Chapter 10 Photosynthesis Power Point Lectures for Biology

Chapter 10 Photosynthesis Power. Point Lectures for Biology, Seventh Edition Neil Campbell and Jane Reece Lectures by Chris Romero Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
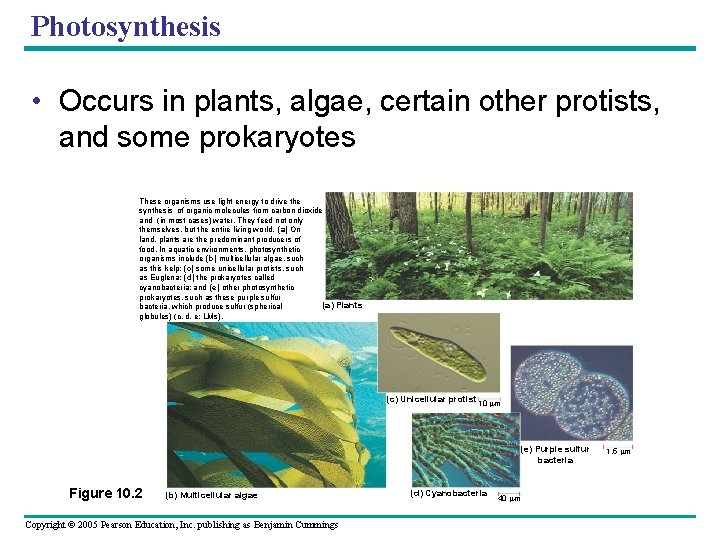
Photosynthesis • Occurs in plants, algae, certain other protists, and some prokaryotes These organisms use light energy to drive the synthesis of organic molecules from carbon dioxide and (in most cases) water. They feed not only themselves, but the entire living world. (a) On land, plants are the predominant producers of food. In aquatic environments, photosynthetic organisms include (b) multicellular algae, such as this kelp; (c) some unicellular protists, such as Euglena; (d) the prokaryotes called cyanobacteria; and (e) other photosynthetic prokaryotes, such as these purple sulfur (a) Plants bacteria, which produce sulfur (spherical globules) (c, d, e: LMs). (c) Unicellular protist 10 m (e) Purple sulfur bacteria Figure 10. 2 (b) Multicellular algae Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings (d) Cyanobacteria 40 m 1. 5 m

Concept 10. 1: Photosynthesis converts light energy to the chemical energy of food • Plants and other autotrophs are the producers of the biosphere Plants are photoautotrophs They use the energy of sunlight to make organic molecules from water and carbon dioxide Figure 10. 1 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Photosynthesis is vital to all life on earth • Heterotrophs – Obtain their organic material from other organisms – Are the consumers of the biosphere – Photosynthetically-produced carbohydrates provide fuel for all Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
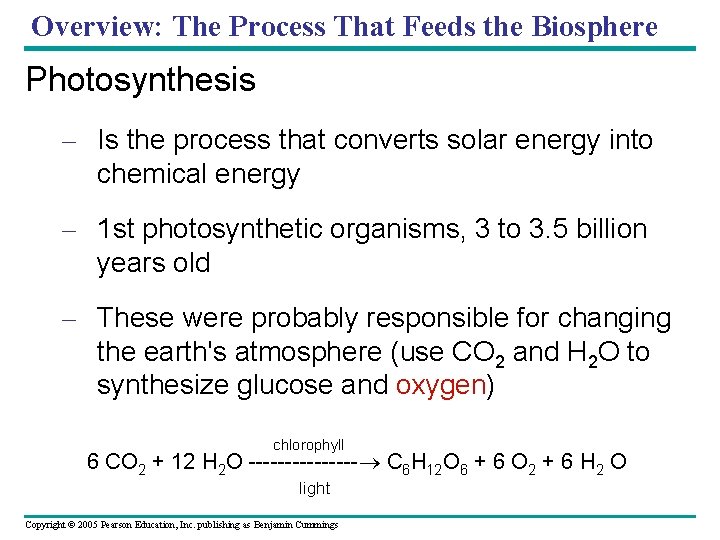
