Chapter 10 The Mole 6 5 Molar Mass


- Slides: 21

Chapter 10 The Mole 6. 5 Molar Mass General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 1

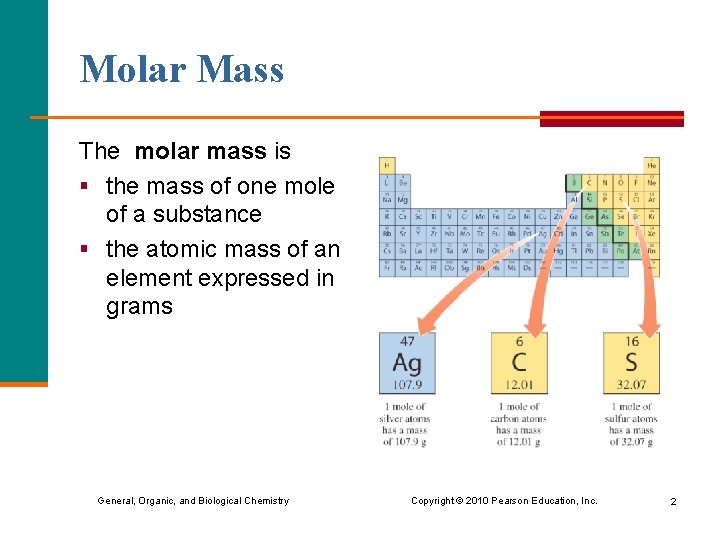
Molar Mass The molar mass is § the mass of one mole of a substance § the atomic mass of an element expressed in grams General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 2

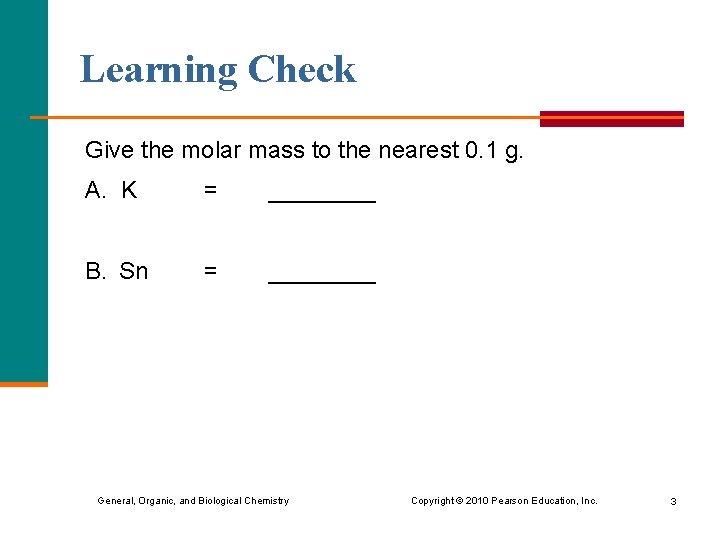
Learning Check Give the molar mass to the nearest 0. 1 g. A. K = ____ B. Sn = ____ General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 3

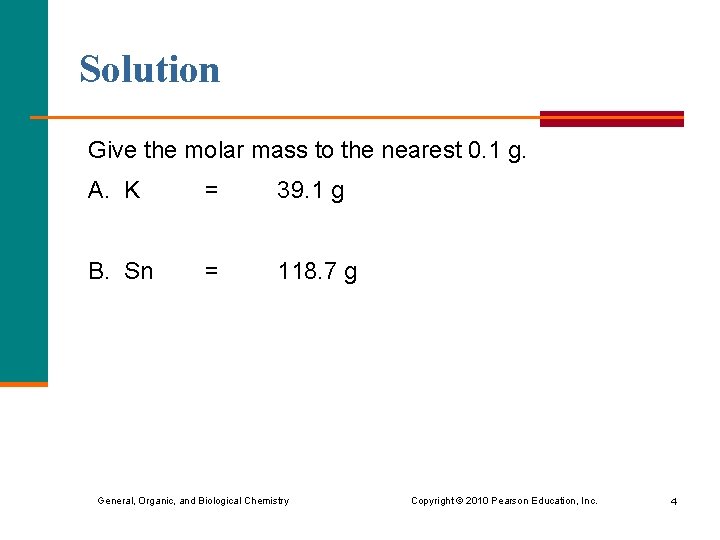
Solution Give the molar mass to the nearest 0. 1 g. A. K = 39. 1 g B. Sn = 118. 7 g General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 4

