Chapter 11 ALKANES Alkanes Are hydrocarbons that contain

Chapter 11 ALKANES

Alkanes • Are hydrocarbons that contain only C-C single bonds • General Formula: Cn. H 2 n + 2 for compounds that contain only C and H atoms

Condensed vs Structural Formula • Condensed Formula: Ex. CH 4 • Structural Formula: shows all bonds • Ex. H | H - C - H | H
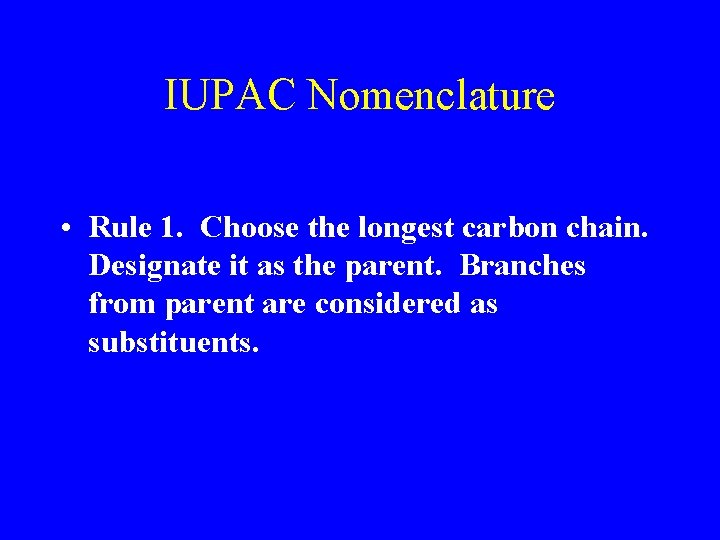
IUPAC Nomenclature • Rule 1. Choose the longest carbon chain. Designate it as the parent. Branches from parent are considered as substituents.
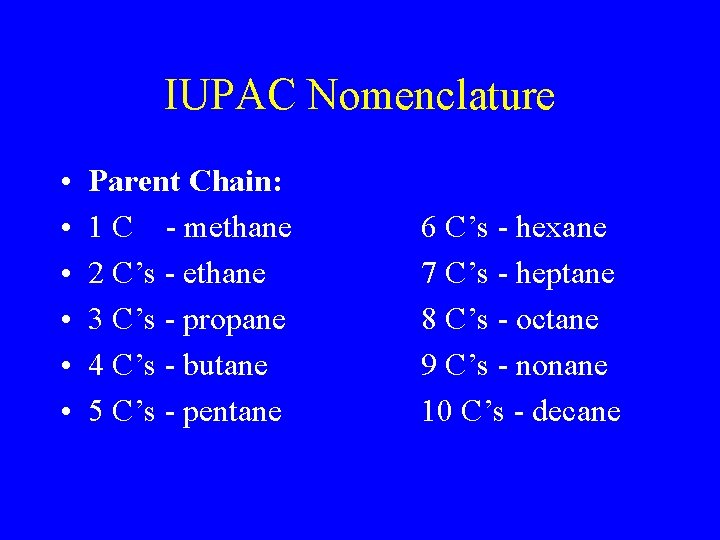
IUPAC Nomenclature • • • Parent Chain: 1 C - methane 2 C’s - ethane 3 C’s - propane 4 C’s - butane 5 C’s - pentane 6 C’s - hexane 7 C’s - heptane 8 C’s - octane 9 C’s - nonane 10 C’s - decane