Overview: The Process That Feeds the Biosphere Photosynthesis – Is the process that converts solar energy into chemical energy – 1 st photosynthetic organisms, 3 to 3. 5 billion years old – These were probably responsible for changing the earth's atmosphere (use CO 2 and H 2 O to synthesize glucose and oxygen) chlorophyll 6 CO 2 + 12 H 2 O -------- C 6 H 12 O 6 + 6 O 2 + 6 H 2 O light Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
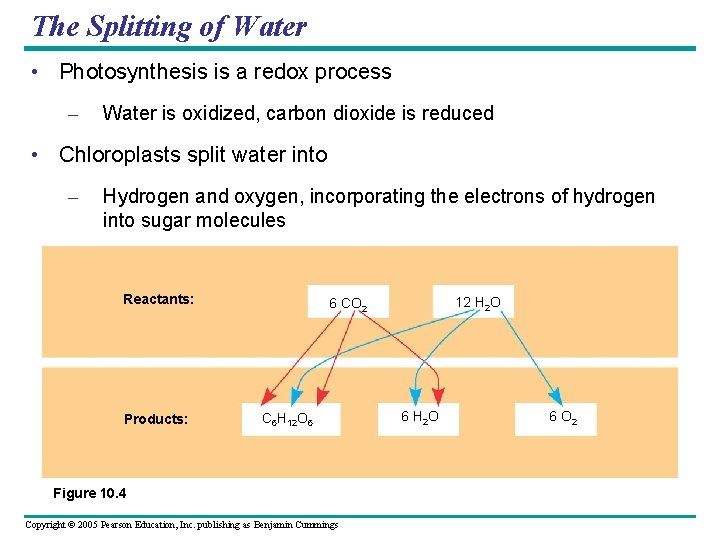
The Splitting of Water • Photosynthesis is a redox process – Water is oxidized, carbon dioxide is reduced • Chloroplasts split water into – Hydrogen and oxygen, incorporating the electrons of hydrogen into sugar molecules Reactants: Products: 12 H 2 O 6 CO 2 C 6 H 12 O 6 Figure 10. 4 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings 6 H 2 O 6 O 2
Oxygen comes from Splitting Water • Van Niel's Hypothesis (Stanford U. , 1930's) • previously, it had been believed that the O 2 given off in light reactions came from the splitting of CO 2 – Van Niel studied autotrophic bacteria "purple sulfur bacteria" which did NOT use H 2 O in their food-making proceses: – CO 2 + 2 H 2 S -------light----> (CH 2 O)n + H 2 O + 2 S So general equation is: – CO 2 + 2 H 2 A ------------> (CH 2 O)n + H 2 O + 2 A – Which proves it is the " H 2 A" that is, in fact, split to release the gas – Later, "heavy" oxygen 18(radioactive isotope) was traced through a plant to prove it. Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
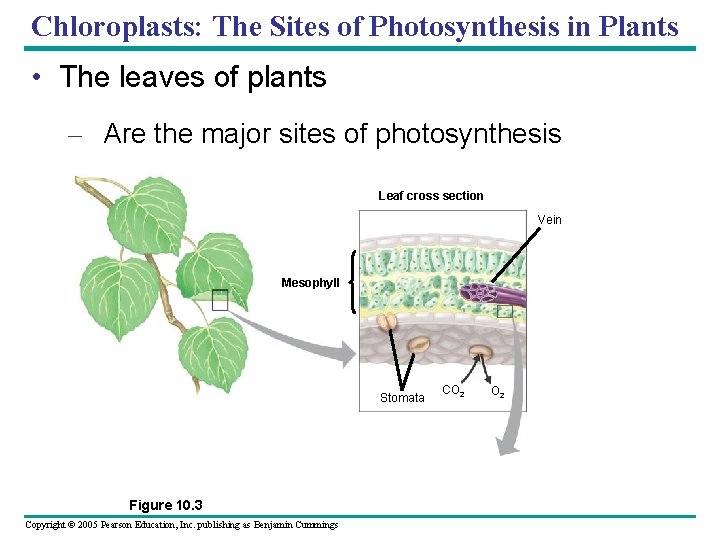
Chloroplasts: The Sites of Photosynthesis in Plants • The leaves of plants – Are the major sites of photosynthesis Leaf cross section Vein Mesophyll Stomata Figure 10. 3 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings CO 2
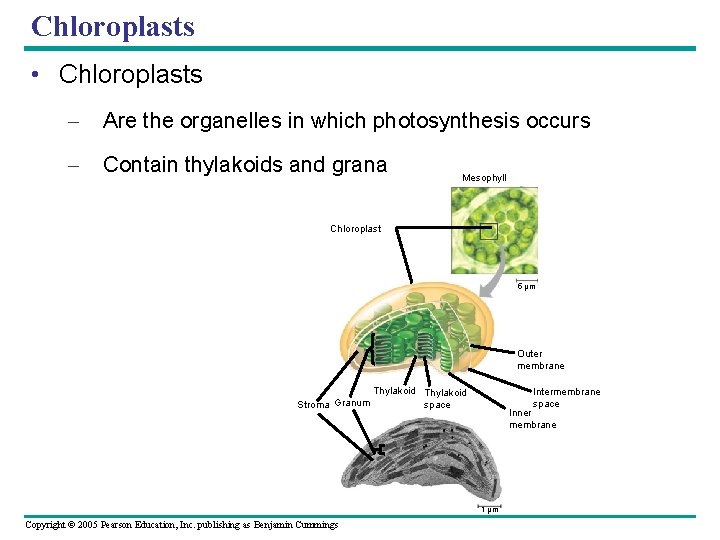
