Chapter 10 Polymers Image source http www ipt




- Slides: 20

Chapter 10 Polymers Image source: http: //www. ipt. arc. nasa. gov/cntpolymer. html

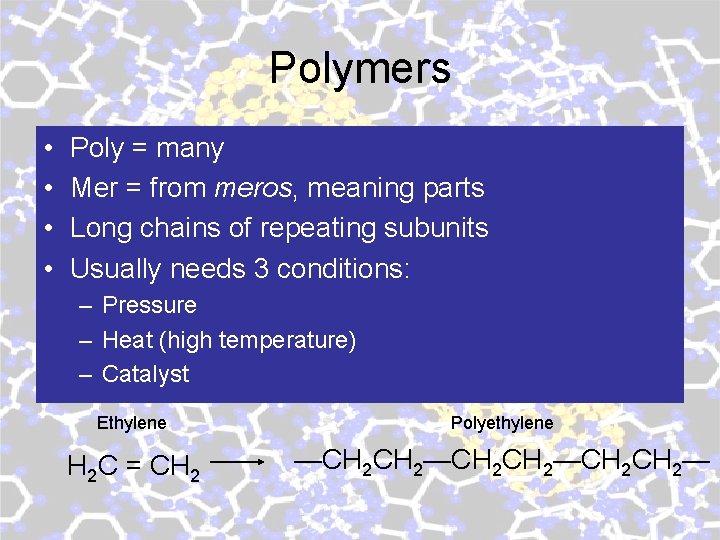
Polymers • • Poly = many Mer = from meros, meaning parts Long chains of repeating subunits Usually needs 3 conditions: – Pressure – Heat (high temperature) – Catalyst Ethylene Polyethylene H 2 C = CH 2 —CH 2 CH 2—CH 2—

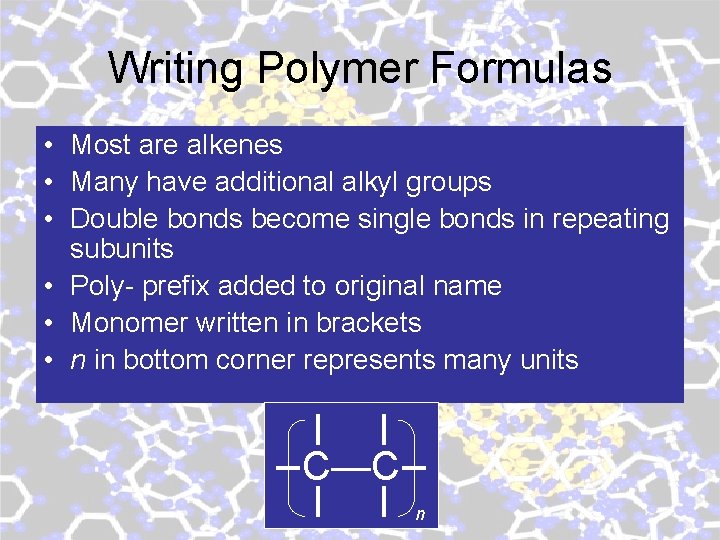
Writing Polymer Formulas • Most are alkenes • Many have additional alkyl groups • Double bonds become single bonds in repeating subunits • Poly- prefix added to original name • Monomer written in brackets • n in bottom corner represents many units C—C n

