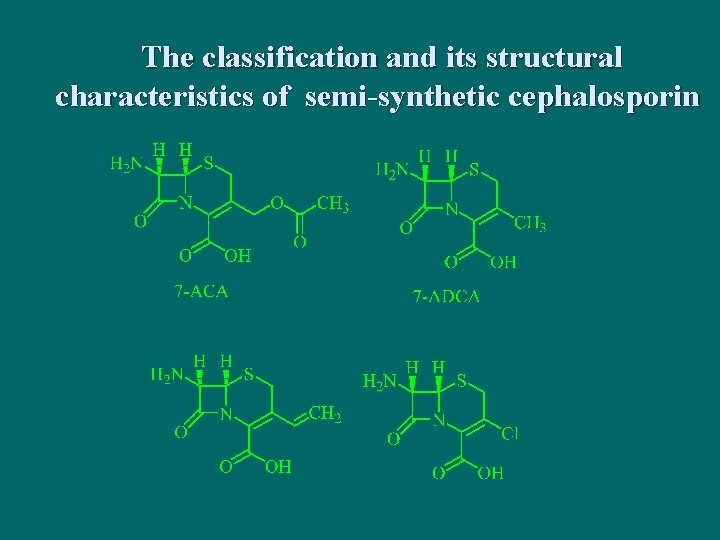
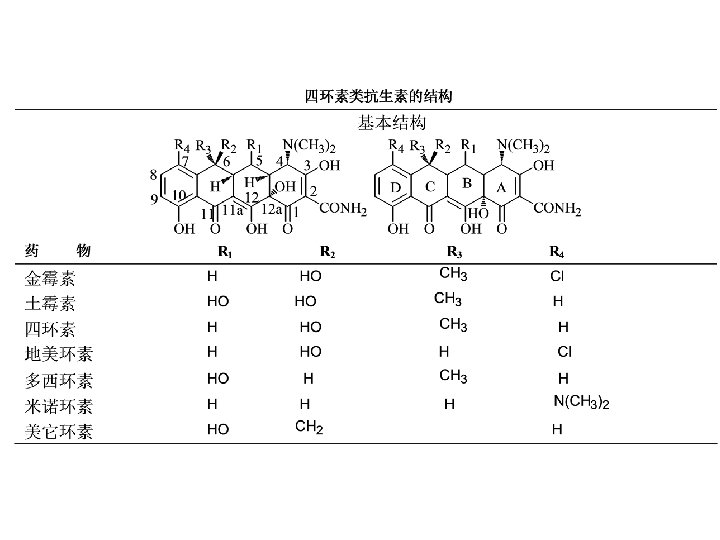
The classification and its structural characteristics of semi-synthetic cephalosporin
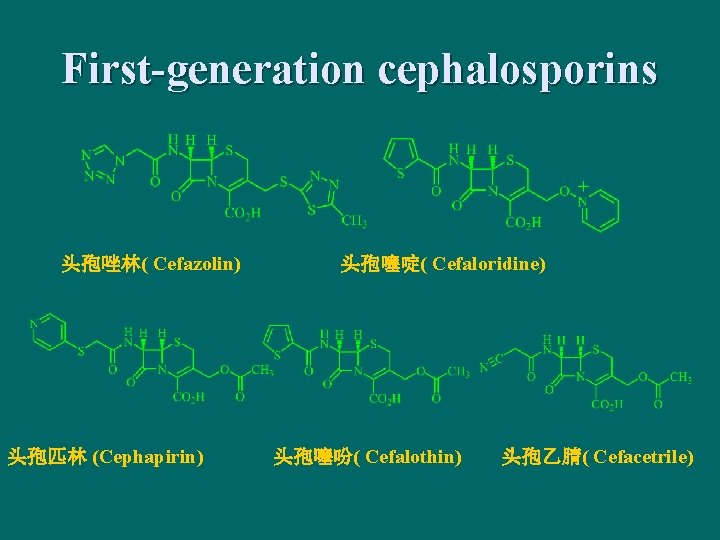
First-generation cephalosporins 头孢唑林( Cefazolin) 头孢匹林 (Cephapirin) 头孢噻啶( Cefaloridine) 头孢噻吩( Cefalothin) 头孢乙腈( Cefacetrile)

头孢氨苄 (Cefalexin) 头孢羟氨苄 (Cefadroxil) 头孢拉定( Cefradinel) First-generation cephalosporins was penicillin enzymeresistant, but intolerance to ß-lactamase: 主要用于耐青 霉素酶的金黄色葡萄球菌等敏感革兰阳性球菌和某些 革兰阴性球菌的感染。
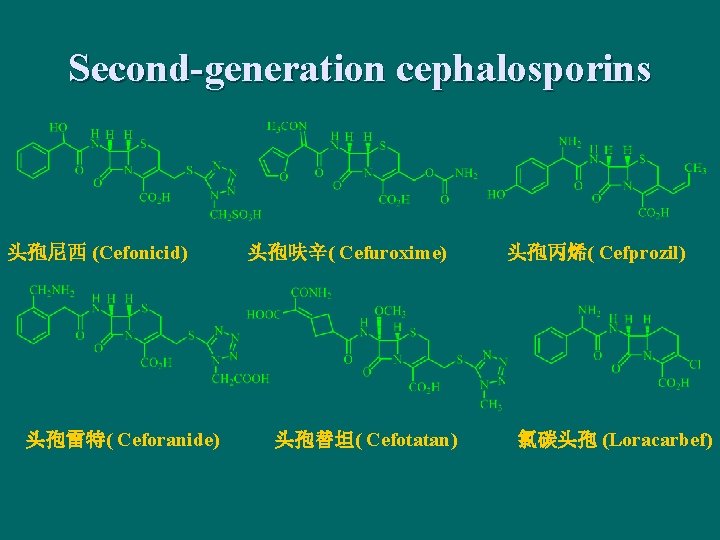
Second-generation cephalosporins 头孢尼西 (Cefonicid) 头孢雷特( Ceforanide) 头孢呋辛( Cefuroxime) 头孢替坦( Cefotatan) 头孢丙烯( Cefprozil) 氯碳头孢 (Loracarbef)
Characteristics of second-generation cephalosporin n Second-generation cephalosporins has notable distinction with the first-generation cephalosporins in the chemical structure. It is stable to the majority of ß-lactamase and with broader antimicrobial spectrum, stronger activity to gram-negative bacteria than the first generation, but it is weaker to anti-Gram-positive.
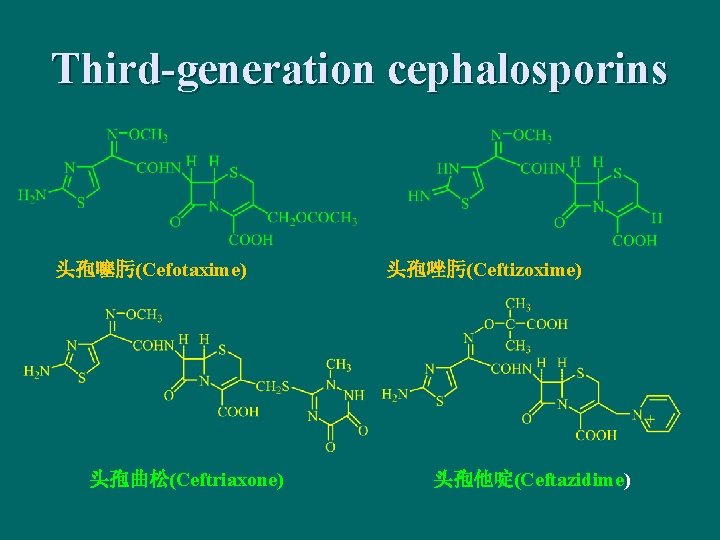
Third-generation cephalosporins 头孢噻肟(Cefotaxime) 头孢曲松(Ceftriaxone) 头孢唑肟(Ceftizoxime) 头孢他啶(Ceftazidime)
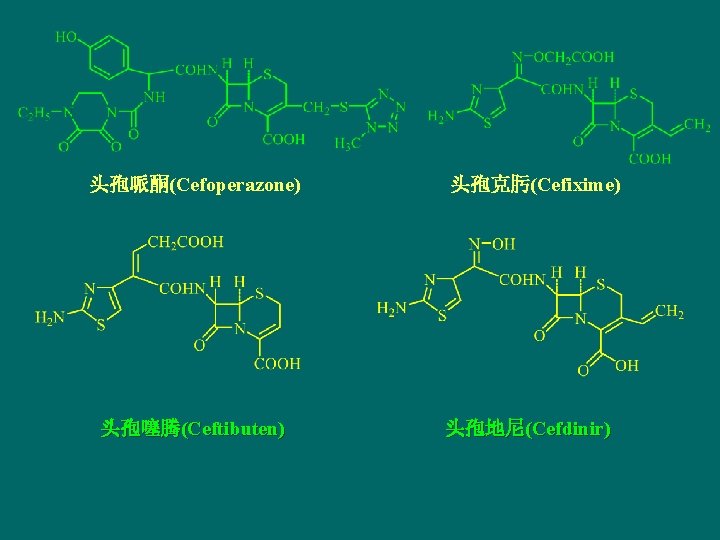
头孢哌酮(Cefoperazone) 头孢噻腾(Ceftibuten) 头孢克肟(Cefixime) 头孢地尼(Cefdinir)
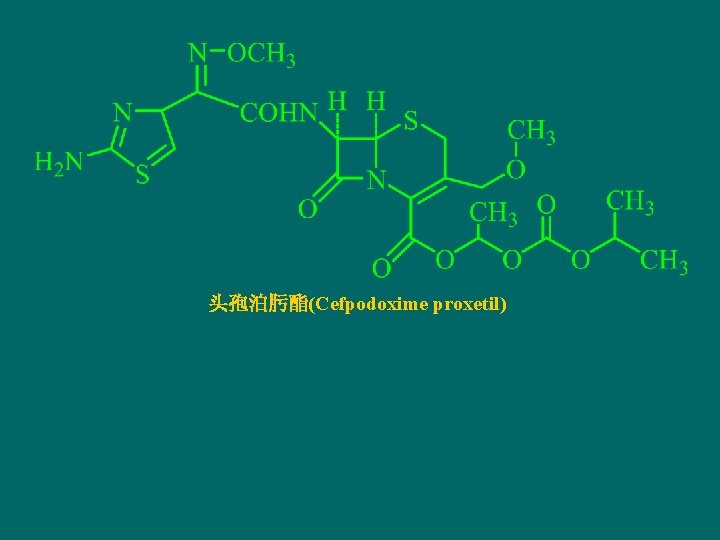
头孢泊肟酯(Cefpodoxime proxetil)
Characteristics of third-generation cephalosporin Third-generation cephalosporins has much broader antibacterial spectrum, with the stronger effect to gram-negative bacteria, but the weaker effect to Gram-positive bacteria than first generation, some drug has strong activity resistance toward Pseudomonas aeruginosa.
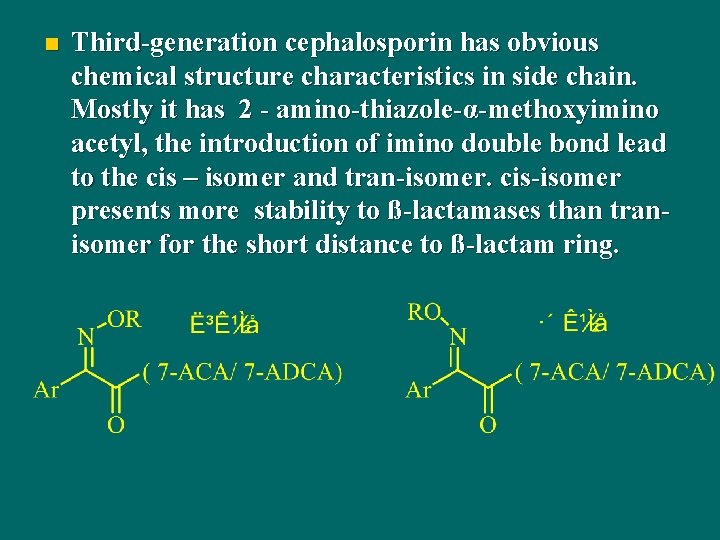
n Third-generation cephalosporin has obvious chemical structure characteristics in side chain. Mostly it has 2 - amino-thiazole-α-methoxyimino acetyl, the introduction of imino double bond lead to the cis – isomer and tran-isomer. cis-isomer presents more stability to ß-lactamases than tranisomer for the short distance to ß-lactam ring.
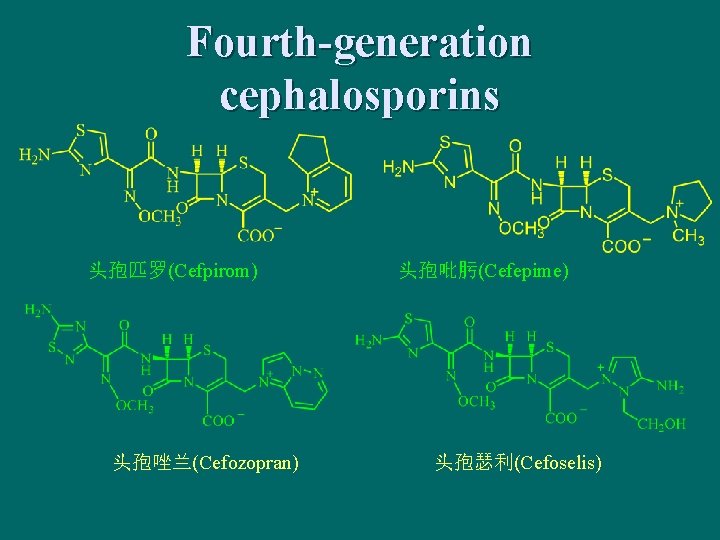
Fourth-generation cephalosporins 头孢匹罗(Cefpirom) 头孢唑兰(Cefozopran) 头孢吡肟(Cefepime) 头孢瑟利(Cefoselis)