Chloroplasts • Chloroplasts – Are the organelles in which photosynthesis occurs – Contain thylakoids and grana Mesophyll Chloroplast 5 µm Outer membrane Stroma Granum Thylakoid space Intermembrane space Inner membrane 1 µm Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Photosynthetic Membranes: the Thylakoid • Thylakoid: the structural unit of photosynthesis is usually a form of a flattened sac, or vesicle. – These form the internal membranes of a CHLOROPLAST. – There can be up to 500, 000 chloroplasts per square mm of leaf surface • The Structure of the CHLOROPLAST – similar in structure to the mitochondria: surrounded by 2 membranes that are separates by an intermembrane space – 3 rd layer inside: grana (stacks of thylakoids), surrounded by a dense solution: the stroma Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
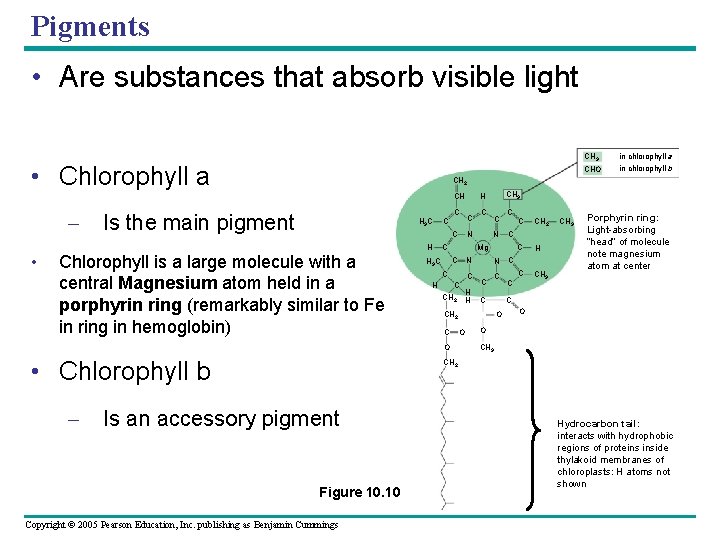
Pigments • Are substances that absorb visible light • Chlorophyll a • C Is the main pigment H 3 C C CHO in chlorophyll b H C N C CH 2 C N C C H H C CH 2 C O • Chlorophyll b C Mg C H C N C H 3 C CH 3 H C C Chlorophyll is a large molecule with a central Magnesium atom held in a porphyrin ring (remarkably similar to Fe in ring in hemoglobin) – in chlorophyll a CH 2 CH – CH 3 CH 2 C H C CH 3 Porphyrin ring: Light-absorbing “head” of molecule note magnesium atom at center C O O CH 3 CH 2 Is an accessory pigment Figure 10. 10 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Hydrocarbon tail: interacts with hydrophobic regions of proteins inside thylakoid membranes of chloroplasts: H atoms not shown
Chlorophyll and Other Pigments • When pigments absorb light, electrons within the pigment molecules are boosted to a higher energy level. This is what powers photosynthesis. • Pigments absorb light of certain wavelengths (reflect some wave lengths back, or transmit other wavelengths). Different pigments absorb light energy at different wavelengths = “absorption spectrum” • In plants, chlorophyll a is directly involved in the transformation of light energy into chemical