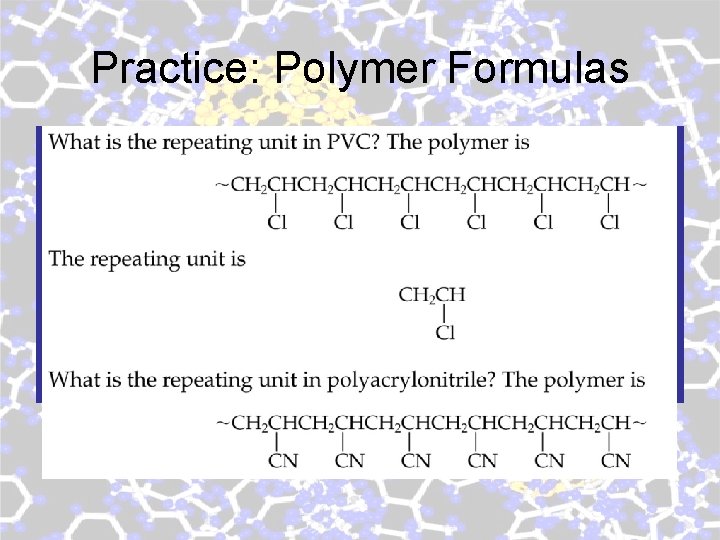
Practice: Polymer Formulas

Addition Polymerization • Subunits are added into a single chain • Product contains all the atoms of the starting monomers • Common examples: – – – – Polyethylene Polypropylene Polyvinyl chloride (PVC) Polyvinylidene chloride (PVDC) Polytetrafluoroethylene (Teflon) Polyacrylate Polyvinyl acetate

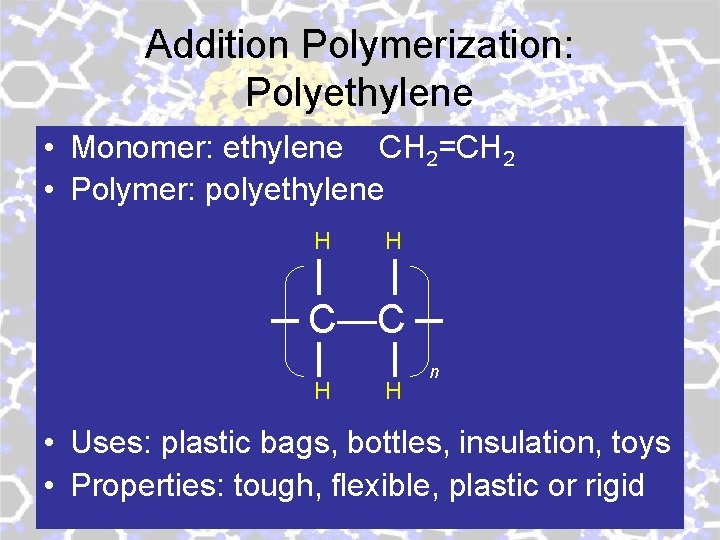
Addition Polymerization: Polyethylene • Monomer: ethylene CH 2=CH 2 • Polymer: polyethylene H H C—C H H n • Uses: plastic bags, bottles, insulation, toys • Properties: tough, flexible, plastic or rigid

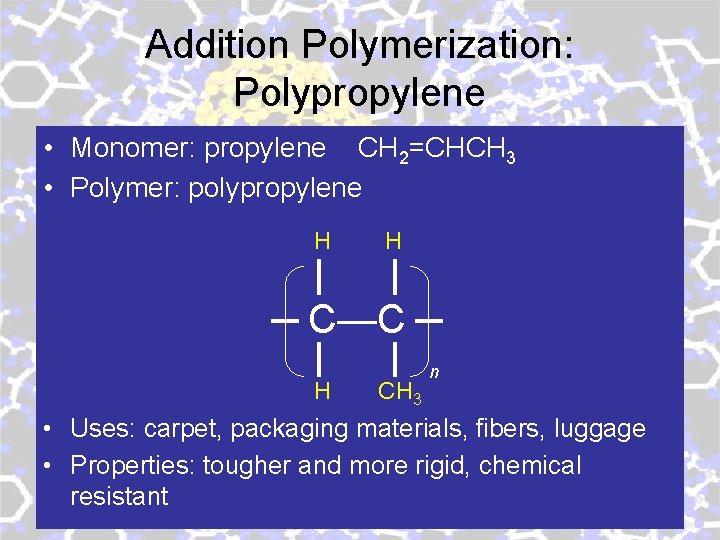
Addition Polymerization: Polypropylene • Monomer: propylene CH 2=CHCH 3 • Polymer: polypropylene H H C—C H CH 3 n • Uses: carpet, packaging materials, fibers, luggage • Properties: tougher and more rigid, chemical resistant