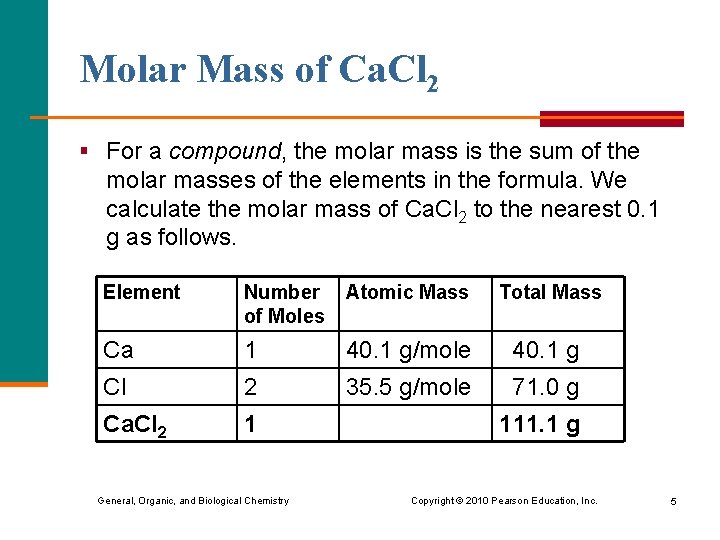
Molar Mass of Ca. Cl 2 § For a compound, the molar mass is the sum of the molar masses of the elements in the formula. We calculate the molar mass of Ca. Cl 2 to the nearest 0. 1 g as follows. Element Number of Moles Atomic Mass Total Mass Ca 1 40. 1 g/mole 40. 1 g Cl 2 35. 5 g/mole 71. 0 g Ca. Cl 2 1 General, Organic, and Biological Chemistry 111. 1 g Copyright © 2010 Pearson Education, Inc. 5

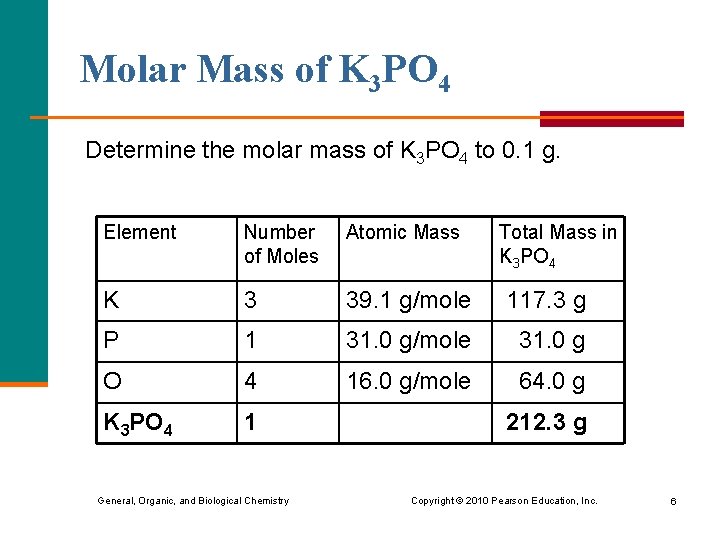
Molar Mass of K 3 PO 4 Determine the molar mass of K 3 PO 4 to 0. 1 g. Element Number of Moles Atomic Mass K 3 39. 1 g/mole 117. 3 g P 1 31. 0 g/mole 31. 0 g O 4 16. 0 g/mole 64. 0 g K 3 PO 4 1 General, Organic, and Biological Chemistry Total Mass in K 3 PO 4 212. 3 g Copyright © 2010 Pearson Education, Inc. 6

One-Mole Quantities 32. 1 g 55. 9 g General, Organic, and Biological Chemistry 58. 5 g 294. 2 g 342. 3 g Copyright © 2010 Pearson Education, Inc. 7