energy • Most photosynthetic cells also contain “accessory pigments”, chlorophyll b and/or carotenoids (red, orange, yellow)…most prevalent is beta-carotene • Xanthophylls = yellow • Anthocyanins = red purple • The presence of the " secondary“ (accessory) pigments allow photosynthetic cells to capitalize on the greatest range of l's • These other pigments absorb different wavelengths of light and pass the energy to chlorophyll a Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
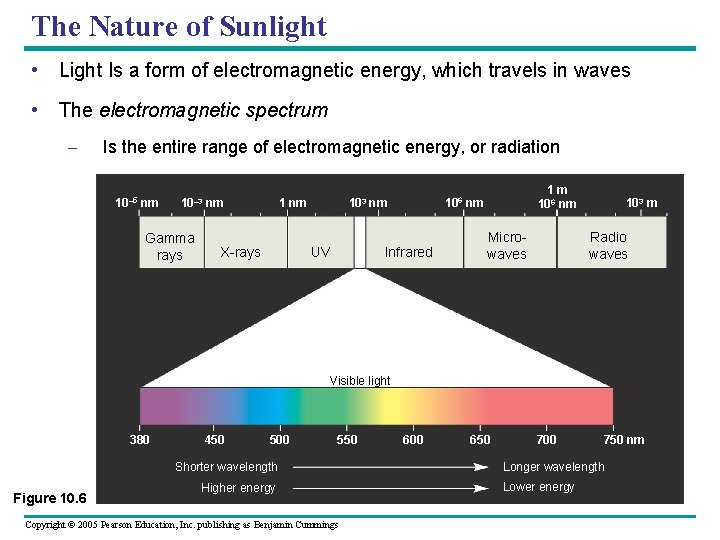
The Nature of Sunlight • Light Is a form of electromagnetic energy, which travels in waves • The electromagnetic spectrum – Is the entire range of electromagnetic energy, or radiation 10– 5 nm 10– 3 nm Gamma rays X-rays UV 1 m 106 nm 103 nm Infrared Microwaves 103 m Radio waves Visible light 380 450 500 550 Shorter wavelength Figure 10. 6 Higher energy Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings 600 650 700 750 nm Longer wavelength Lower energy
Light • Wavelength l – Is the distance between the crests of waves – Determines the type of electromagnetic energy • Isaac Newton - discovered prism divides white light into the visible light spectrum • The visible light spectrum includes the colors of light we can see; includes the wavelengths that drive photosynthesis – Range is from about 380 -750 nm • James Maxwell - discovered light (visible) is only a small part of a larger electromagetic spectrum • all light travels at approx. 300, 000 km/sec • variation in this WAVELENGTH makes different lights. • visible light l is measured in VERY small units, called nanometers • 1 nm = 10 -9 m Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Light – Albert Einstein (1905) proposed that light travels in both waves and particles "photons" (packets of energy) – Photons for different light are inversely energetic to the wavelength – ex: Violet light has short l, but large amounts of energy in each photon Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