Structural transformation of Cephalosporins n n Amide substituent at 7 is essential to antibacterial spectrum, the structural modification at 7 can expand the antibacterial spectrum and enhance antibacterial activity. Methoxy substitutes the hydrogen atom at 7 can improve the stability ofβ-lactam ring. S is replaced by O or C can improve the antibacterial activity. Substituent at 3 can not only improve antibacterial activity, but also affect pharmacokinetics.

SQR of Semi-synthetic cephalosporin n n ① The introduction of lipophilic group at side chain of 7 such as benzene, thiophene, heterocyclic ring with nitrogen, and the introduction of heterocyclic ring at 3 can expand the antibacterial spectrum, enhance the antibacterial activity. 如第一代的头孢菌素的头孢噻吩、头 孢噻啶、头孢唑林和头孢匹林。 The introduction of hydrophilic group at 7 α-amide group such as -SO 3 H, -NH 2, -COOH, can expand anti-bacterial spectrum to pruduce broad-spectrum cephalosporins, 此类 药物对绿脓杆菌的外壁有很高的渗透作用,此基团的引入 既增加了口服吸收,也极大地改变抗菌活性对酶的稳定性。 若同时用-CH 3、- Cl或含氮杂环取代基替代 3位上的取代 基,除改进口服吸收外还可使其对革兰氏阴性菌和绿脓杆 菌都有效。

n n ③ 7 -ß cis-methoxy imino -2 - thiazolyl ammonia can increase stability to ß-lactamase. And expand the antimicrobial spectrum by reason of enhancing penetration to outer membrane of gram-negative bacteria. ④ Oxime methoxy of side-chain at 7 changes into carboxy can avoid cross-allergy,如将头 孢噻肟改造成头孢他啶,头孢克肟, 口服后血药浓 度高,持续时间长,具有良好的生物利用度。


n n n ⑥ Carboxyl group at 2 is anti-bacterial activity group, can not be changed. Esters of prodrug can be made in order to improve the pharmacokinetics, prodrug esters improve the oral absorption and bioavailability. In the body the prodrug can be hydrolyzed quickly by non-specific esterase to give out the original drugs so can extend the acting time. ⑦For fourth-generation cephalosporins, positively charged quaternary ammonium group at 3 of drugs increased the penetration through the cell membrane and showed a low affinity to βlactamase. ⑧ The introduction of methoxy of 7 produces cephamycin derivatives, the stereochemistry of methoxy can block the approaching of lactam to enzyme, so increase the stability toβ-lactamase and thus enhance the activity to anaerobic bacteria.