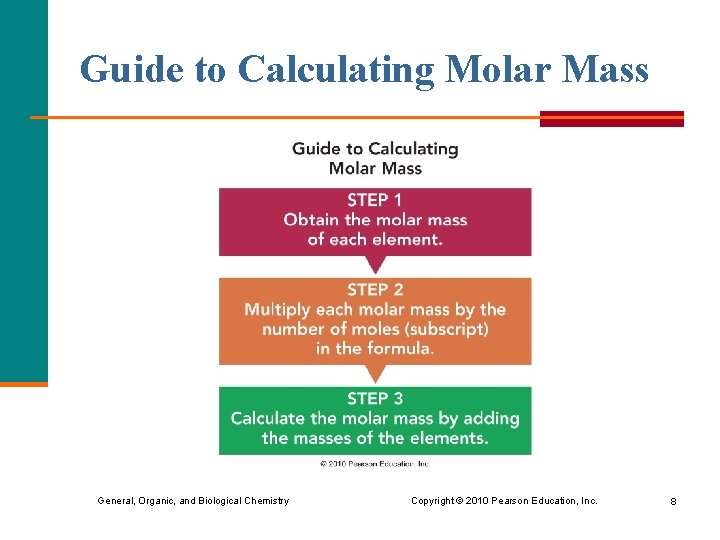
Guide to Calculating Molar Mass General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 8

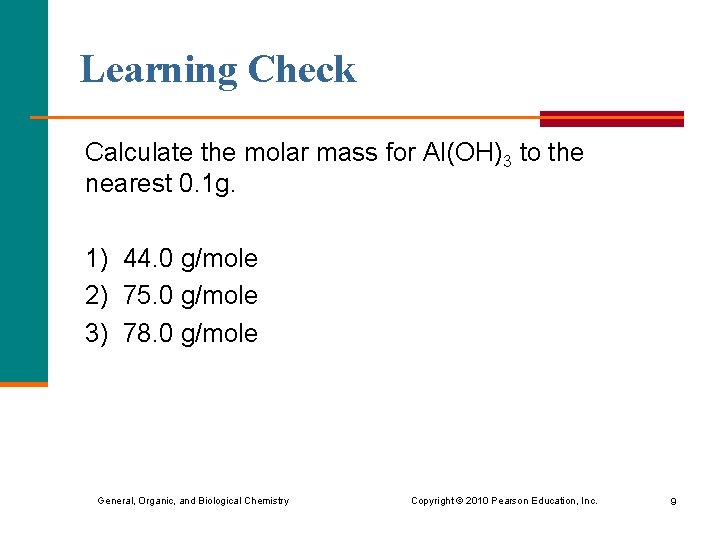
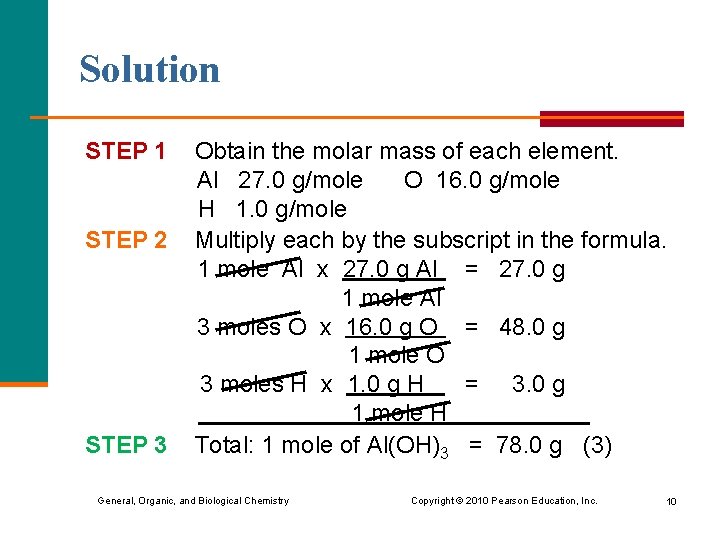
Learning Check Calculate the molar mass for Al(OH)3 to the nearest 0. 1 g. 1) 44. 0 g/mole 2) 75. 0 g/mole 3) 78. 0 g/mole General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 9