" Why does so narrow a region of the EM spectrum have such an important impact on life? " • Biorhythms, seasons, photosynthesis, visible colors, etc 1. Life forms are held together with weak H-bonds, etc. Easily disrupted by strong light (such as high photon energy or low l, such as UV light) drives e- out of atoms. Conversely, low photon energy or high l (such as IR light) is absorbed by H 2 O in cells to heat up 2. Most radiation that reaches thru earths atmosphere is in the spectrum higher energy is filtered out by O 2 & O 3 lower energy l screened by CO 2 and H 2 O in clouds Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
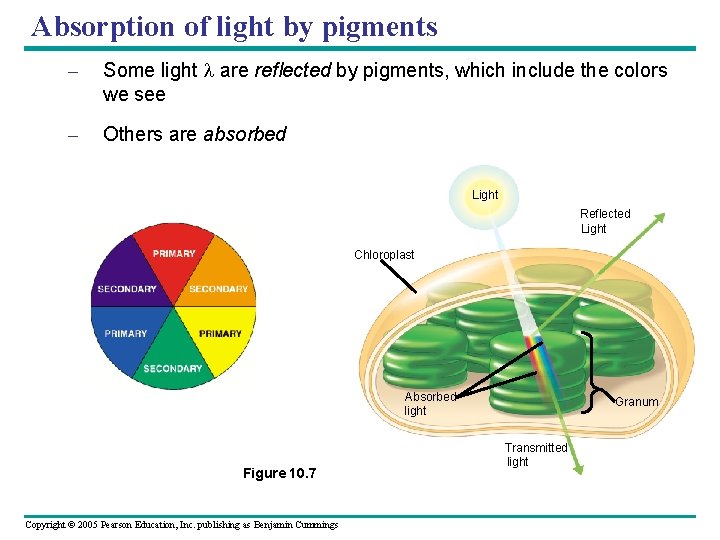
Absorption of light by pigments – Some light l are reflected by pigments, which include the colors we see – Others are absorbed Light Reflected Light Chloroplast Absorbed light Figure 10. 7 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Granum Transmitted light
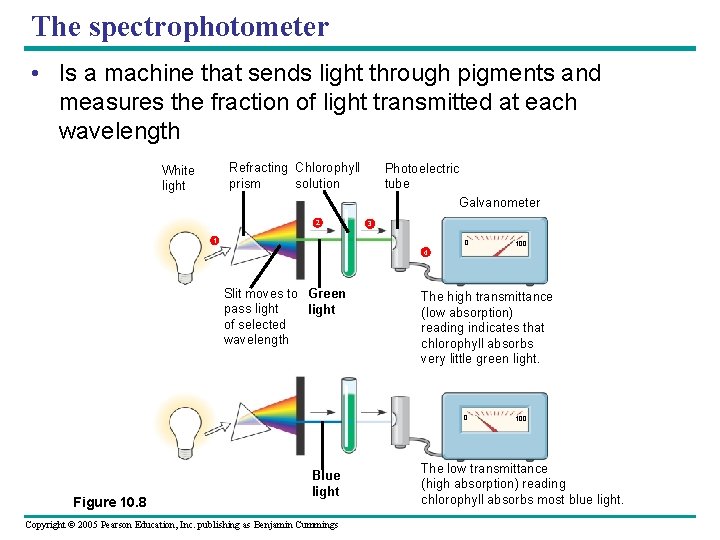
The spectrophotometer • Is a machine that sends light through pigments and measures the fraction of light transmitted at each wavelength Refracting Chlorophyll prism solution White light 2 Photoelectric tube Galvanometer 3 1 0 100 4 Slit moves to Green pass light of selected wavelength The high transmittance (low absorption) reading indicates that chlorophyll absorbs very little green light. 0 Figure 10. 8 Blue light Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings 100 The low transmittance (high absorption) reading chlorophyll absorbs most blue light.
• The absorption spectra of chloroplast pigments – Provide clues to the relative effectiveness of different wavelengths for driving photosynthesis Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