n ⑨ Oxygen cephalosporin and carbon cephalosporin vinyl would be produced if S was replaced by bio-electronic body-O- and -CH 2 -, respectively. 碳头孢烯由于立体位阻 作用使药物耐β-内酰胺酶,具有广谱、耐酶、 长效的性质。-CH 2 -取代S原子后,增加了 药物在体内的稳定性。
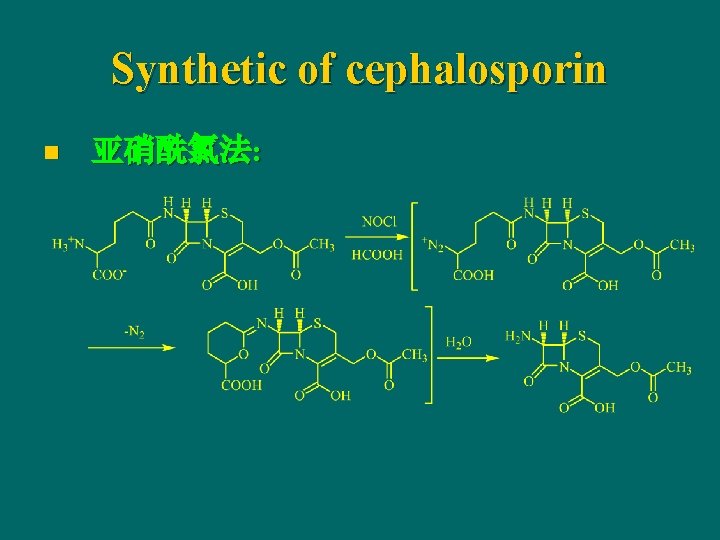
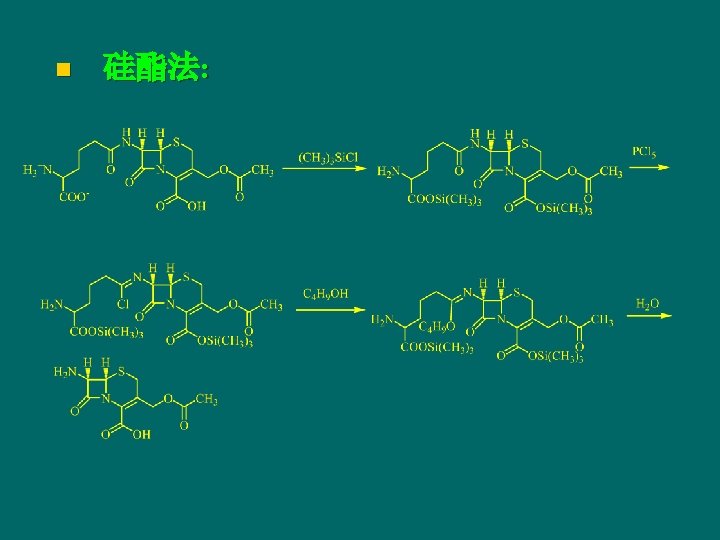
Synthetic of cephalosporin n 亚硝酰氯法:
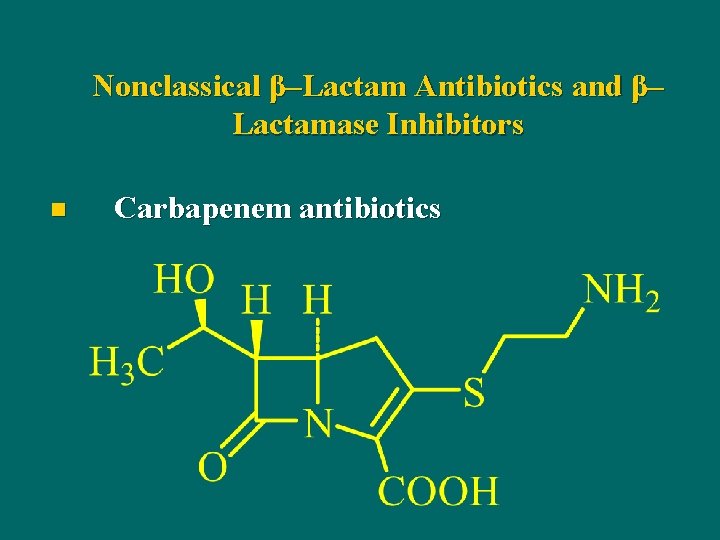
Nonclassical β–Lactam Antibiotics and β– Lactamase Inhibitors n Carbapenem antibiotics
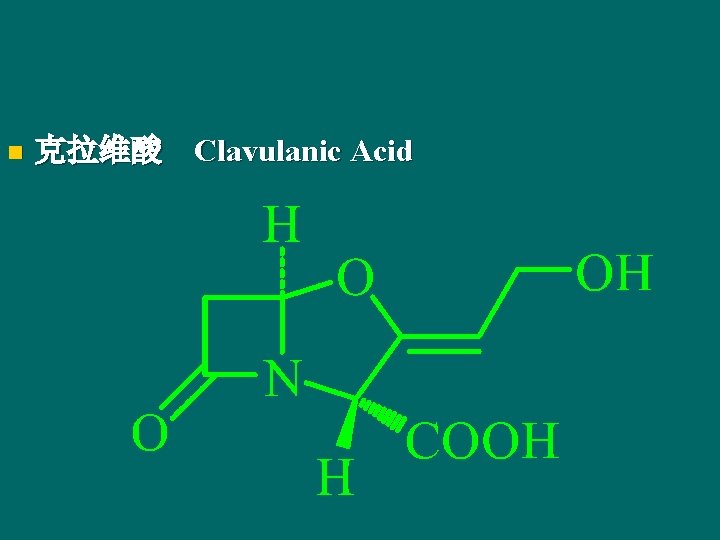
n 克拉维酸 Clavulanic Acid
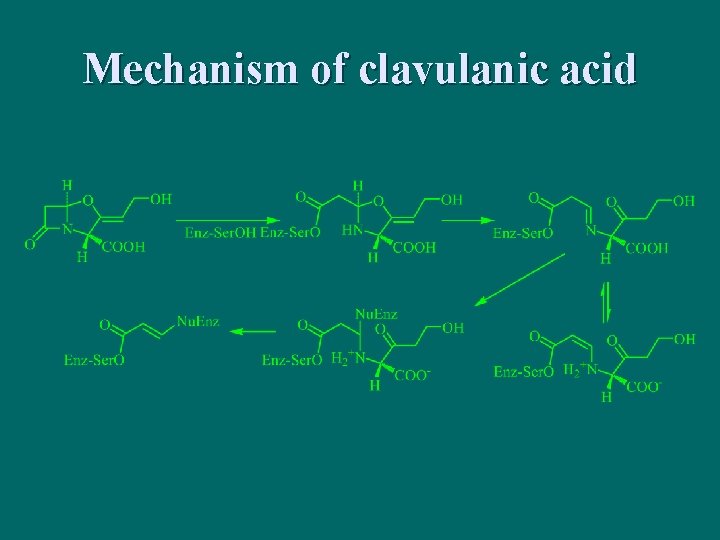
Mechanism of clavulanic acid
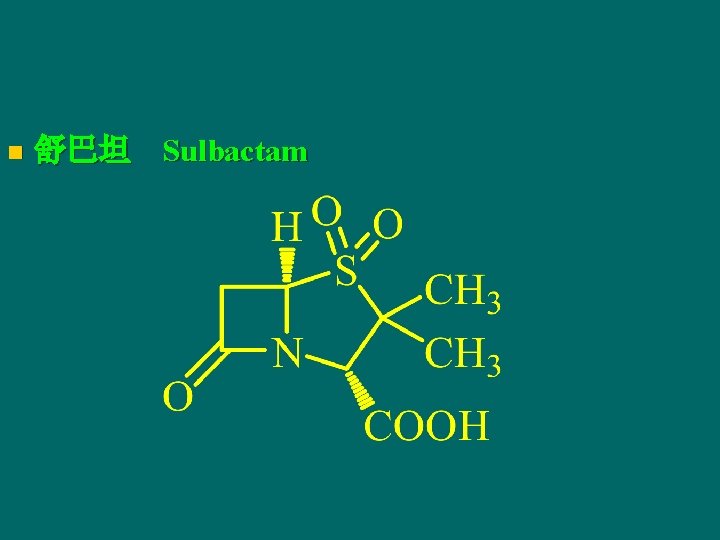
n 舒巴坦 Sulbactam
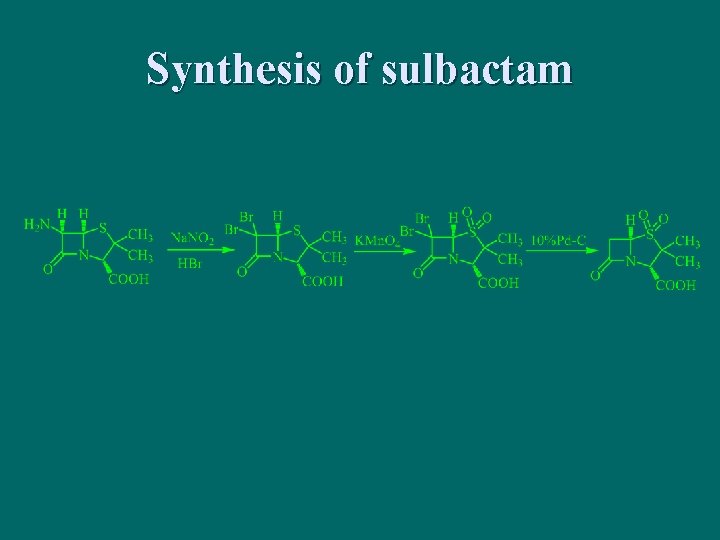
Synthesis of sulbactam
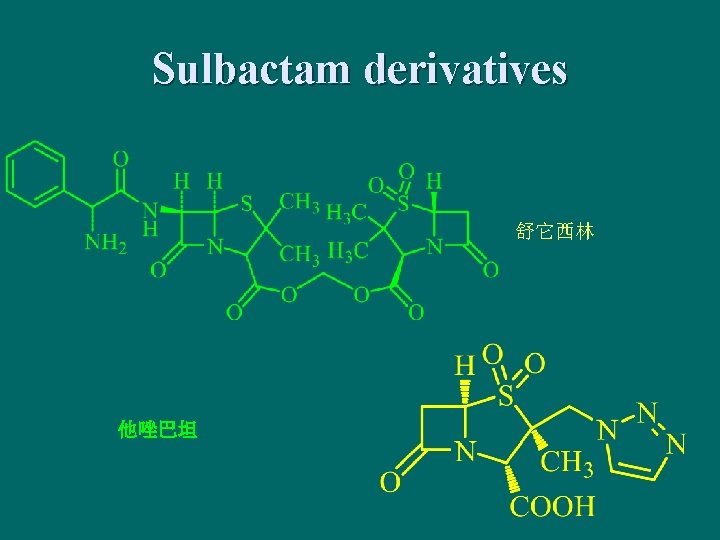
Sulbactam derivatives 舒它西林 他唑巴坦
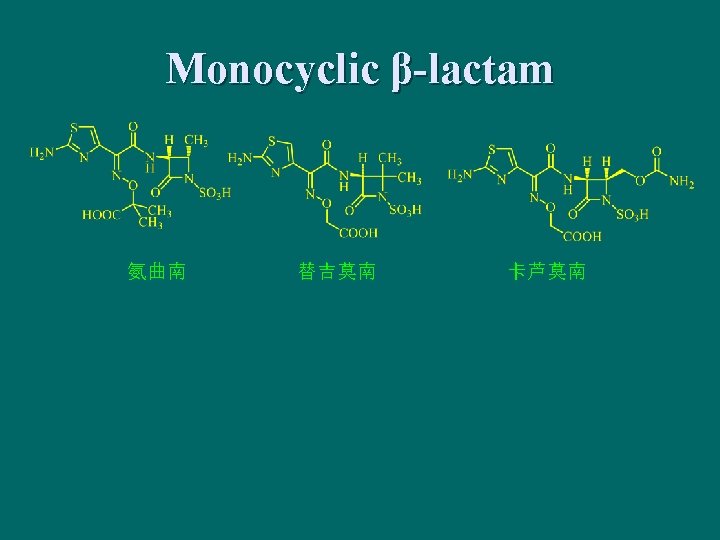