Solution STEP 1 STEP 2 STEP 3 Obtain the molar mass of each element. Al 27. 0 g/mole O 16. 0 g/mole H 1. 0 g/mole Multiply each by the subscript in the formula. 1 mole Al x 27. 0 g Al = 27. 0 g 1 mole Al 3 moles O x 16. 0 g O = 48. 0 g 1 mole O 3 moles H x 1. 0 g H = 3. 0 g 1 mole H Total: 1 mole of Al(OH)3 = 78. 0 g (3) General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 10

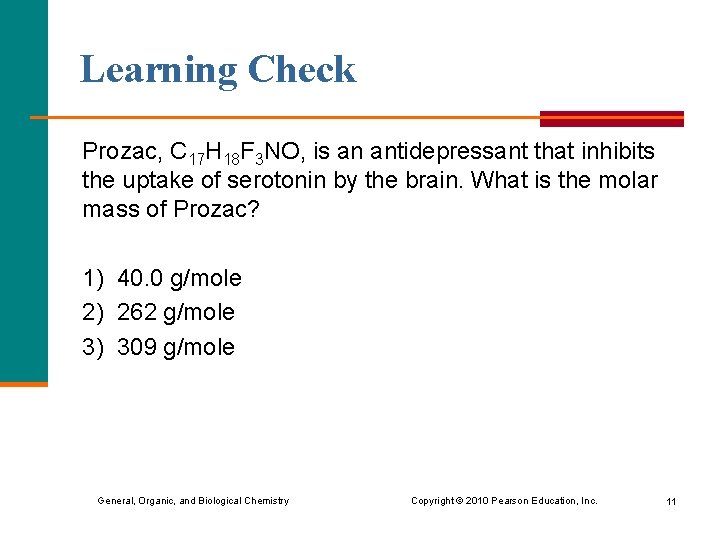
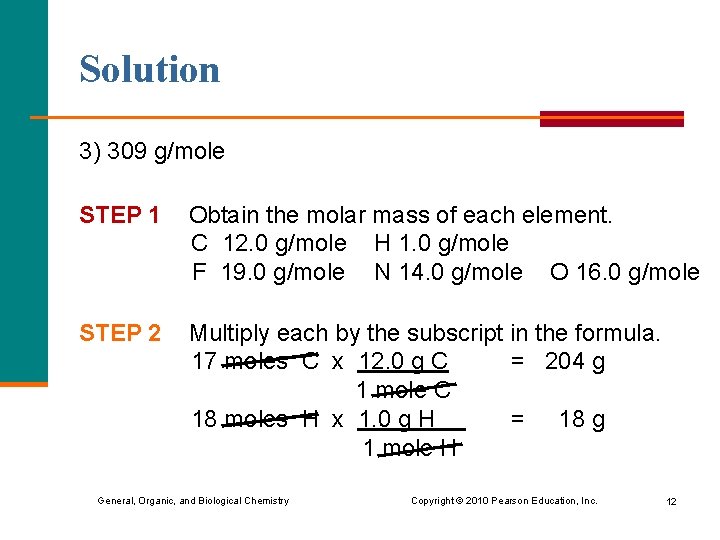
Learning Check Prozac, C 17 H 18 F 3 NO, is an antidepressant that inhibits the uptake of serotonin by the brain. What is the molar mass of Prozac? 1) 40. 0 g/mole 2) 262 g/mole 3) 309 g/mole General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 11