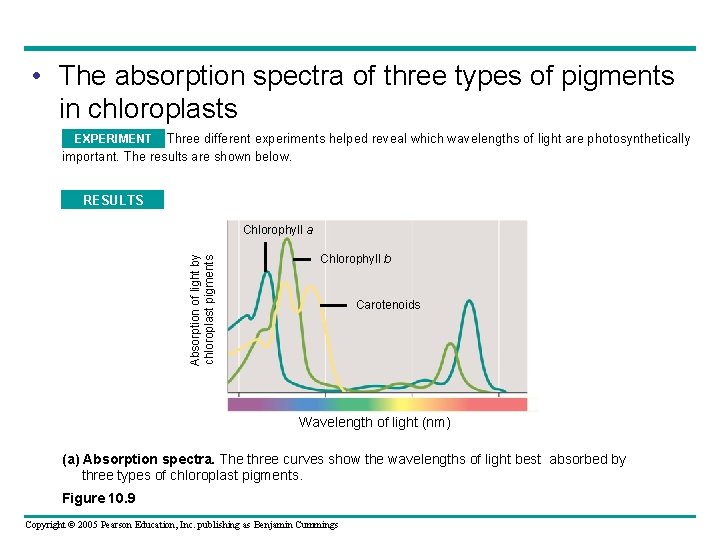
• The absorption spectra of three types of pigments in chloroplasts Three different experiments helped reveal which wavelengths of light are photosynthetically EXPERIMENT important. The results are shown below. RESULTS Absorption of light by chloroplast pigments Chlorophyll a Chlorophyll b Carotenoids Wavelength of light (nm) (a) Absorption spectra. The three curves show the wavelengths of light best absorbed by three types of chloroplast pigments. Figure 10. 9 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
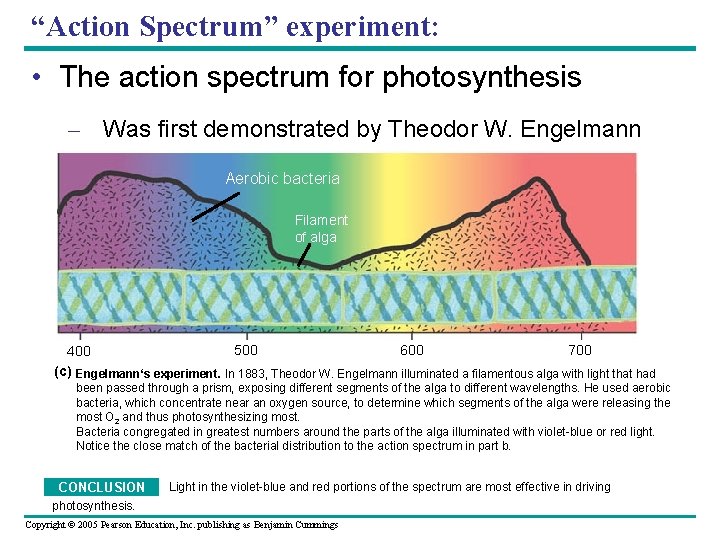
“Action Spectrum” experiment: • The action spectrum for photosynthesis – Was first demonstrated by Theodor W. Engelmann Aerobic bacteria Filament of alga 500 600 700 400 (c) Engelmann‘s experiment. In 1883, Theodor W. Engelmann illuminated a filamentous alga with light that had been passed through a prism, exposing different segments of the alga to different wavelengths. He used aerobic bacteria, which concentrate near an oxygen source, to determine which segments of the alga were releasing the most O 2 and thus photosynthesizing most. Bacteria congregated in greatest numbers around the parts of the alga illuminated with violet-blue or red light. Notice the close match of the bacterial distribution to the action spectrum in part b. Light in the violet-blue and red portions of the spectrum are most effective in driving CONCLUSION photosynthesis. Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
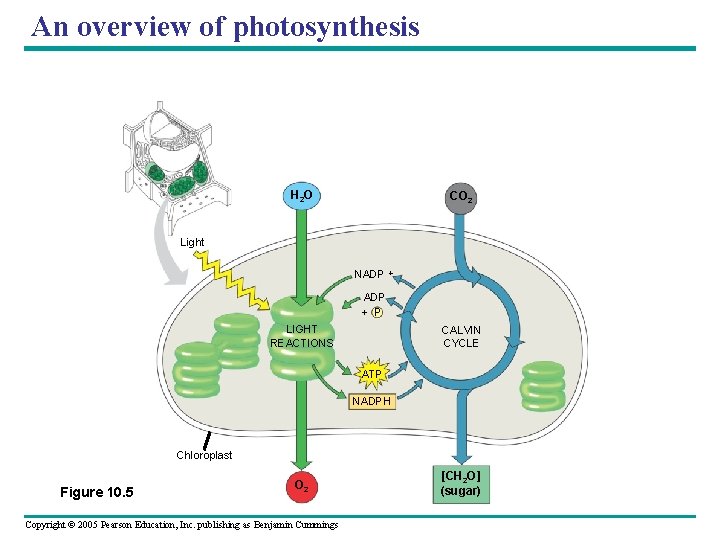
An overview of photosynthesis H 2 O CO 2 Light NADP + P LIGHT REACTIONS CALVIN CYCLE ATP NADPH Chloroplast Figure 10. 5 O 2 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings [CH 2 O] (sugar)