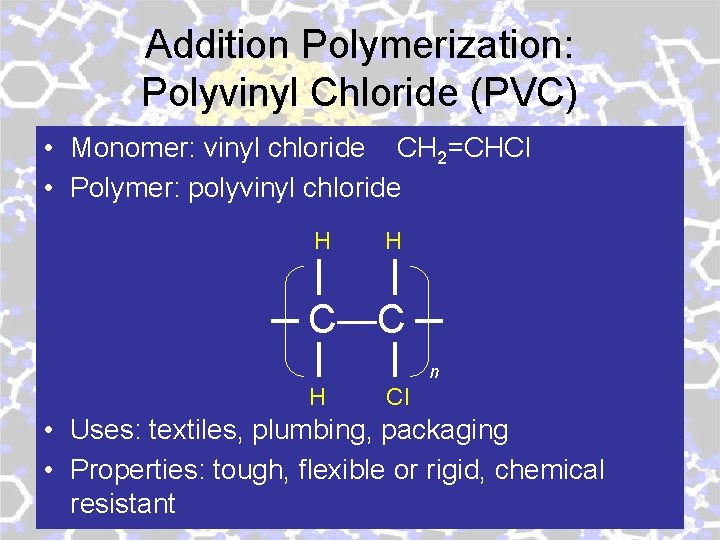
Addition Polymerization: Polyvinyl Chloride (PVC) • Monomer: vinyl chloride CH 2=CHCl • Polymer: polyvinyl chloride H H C—C n H Cl • Uses: textiles, plumbing, packaging • Properties: tough, flexible or rigid, chemical resistant

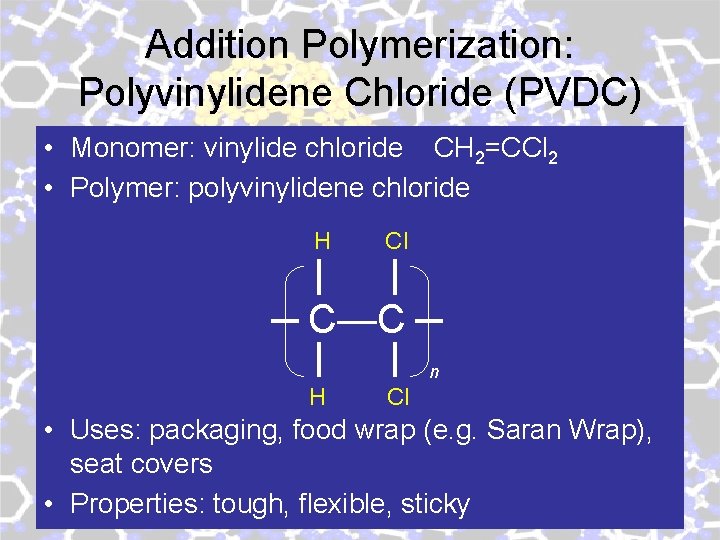
Addition Polymerization: Polyvinylidene Chloride (PVDC) • Monomer: vinylide chloride CH 2=CCl 2 • Polymer: polyvinylidene chloride H Cl C—C n H Cl • Uses: packaging, food wrap (e. g. Saran Wrap), seat covers • Properties: tough, flexible, sticky