11. 3 Tetracycline Antibiotics n Naphthacene derivatives with (1, 2, 3, 4, 4 a, 5, 5 a, 6, 11 a, 12 a) -odecahydronaphthacene
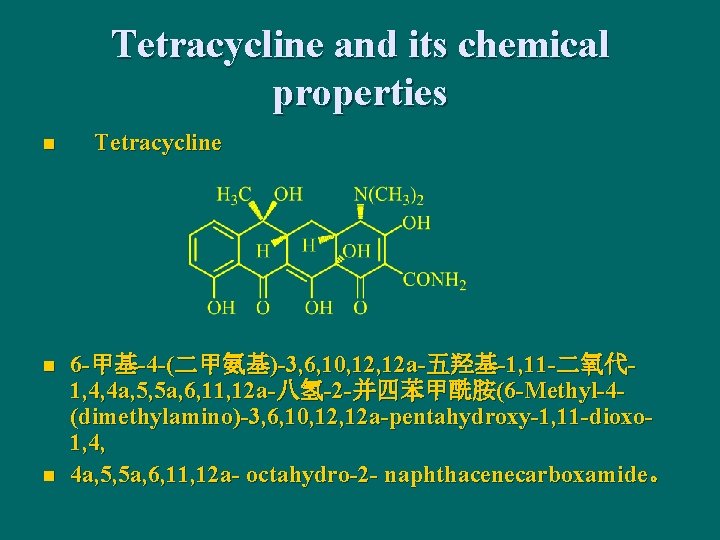
Tetracycline and its chemical properties n n n Tetracycline 6 -甲基-4 -(二甲氨基)-3, 6, 10, 12 a-五羟基-1, 11 -二氧代 1, 4, 4 a, 5, 5 a, 6, 11, 12 a-八氢-2 -并四苯甲酰胺(6 -Methyl-4(dimethylamino)-3, 6, 10, 12 a-pentahydroxy-1, 11 -dioxo 1, 4, 4 a, 5, 5 a, 6, 11, 12 a- octahydro-2 - naphthacenecarboxamide。

Tetracycline antibiotics has both the acid group of phenolic and enol hydroxyls and alkaline dimethylamino, so with the properties of acid and alkaline presenting three p. Ka values of 2. 8 ~ 3. 4, 7. 2 ~ 7. 8, 9. 1 ~ 9. 7. n Tetracyclines are relatively stable in solid state and dry condition, but may change color meeting sunlight. In acidic and alkaline conditions are not stable and prone to hydrolyze. n
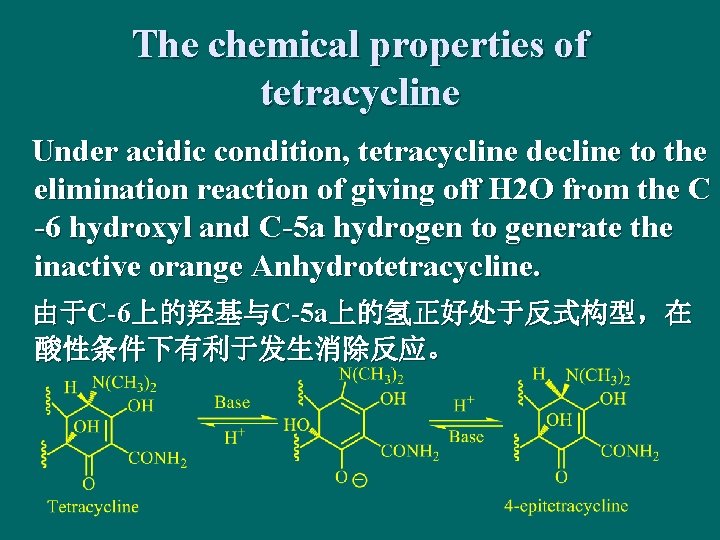
The chemical properties of tetracycline Under acidic condition, tetracycline decline to the elimination reaction of giving off H 2 O from the C -6 hydroxyl and C-5 a hydrogen to generate the inactive orange Anhydrotetracycline. 由于C-6上的羟基与C-5 a上的氢正好处于反式构型,在 酸性条件下有利于发生消除反应。

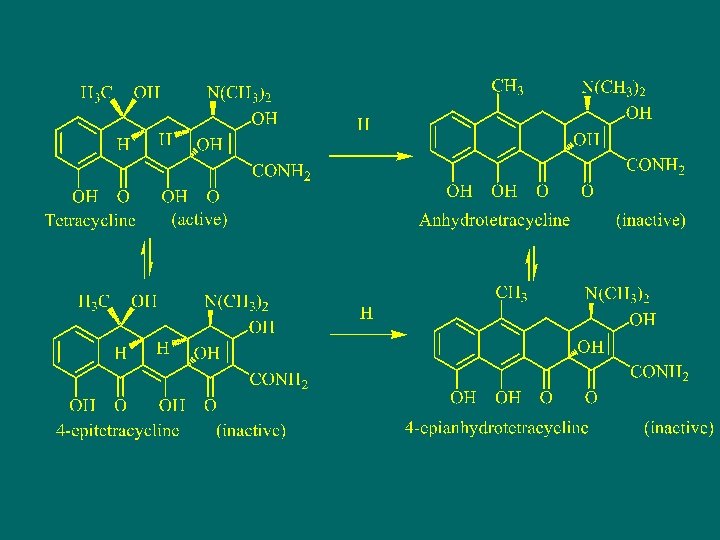
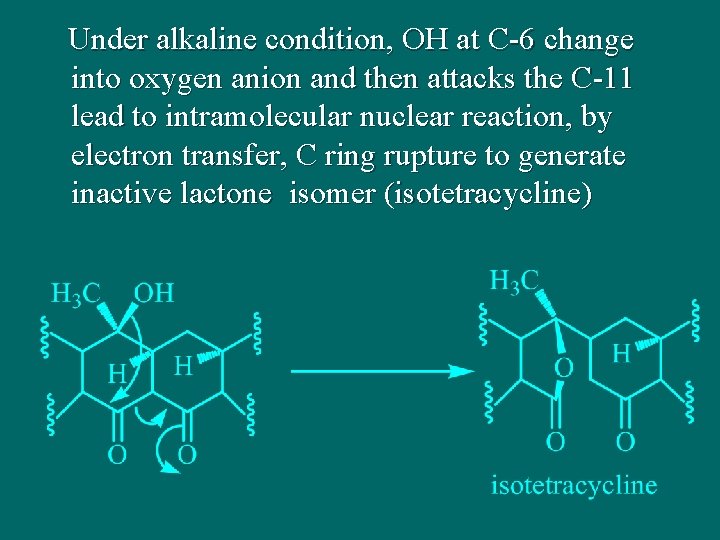
Under alkaline condition, OH at C-6 change into oxygen anion and then attacks the C-11 lead to intramolecular nuclear reaction, by electron transfer, C ring rupture to generate inactive lactone isomer (isotetracycline)
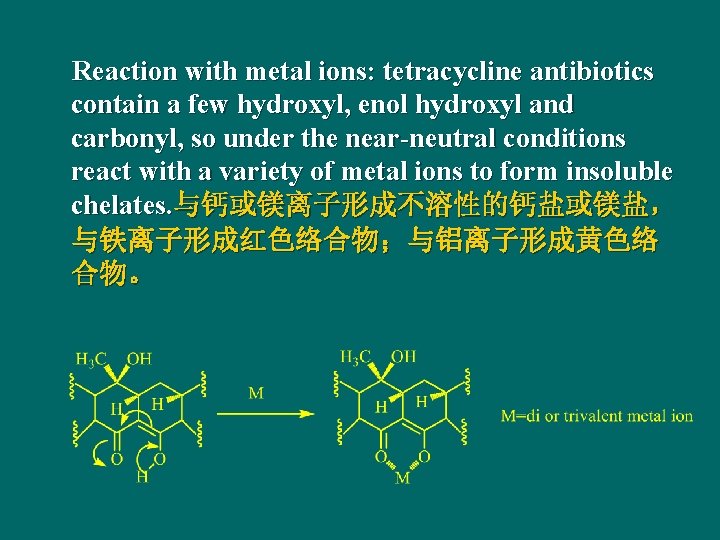
Reaction with metal ions: tetracycline antibiotics contain a few hydroxyl, enol hydroxyl and carbonyl, so under the near-neutral conditions react with a variety of metal ions to form insoluble chelates. 与钙或镁离子形成不溶性的钙盐或镁盐, 与铁离子形成红色络合物;与铝离子形成黄色络 合物。
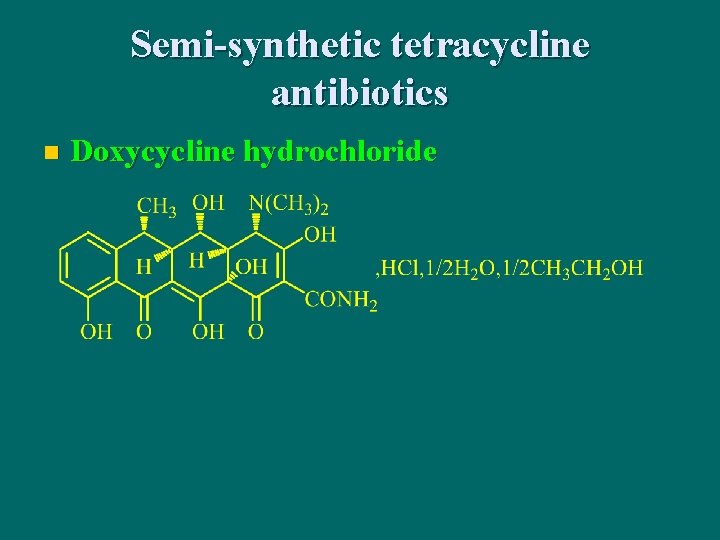
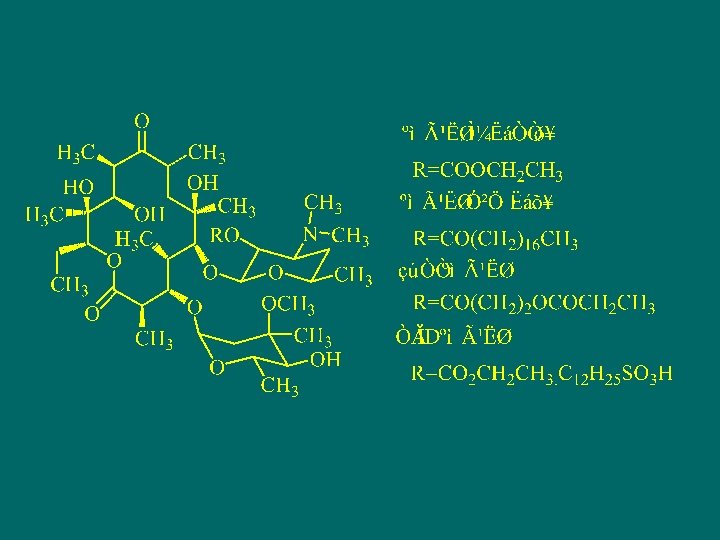
Semi-synthetic tetracycline antibiotics n Doxycycline hydrochloride
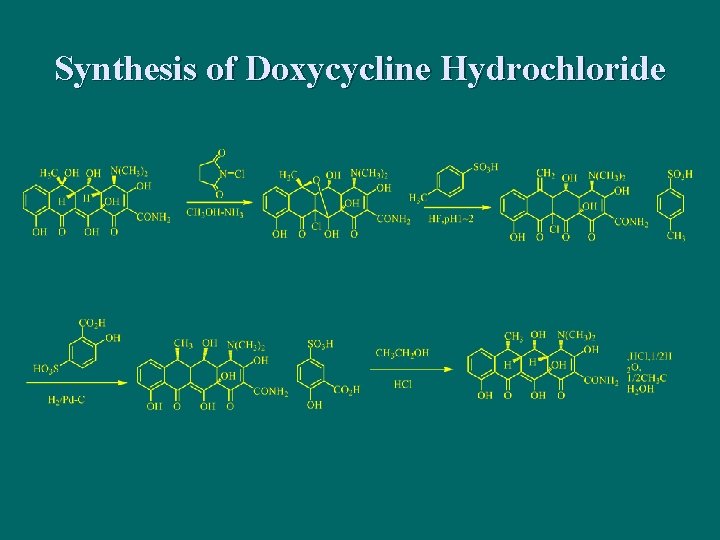
Synthesis of Doxycycline Hydrochloride
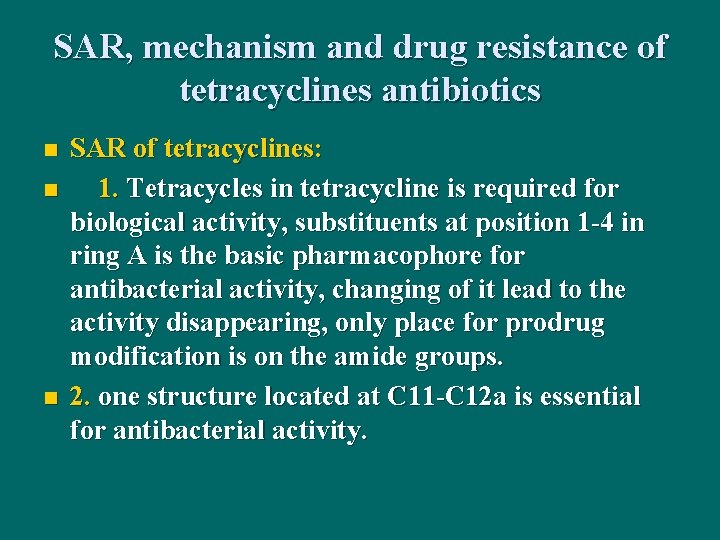
SAR, mechanism and drug resistance of tetracyclines antibiotics n n n SAR of tetracyclines: 1. Tetracycles in tetracycline is required for biological activity, substituents at position 1 -4 in ring A is the basic pharmacophore for antibacterial activity, changing of it lead to the activity disappearing, only place for prodrug modification is on the amide groups. 2. one structure located at C 11 -C 12 a is essential for antibacterial activity.