The Two Stages of Photosynthesis: A Preview • Photosynthesis consists of two processes I. The “light” reactions: – "Light-Dependent Reactions" (aka Energy-Capturing Rxns) need light energy to occur. – trap light energy by exciting electrons in chlorophyll --> energy is used to form ATP from ADP, and to reduce NADP+ to NADPH. Water molecules also broken down. – Occurs in the thylakoids. II. The Calvin cycle: – "Light-Independent Reactions" (aka the Carbon-Fixing Rxns) are enzymatic; can take place in/out of light, but need the products of light rxns to work. – Energy in the form of ATP & NADPH (from previous set of rxns) used to reduce carbon (from CO 2) into sugar molecules (" carbon fixation") – Occurs in the stroma. Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
A Photosystem: A Reaction Center Associated with Light-Harvesting Complexes In the thylakoids: • Chlorophyll a (reaction center) and other molecules (antennas) are packed into units called photosystems, made up of 250 -400 pigment molecules each. • Photons of light are absorbed by pigments and passed to the reaction center. • When a reaction-center chlorophyll molecule absorbs energy, one of its electrons gets bumped up to a primary electron acceptor Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
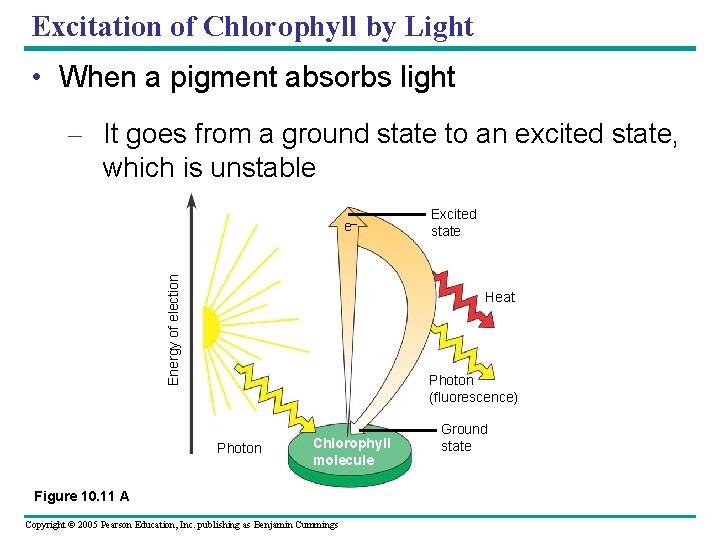
Excitation of Chlorophyll by Light • When a pigment absorbs light – It goes from a ground state to an excited state, which is unstable Energy of election e– Excited state Heat Photon (fluorescence) Photon Chlorophyll molecule Figure 10. 11 A Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Ground state
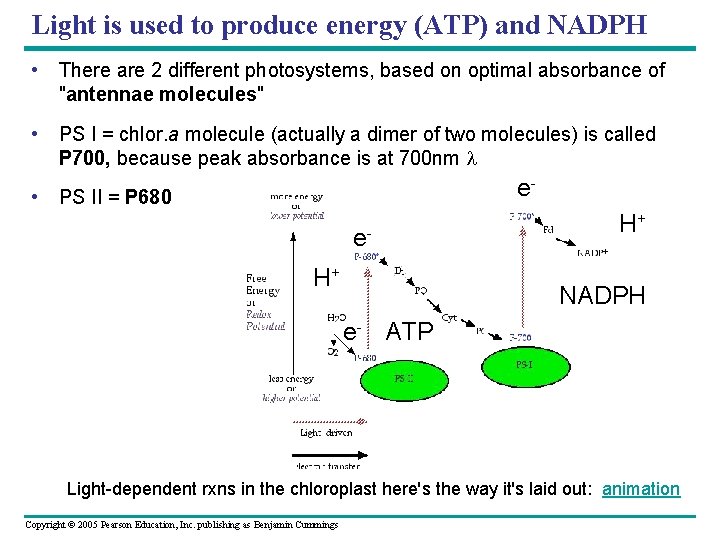
Light is used to produce energy (ATP) and NADPH • There are 2 different photosystems, based on optimal absorbance of "antennae molecules" • PS I = chlor. a molecule (actually a dimer of two molecules) is called P 700, because peak absorbance is at 700 nm l e- • PS II = P 680 e. H+ H+ NADPH e- ATP Light-dependent rxns in the chloroplast here's the way it's laid out: animation Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