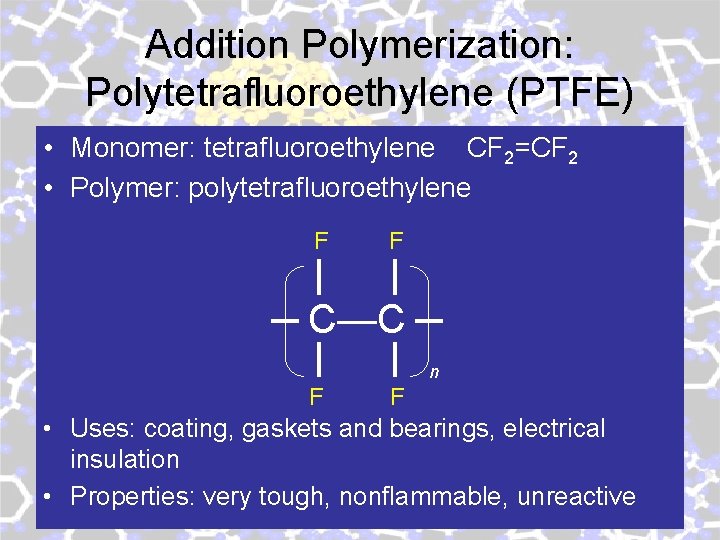
Addition Polymerization: Polytetrafluoroethylene (PTFE) • Monomer: tetrafluoroethylene CF 2=CF 2 • Polymer: polytetrafluoroethylene F F C—C n F F • Uses: coating, gaskets and bearings, electrical insulation • Properties: very tough, nonflammable, unreactive

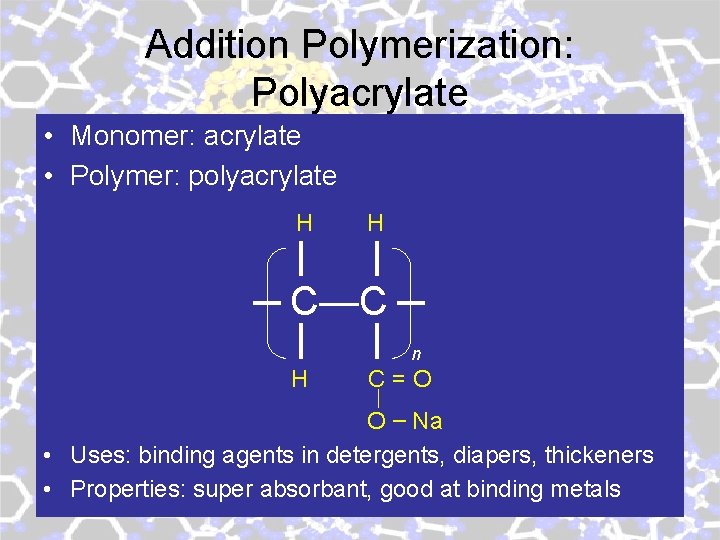
Addition Polymerization: Polyacrylate • Monomer: acrylate • Polymer: polyacrylate H H C—C n H C=O O – Na • Uses: binding agents in detergents, diapers, thickeners • Properties: super absorbant, good at binding metals

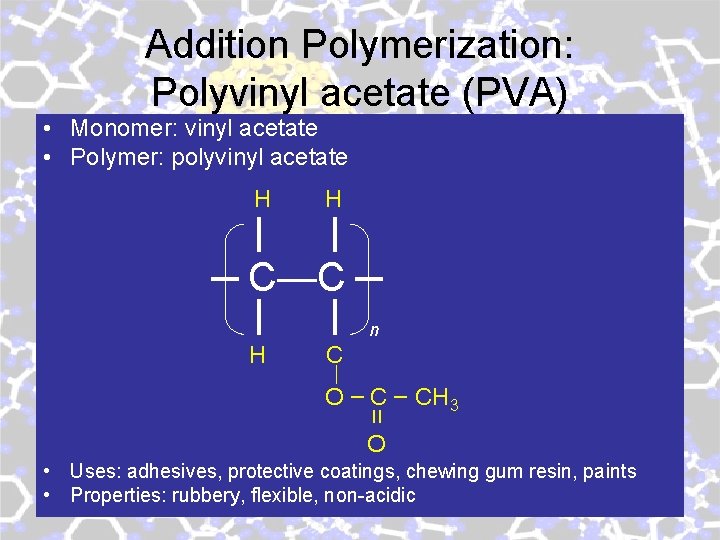
Addition Polymerization: Polyvinyl acetate (PVA) • Monomer: vinyl acetate • Polymer: polyvinyl acetate H H C—C n H C O – CH 3 װ O • Uses: adhesives, protective coatings, chewing gum resin, paints • Properties: rubbery, flexible, non-acidic