3. Substituents on places 5 -9 are not active groups, its transformation can change the antibacterial activity, chemical stability and pharmacokinetic properties of the drugs. 4. 6 carbon atom at place 6 substituted by sulfur can lead to the drugs with the antibacterial activity superior to doxycycline, and long-lasting, broad spectrum, high concentration after oral taking.

2. Drug resistance and mechanisms n Tetracyclines can inhibit the synthesis ribosomal protein of bacterial to inhibit the growth of bacteria, they are the broadspectrum antibiotics. n Tetracyclines-resistant bacteria has been found because of the long-term use of tetracycline. n

11. 4 Aminoglycoside Antibiotics n Aminoglycoside antibiotics arised from streptomyces, micromonospora and bacteria. The chemical structure of these antibiotics is usually composed of 1, 3 - diamino inositol and some certain amino sugars linked by glucoside bond.
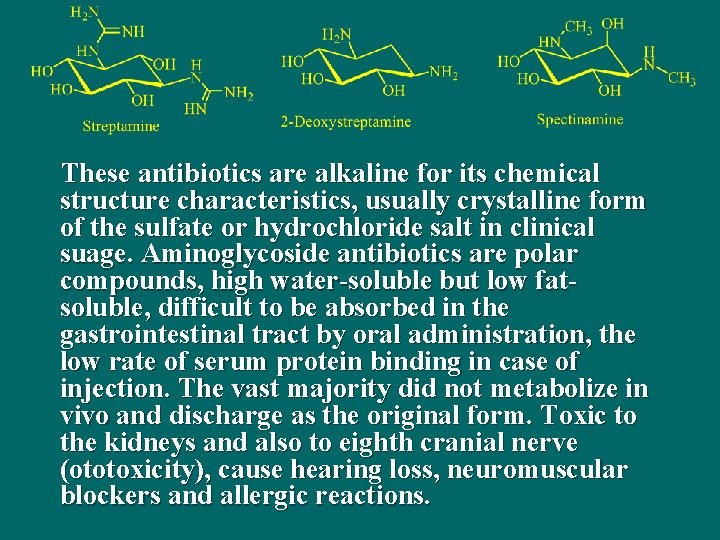
These antibiotics are alkaline for its chemical structure characteristics, usually crystalline form of the sulfate or hydrochloride salt in clinical suage. Aminoglycoside antibiotics are polar compounds, high water-soluble but low fatsoluble, difficult to be absorbed in the gastrointestinal tract by oral administration, the low rate of serum protein binding in case of injection. The vast majority did not metabolize in vivo and discharge as the original form. Toxic to the kidneys and also to eighth cranial nerve (ototoxicity), cause hearing loss, neuromuscular blockers and allergic reactions.

Mechanism of aminoglycoside antibiotics n Inhibit biosynthesis of bacterial protein to kill them.