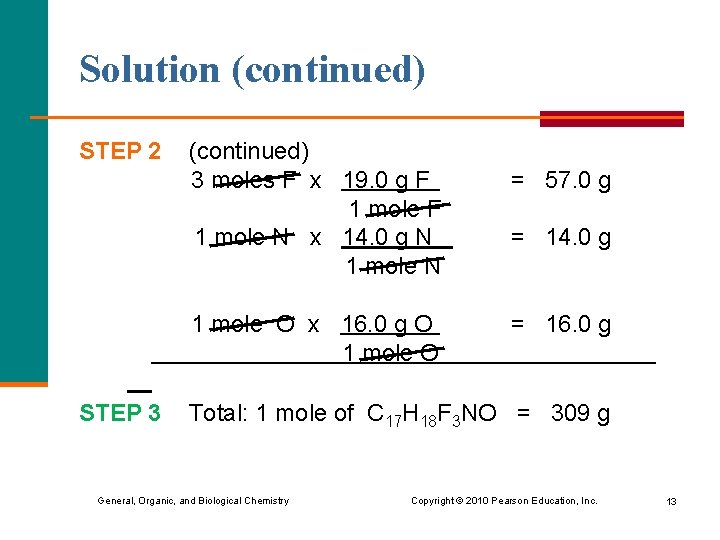
Solution 3) 309 g/mole STEP 1 Obtain the molar mass of each element. C 12. 0 g/mole H 1. 0 g/mole F 19. 0 g/mole N 14. 0 g/mole O 16. 0 g/mole STEP 2 Multiply each by the subscript in the formula. 17 moles C x 12. 0 g C = 204 g 1 mole C 18 moles H x 1. 0 g H = 18 g 1 mole H General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 12

Solution (continued) STEP 2 (continued) 3 moles F x 19. 0 g F 1 mole N x 14. 0 g N 1 mole O x 16. 0 g O 1 mole O STEP 3 = 57. 0 g = 14. 0 g = 16. 0 g Total: 1 mole of C 17 H 18 F 3 NO = 309 g General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 13

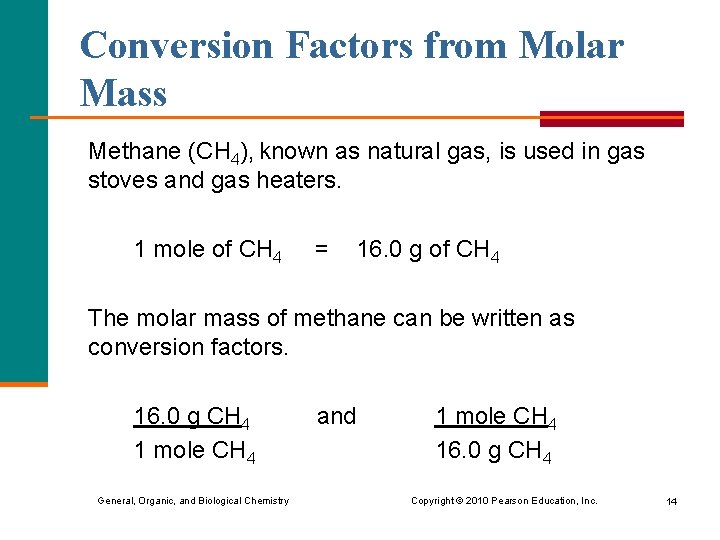
Conversion Factors from Molar Mass Methane (CH 4), known as natural gas, is used in gas stoves and gas heaters. 1 mole of CH 4 = 16. 0 g of CH 4 The molar mass of methane can be written as conversion factors. 16. 0 g CH 4 1 mole CH 4 General, Organic, and Biological Chemistry and 1 mole CH 4 16. 0 g CH 4 Copyright © 2010 Pearson Education, Inc. 14

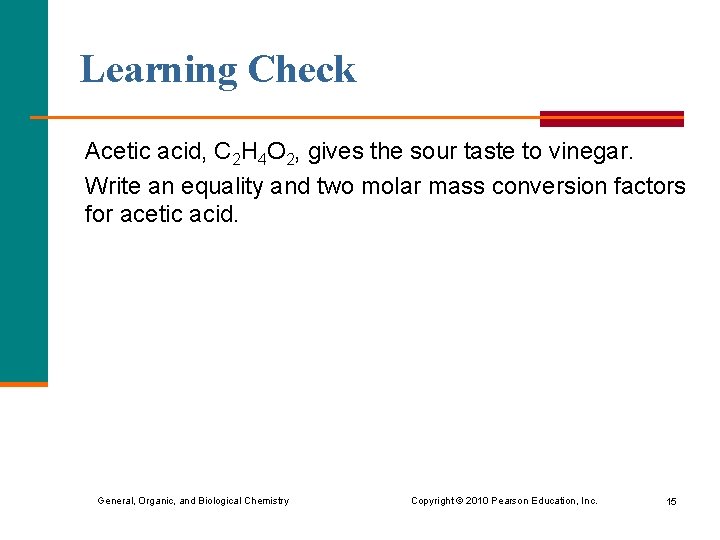
Learning Check Acetic acid, C 2 H 4 O 2, gives the sour taste to vinegar. Write an equality and two molar mass conversion factors for acetic acid. General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 15