IUPAC Nomenclature • • • Substituent: (ends in -yl) 1 C - methyl 6 C’s - hexyl 2 C’s - ethyl 7 C’s - heptyl 3 C’s - propyl 8 C’s - octyl 4 C’s - butyl 9 C’s - nonyl 5 C’s - pentyl 10 C’s - decyl
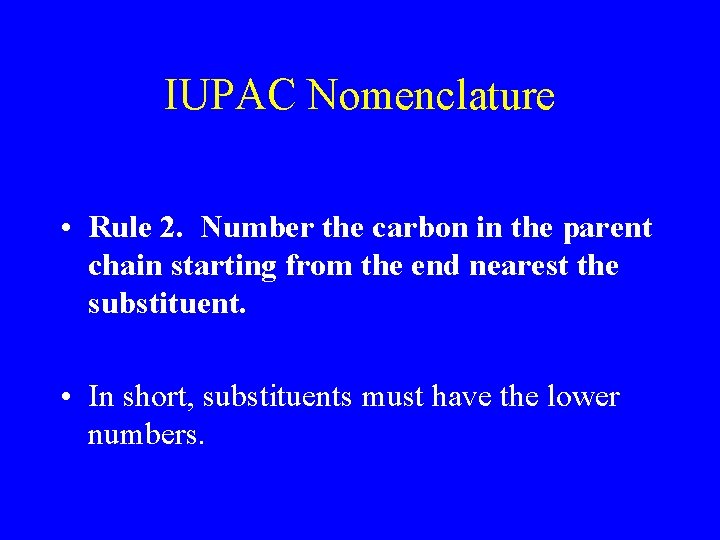
IUPAC Nomenclature • Rule 2. Number the carbon in the parent chain starting from the end nearest the substituent. • In short, substituents must have the lower numbers.
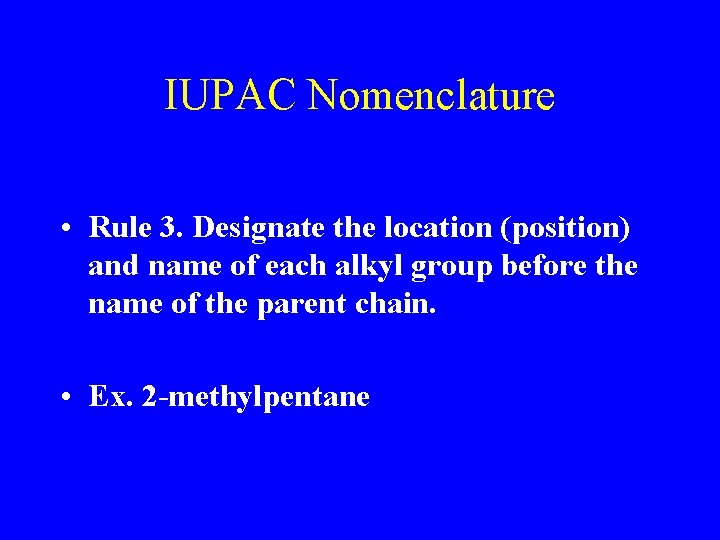
IUPAC Nomenclature • Rule 3. Designate the location (position) and name of each alkyl group before the name of the parent chain. • Ex. 2 -methylpentane

IUPAC Nomenclature • Rule 4. List the substituents in alphabetical order
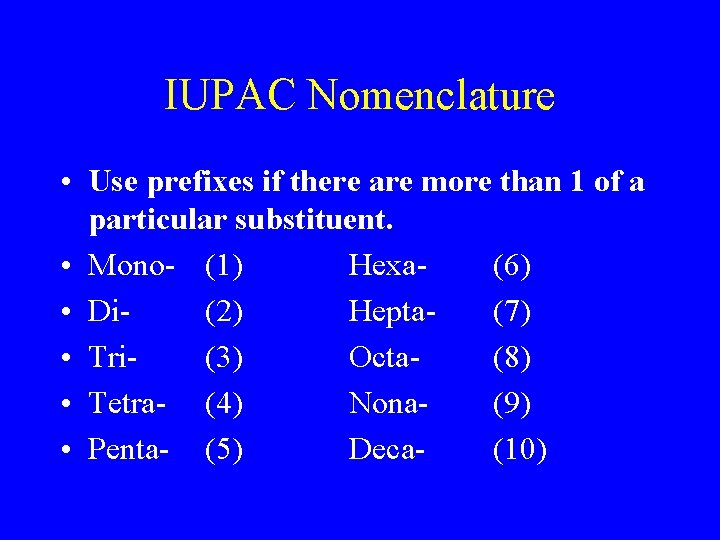
IUPAC Nomenclature • Use prefixes if there are more than 1 of a particular substituent. • Mono- (1) Hexa(6) • Di(2) Hepta(7) • Tri(3) Octa(8) • Tetra- (4) Nona(9) • Penta- (5) Deca(10)

Haloalkanes and Cycloalkanes • Nomenclature for haloalkanes: Same as simple alkanes • • Nomenclature of cycloalkanes: Same as simple alkanes, however, add the term “cyclo” before parent name

Chemical Properties • The greater the number of C atoms, the higher the boiling point.
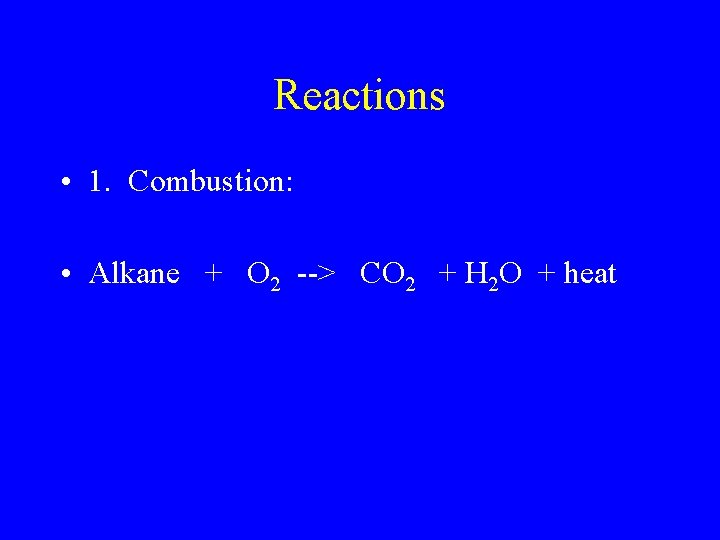
Reactions • 1. Combustion: • Alkane + O 2 --> CO 2 + H 2 O + heat
- Slides: 13