Mechanism of drug resistance n To produce inactivating enzyme to change the structure of aminoglycosides leading to the loss of antibacterial activity, or by changing the permeability of bacterial cell membrane lead to non-specific drug resistance. Aminoglycoside antibiotics usually have cross-resistance.
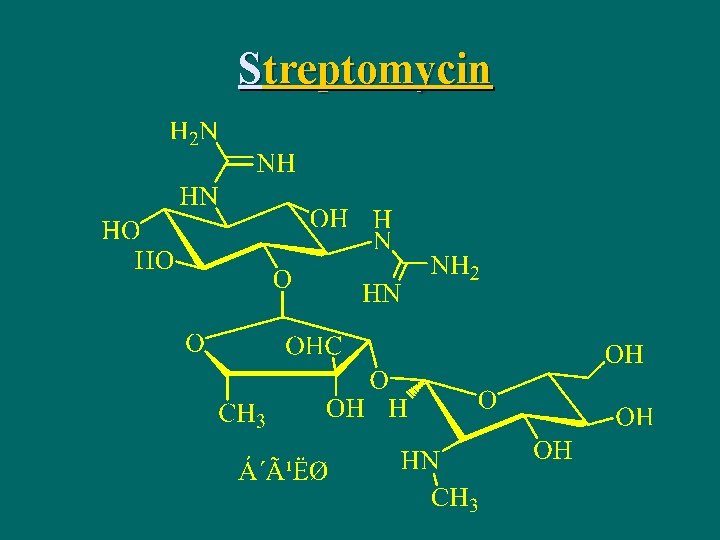
Streptomycin
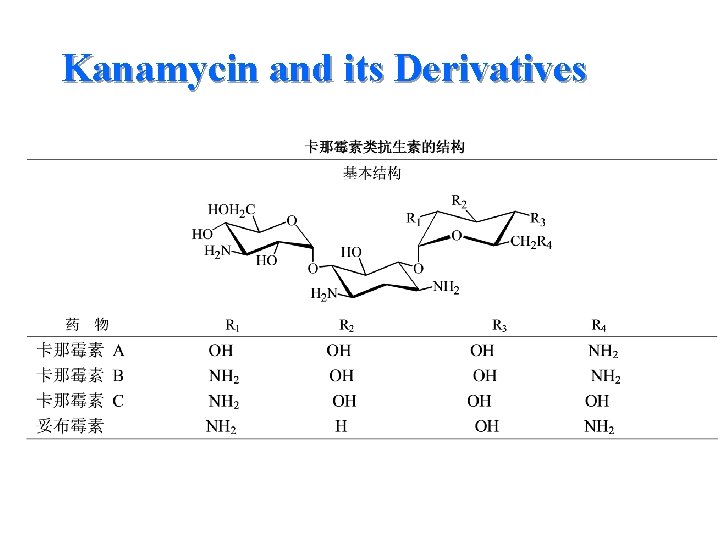
Kanamycin and its Derivatives
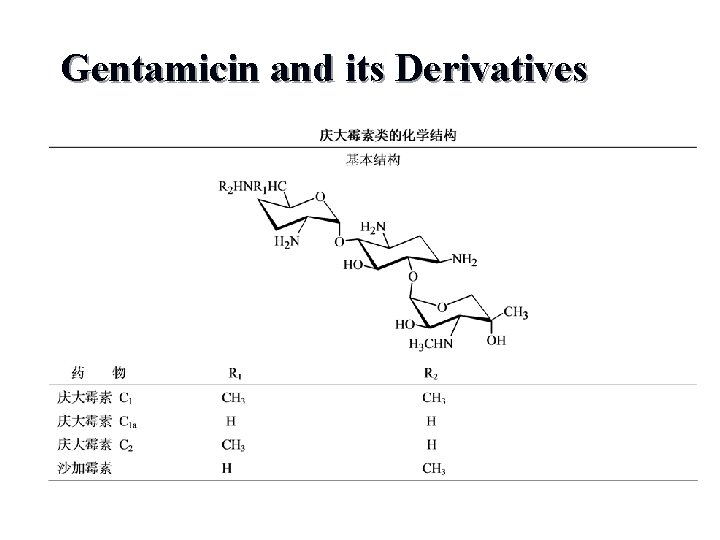
Gentamicin and its Derivatives

11. 5 Macrolide Antibiotics n n n Structural characteristic, physicochemical property、and mechanism of Macrolide Antibiotics 1. Structural characteristic Macrolide antibiotics produced by Streptomyces, a kind of alkalescence antibiotics, the molecular structure containing a lactone macrocycle formed by fourteen or 16 atoms and there is a alkaline glycoside formed by hydroxyl on lactone and amino-deoxy sugar or 6 - deoxy sugar. 此类药物的碱性 较弱,大约为p. H 8,游离的碱不溶水,其葡庚糖酸盐和乳 糖酸盐的水溶解度较大,而其他盐如硬脂酸盐和十二烷硫 酸盐的水溶度降低。

n n n 2. Physicochemical properties Generally macrolide antibiotics are alkaline compounds, colorless, soluble in organic solvents. Can change into salt with acids, the salt is soluble in water, chemically unstable in acidic conditions, the glycoside bond are susceptible to hydrolysis and when breakdown of the lactone ring under alkali conditions. 大环内酯类抗生素在微生物合成过程中往往产生 结构近似、性质相仿的多种成分。当菌种或生产 艺不同时,常使产品中各成分的比例有明显不 同,影响产品的质量。


Erythromycin and its Derivatives n Erythromycin was discovered in 1952 from Streptomyces erythreus, including erythromycin A, B and C. 红霉素A为抗菌 主要成分,C的活性较弱,只为A的1/5,而 毒性则为 5倍,B不仅活性低且毒性大。
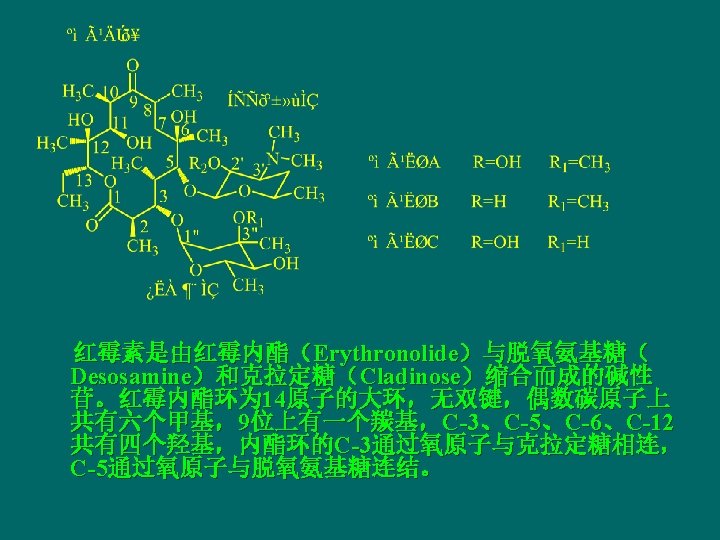
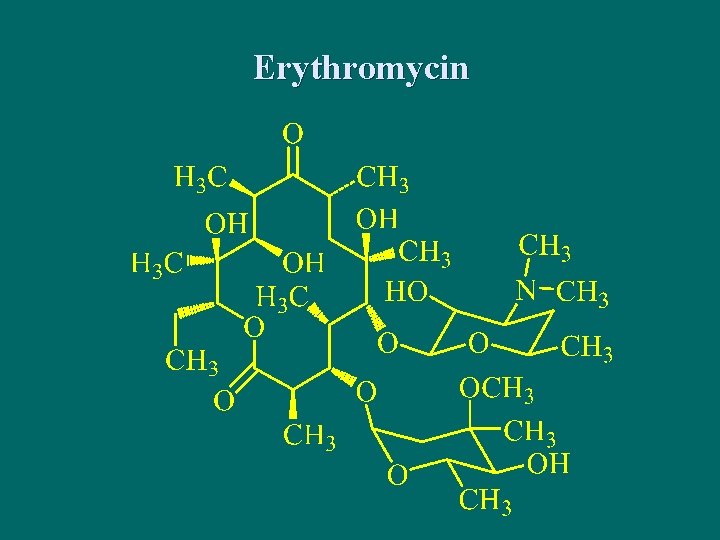
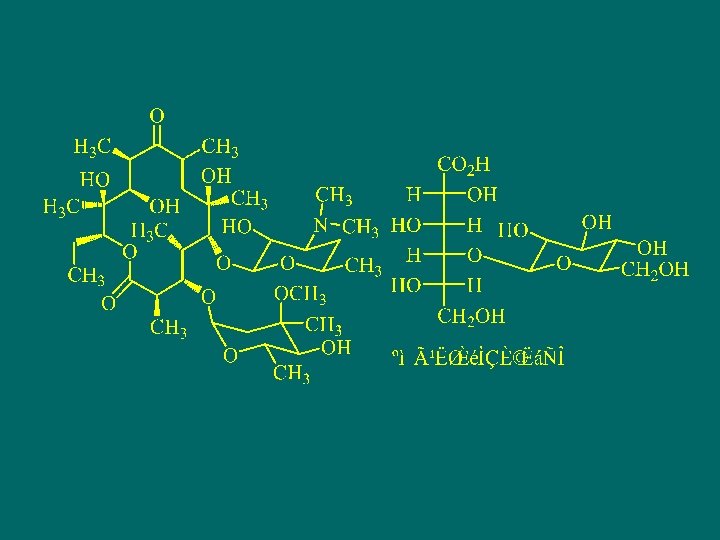
Erythromycin
![3 -[(2, 6 -二脱氧-3 -C-甲基-3 -O-甲基l-α-L-核-己吡喃 糖基)氧] -13 -乙基- 6, 11, 12 -三羟基-2, 4, 3 -[(2, 6 -二脱氧-3 -C-甲基-3 -O-甲基l-α-L-核-己吡喃 糖基)氧] -13 -乙基- 6, 11, 12 -三羟基-2, 4,](http://slidetodoc.com/presentation_image_h2/d3e7130fccffc667168d50e30fb3ab6d/image-97.jpg)
3 -[(2, 6 -二脱氧-3 -C-甲基-3 -O-甲基l-α-L-核-己吡喃 糖基)氧] -13 -乙基- 6, 11, 12 -三羟基-2, 4, 6, 8, 10, 12 -六甲基-5 -[[3, 4, 6 -三脱氧-3 -(二甲氨基)-β-D-木己吡喃糖基]氧] -1 -氧杂环十四烷-1, 9 -二酮 3 -[(2, , 6 Dideoxy-3 -C-methyl-3 -O-methyl-α-L-ribohexopyranosyl)oxy]-13 -ethyl -6, 11, 12 -trihydroxy 2, 4, 6, 8, 10, 12 -hexamethyl-5 -[[3, 4, 6 -trideoxy-3(dimethyl-amino)-β-D-xylo-hexopyranosyl] oxy]oxacyclo- tetradecan-1, 9 -dione。