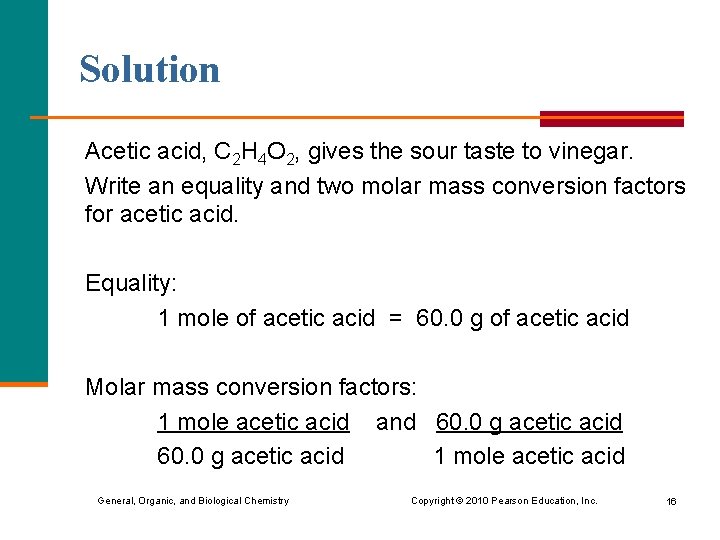
Solution Acetic acid, C 2 H 4 O 2, gives the sour taste to vinegar. Write an equality and two molar mass conversion factors for acetic acid. Equality: 1 mole of acetic acid = 60. 0 g of acetic acid Molar mass conversion factors: 1 mole acetic acid and 60. 0 g acetic acid 1 mole acetic acid General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 16

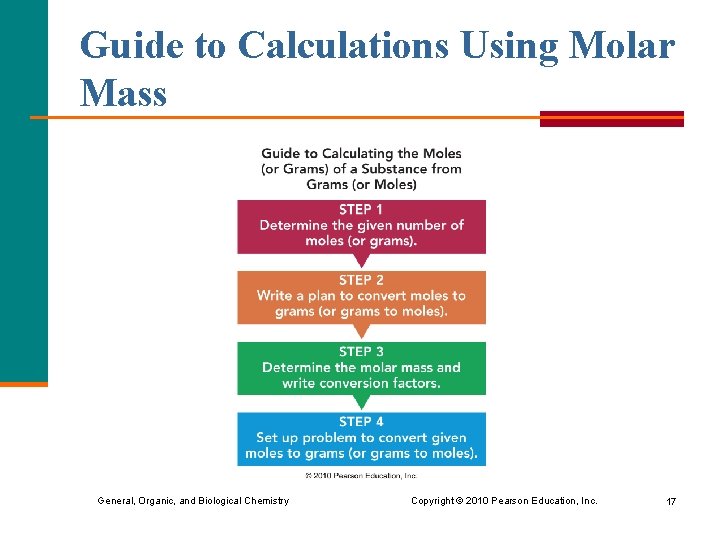
Guide to Calculations Using Molar Mass General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 17

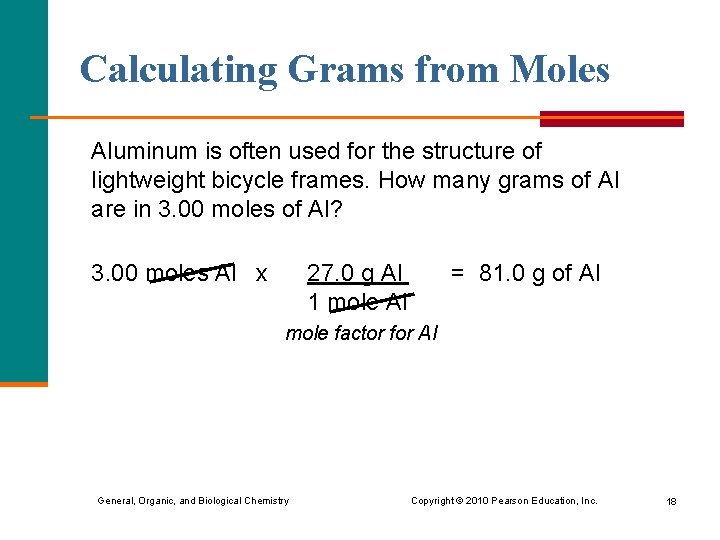
Calculating Grams from Moles Aluminum is often used for the structure of lightweight bicycle frames. How many grams of Al are in 3. 00 moles of Al? 3. 00 moles Al x 27. 0 g Al 1 mole Al = 81. 0 g of Al mole factor for Al General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 18