Condensation Polymerization • Small molecules (usually water) are split out of monomers and they become joined • Common examples: – Polyamides (nylon) – Polyesters – Polyurethanes (“foam rubber”)

Condensation Polymerization: Nylon • Monomer: 6 -carbon carboxylic acid • Polymer: nylon (polyamide) • Uses: fibers for fabrics (some silk-like, some yarn like wool), hoisery, carpeting • Properties: very elastic, fine, strong Image source: img. alibaba. com/photo/10922619/Men_s_100_Nylo. . . Image source: www. threadart. com/images/bonded_nylon. jpg

Condensation Polymerization: Polyesters • Monomers: molecules with alcohol and carboxylic acid functional groups • Polymer: Polyester (ester linkages) • Uses: beverage bottles, audio and videocassette film, fibers • Properties: strong, wrinkle-resistant Image source: www. stillwaterpalladium. com/images/polyester. jpg Image source: www. h 2 oforyou. com/images/bottles. JPG

Natural Polymers • Complex biological molecules • Common examples: – Proteins: repeating amino acid monomers – Nucleic Acids (DNA & RNA): repeating nucleotide monomers – Starch: repeating sugar monomers – Rubber

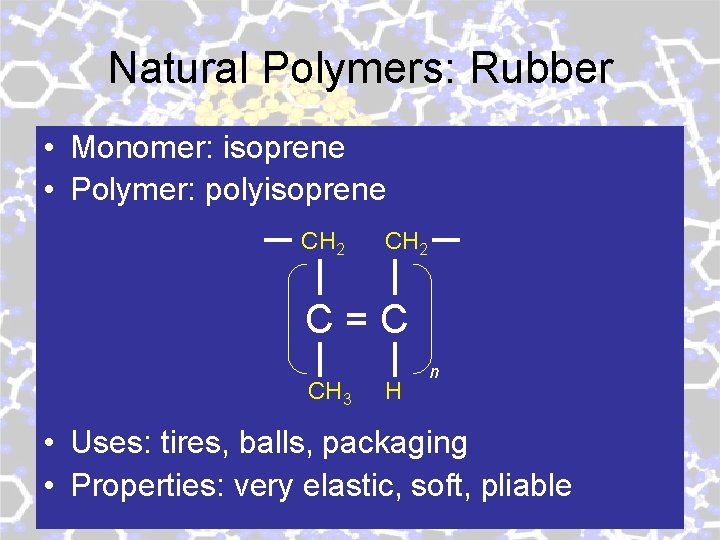
Natural Polymers: Rubber • Monomer: isoprene • Polymer: polyisoprene CH 2 C=C CH 3 H n • Uses: tires, balls, packaging • Properties: very elastic, soft, pliable

Natural Polymers: Rubber • Vulcanization: increasing the strength of rubber • Reaction requires 2 conditions: – Heat – Sulfur • Polyisoprene chains are cross-linked with sulfur bridges • Common uses: car tires, rubber bands, racquetballs

Other Polymers in Your Life • Chewing Gum – Gum base • Elastomers • Resins – Fats – Flavors • Sugar • Extracts Image source: http: //www. ornl. gov/info/ornlreview/v 37_2_04/article 15. shtml

Polymers: What You Should Know 1. 2. 3. 4. What is a polymer? What are the 3 conditions necessary to form a polymer? What is addition polymerization? Name the monomer (write the formula), and give properties and uses of the following polymers: • • 5. 6. 7. 8. 9. Polyethylene Polypropylene Polyvinyl Chloride (PVC) Polyvinylidene Chloride (PVDC) Polytetrafluoroethylene (PTFE) Polyacrylate Polyvinyl acetate What is condensation polymerization? Name common polymers formed from condensation polymerization. Name at least 3 natural polymers. Name and write the formula for the monomer of rubber. What is vulcanization? What does it do? What products is it used in?