The physicochemistry properties of erythromycin n Under acidic conditions, erythromycin is not stable and prone to produce intramolecular dehydration cyclization between carbonyl at 9 and several hydroxys. 在酸性液中,红霉素C- 6上的羟基与C-9的羰基形成半缩酮的羟基,再与 C-8上氢消去一分子水,生成 8,9 -脱水-6,9 -半缩 酮衍生物。然后C-12上的羟基与C-8 -C-9双键加成, 进行分子内环合,生成 6,9,-9,12 -螺旋酮;最 后其C-11羟基与C-10上的氢消去一分子水,同时 水解成红霉胺和克拉定糖。这种降解反应使红霉 素失去抗菌活性
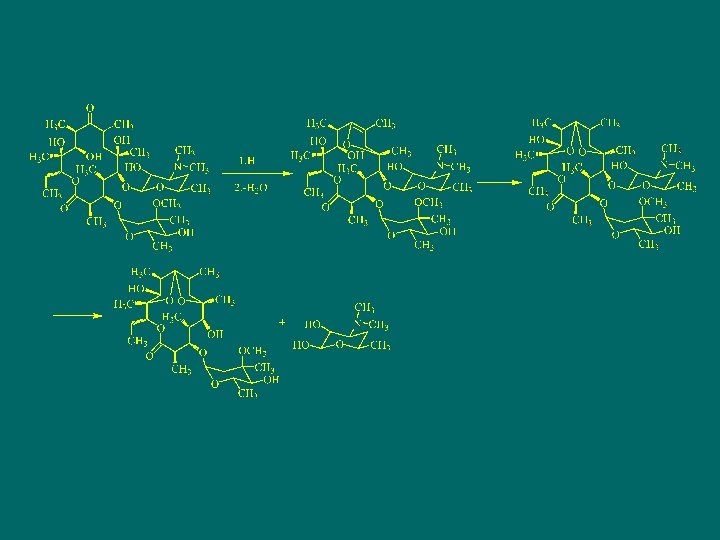
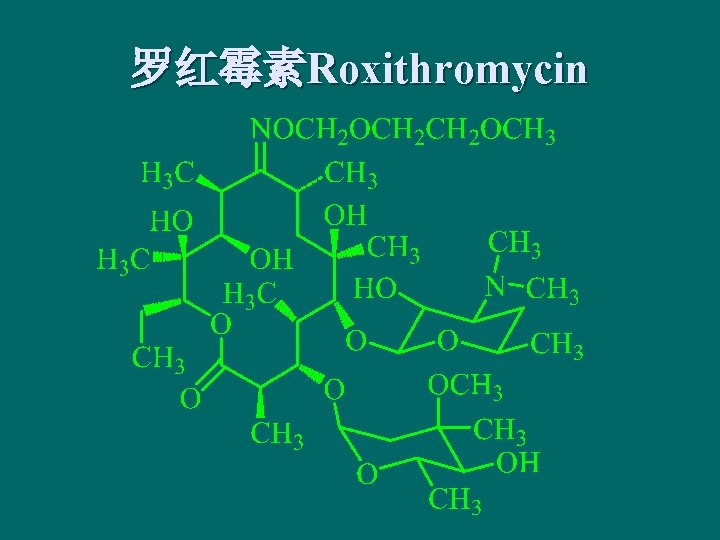
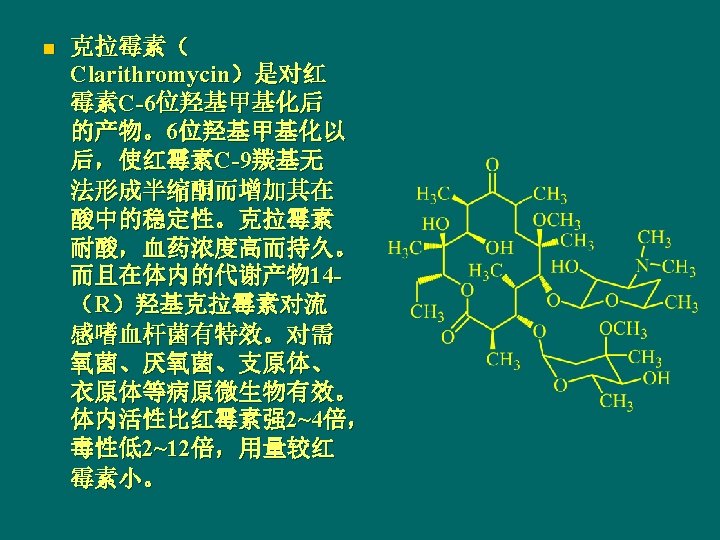
罗红霉素Roxithromycin
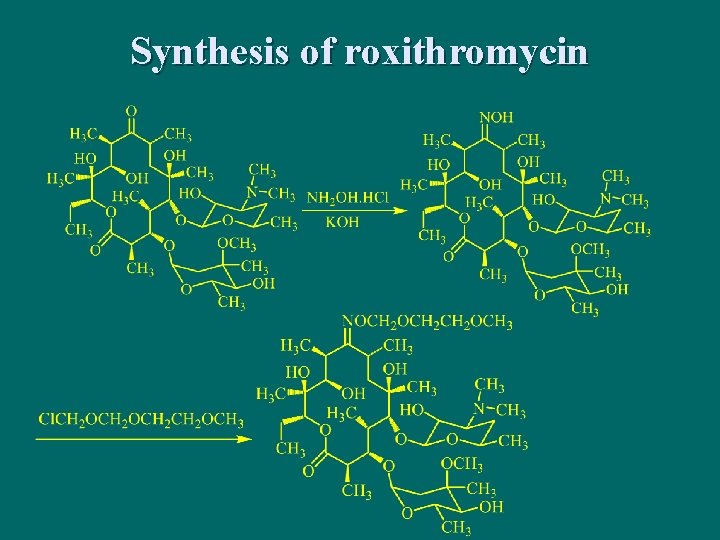
Synthesis of roxithromycin
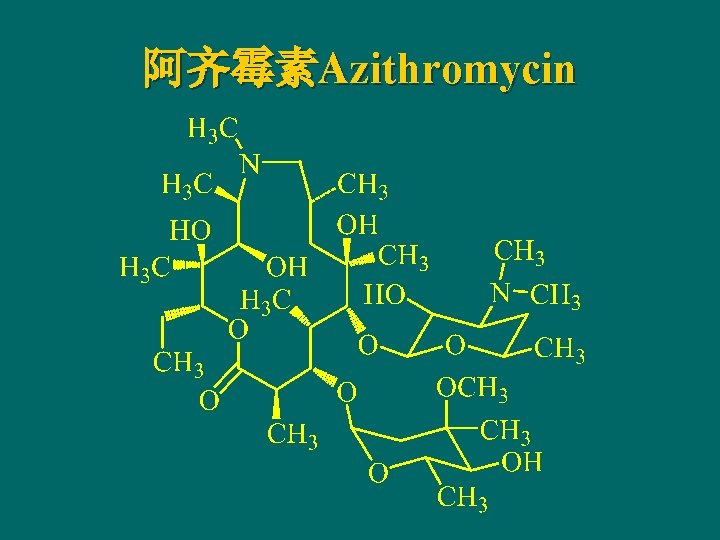
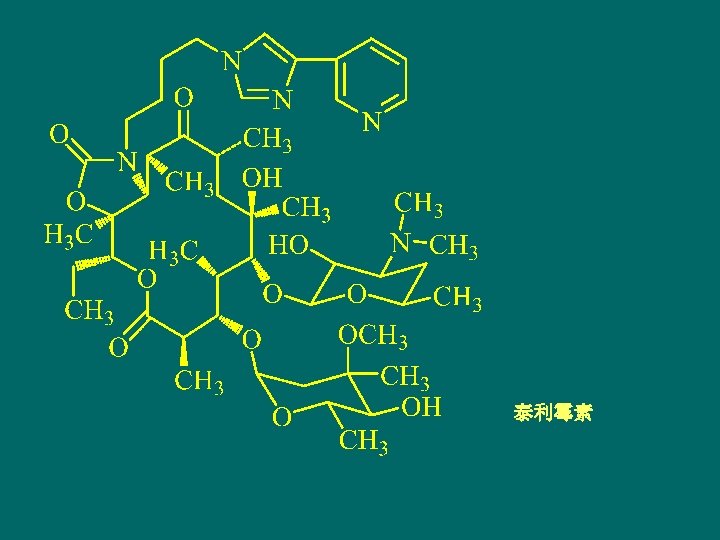
阿齐霉素Azithromycin