Learning Check The artificial sweetener aspartame (Nutra. Sweet), C 14 H 18 N 2 O 5 , is used to sweeten diet foods, coffee, and soft drinks. How many moles of aspartame are present in 225 g of aspartame? General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 19

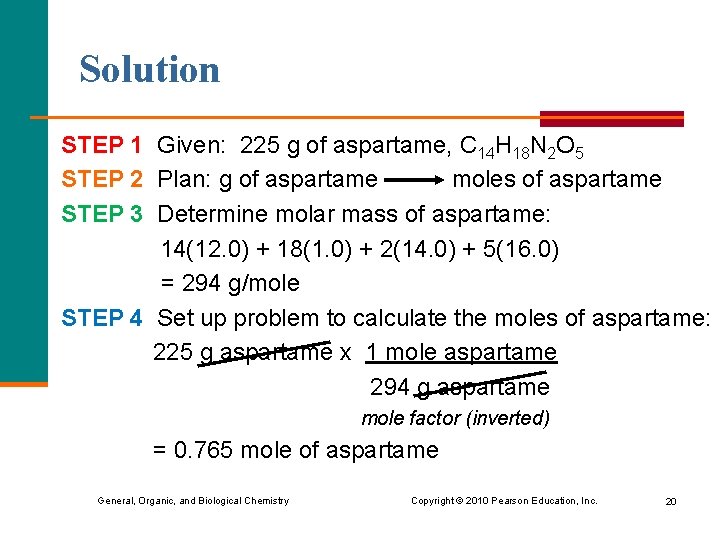
Solution STEP 1 Given: 225 g of aspartame, C 14 H 18 N 2 O 5 STEP 2 Plan: g of aspartame moles of aspartame STEP 3 Determine molar mass of aspartame: 14(12. 0) + 18(1. 0) + 2(14. 0) + 5(16. 0) = 294 g/mole STEP 4 Set up problem to calculate the moles of aspartame: 225 g aspartame x 1 mole aspartame 294 g aspartame mole factor (inverted) = 0. 765 mole of aspartame General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 20

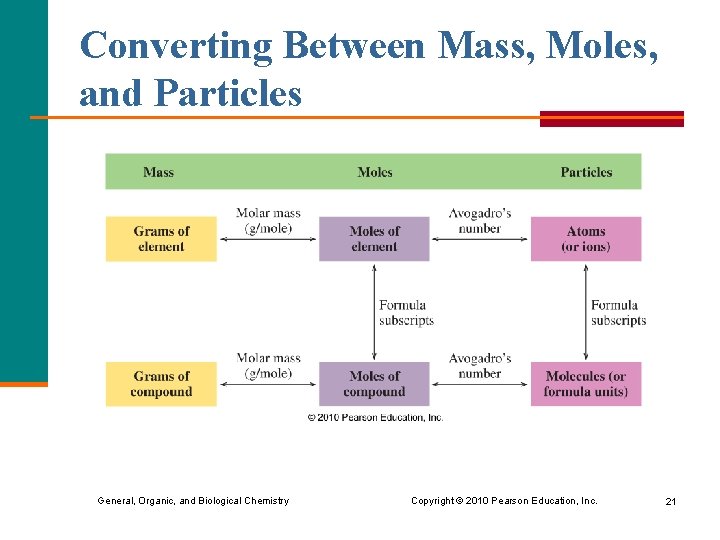
Converting Between Mass, Moles, and Particles General, Organic, and Biological Chemistry Copyright © 2010 Pearson Education, Inc. 21