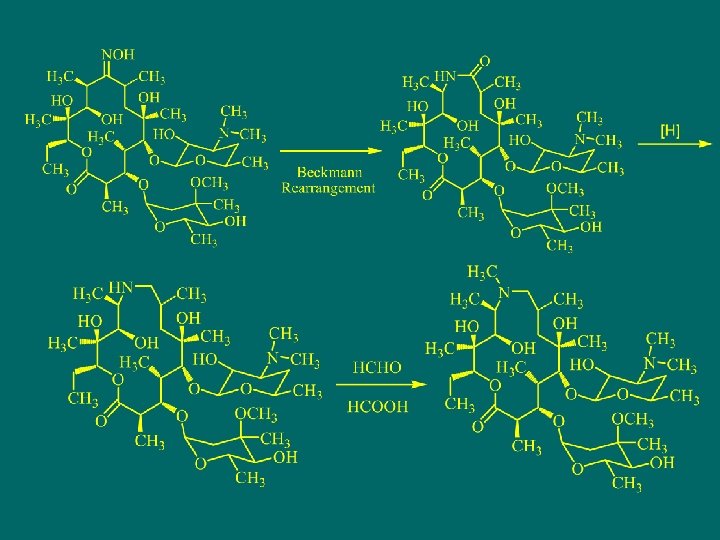
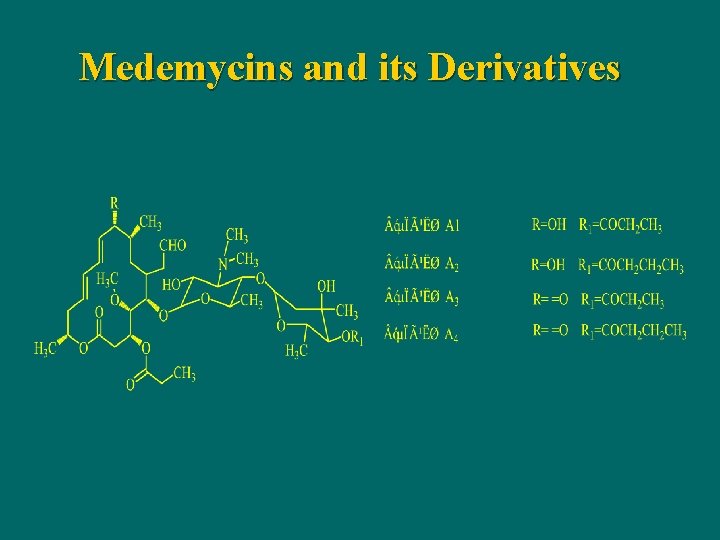
Medemycins and its Derivatives
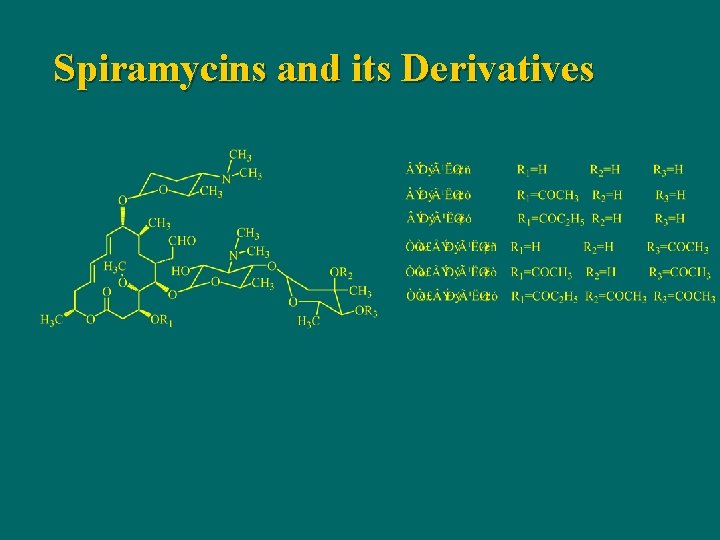
Spiramycins and its Derivatives

11. 6 Miscellaneous Antibiotics n Chloramphenicol and its Derivatives 氯霉素 琥珀氯霉素 甲砜霉素 棕榈氯霉素


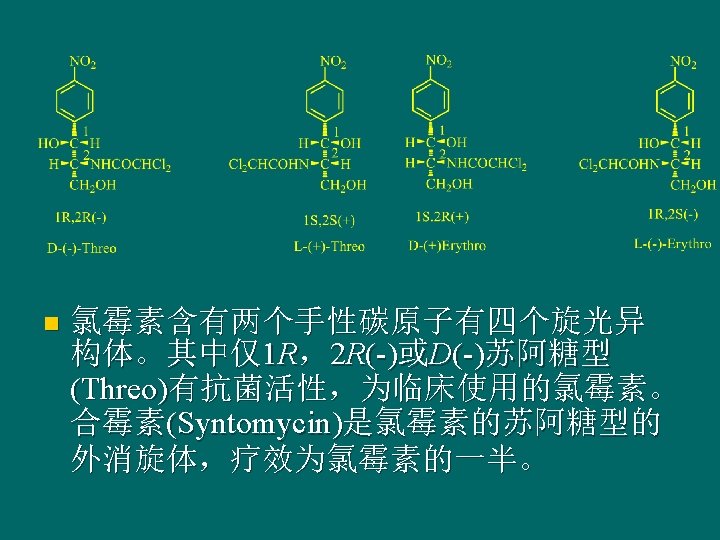
Structure-activity relationships of chloramphenicol n n n Para-nitro of benzene ring is necessary to activity, o -, meta-substituted is no activity. Benzene ring is necessary active group. Chloramphenicol is highly specific stereochemistry, only the 1 R, 2 R-D (-) isomer showed antibacterial activity. The activity is strongest in case of dichloro-acetylamino as side chain, and other substituents decreased activity.
![氯霉素 Chloramphenicol D-苏式-(-)-N-[a-(羟基甲基)-b-羟基对硝基苯 乙基-2, 2 -二氯乙酰胺(D-Threon (-)-N-[a-(hydroxymethyl)-b-hydroxy-pnitrophenethyl-2, 2 -dichloro acetamine])。 n 氯霉素 Chloramphenicol D-苏式-(-)-N-[a-(羟基甲基)-b-羟基对硝基苯 乙基-2, 2 -二氯乙酰胺(D-Threon (-)-N-[a-(hydroxymethyl)-b-hydroxy-pnitrophenethyl-2, 2 -dichloro acetamine])。 n](http://slidetodoc.com/presentation_image_h2/d3e7130fccffc667168d50e30fb3ab6d/image-115.jpg)
氯霉素 Chloramphenicol D-苏式-(-)-N-[a-(羟基甲基)-b-羟基对硝基苯 乙基-2, 2 -二氯乙酰胺(D-Threon (-)-N-[a-(hydroxymethyl)-b-hydroxy-pnitrophenethyl-2, 2 -dichloro acetamine])。 n
The physical properties of chloramphenicol n white or light yellow-green needles, long sheet crystal or crystalline powder, bitter taste; mp. 149 ~ 152 ℃. soluble in methanol, acetone or propylene glycol, slightly soluble in water. In anhydrous ethanol was right rotation +18. 5 ~ +21. 5 °; in ethyl acetate was levorotary -25. 5 °.
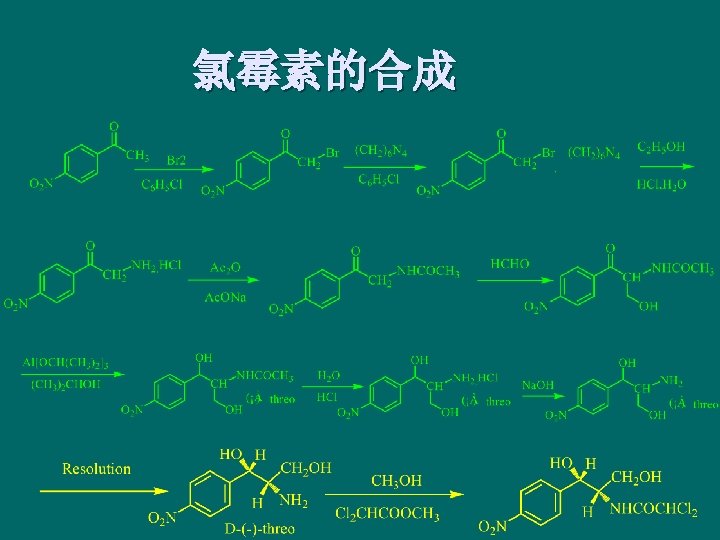
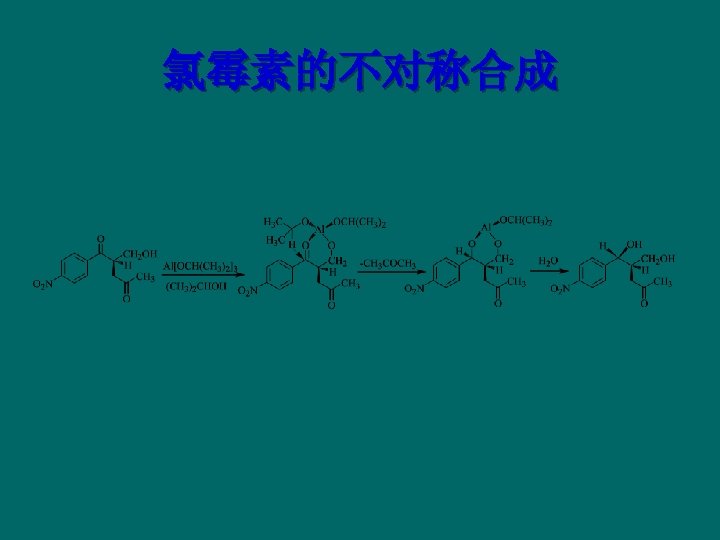

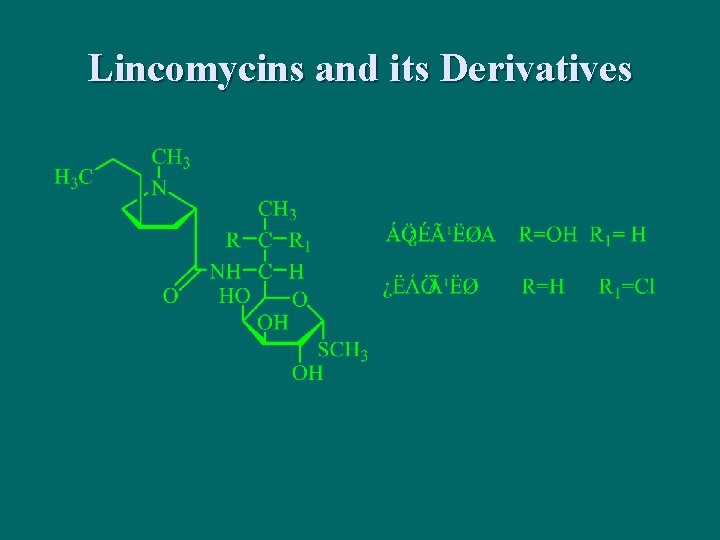
Lincomycins and its Derivatives
- Slides: 121