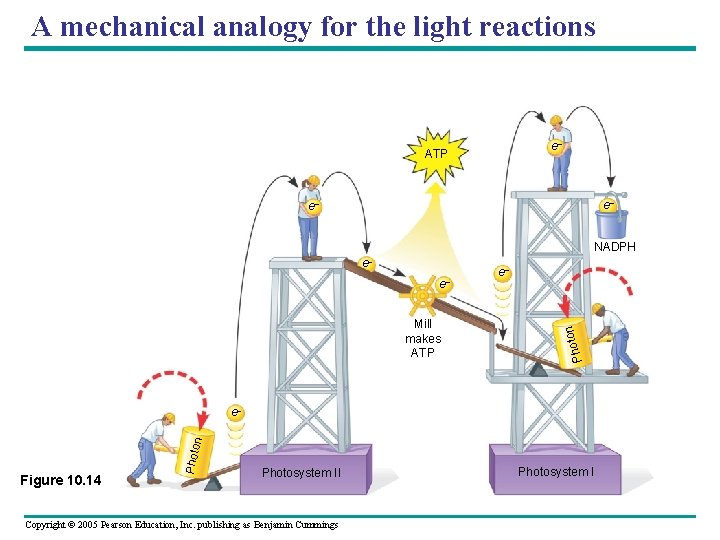
A mechanical analogy for the light reactions e– ATP e– e– NADPH Mill makes ATP e– on e– Phot e– Figure 10. 14 Photo n e– Photosystem II Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Photosystem I
The Light-trapping part: a synopsis • The two photosystems work independently and continuously • The boosted electrons are passed down Electron Transport Chains (remember the last chapter? ) and the energy released is used to turn ADP + Pi-->ATP ="phosphorylation“ • Also, WATER is split into its components • The oxygen is let off and the H+ gets passed through photosystems • This accumulation of hydrogen ions builds up a Chemiosmotic Gradient inside thylakoid Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
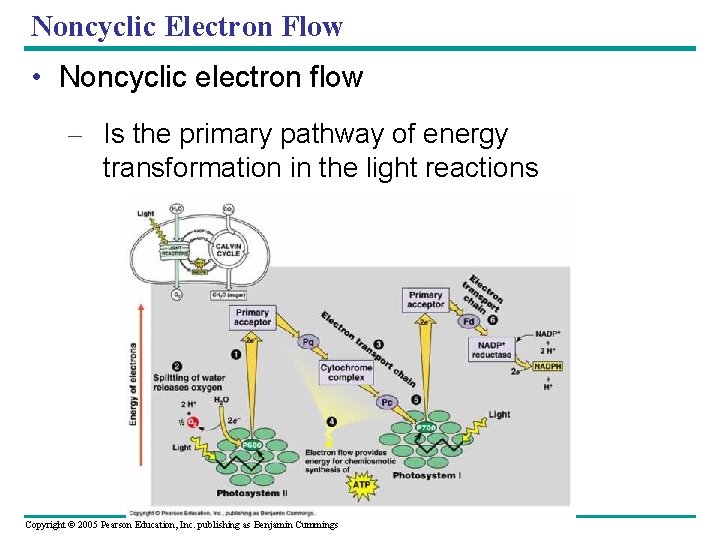
Noncyclic Electron Flow • Noncyclic electron flow – Is the primary pathway of energy transformation in the light reactions Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings

Cyclic Electron Flow • Under certain conditions, photoexcited electrons take an alternative path • In cyclic electron flow – Only photosystem I is used – Only ATP is produced Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
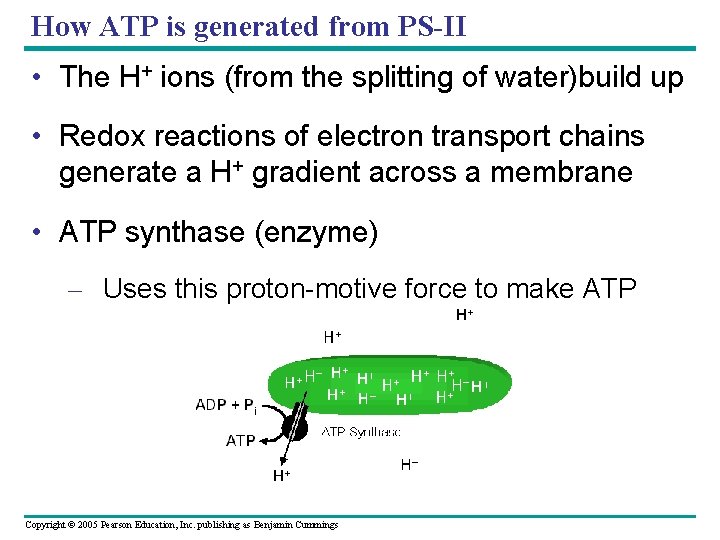
How ATP is generated from PS-II • The H+ ions (from the splitting of water)build up • Redox reactions of electron transport chains generate a H+ gradient across a membrane • ATP synthase (enzyme) – Uses this proton-motive force to make ATP Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
A Comparison of Chemiosmosis in Chloroplasts and Mitochondria • Chloroplasts and mitochondria – Generate ATP by the same basic mechanism: chemiosmosis – But use different sources of energy to accomplish this Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
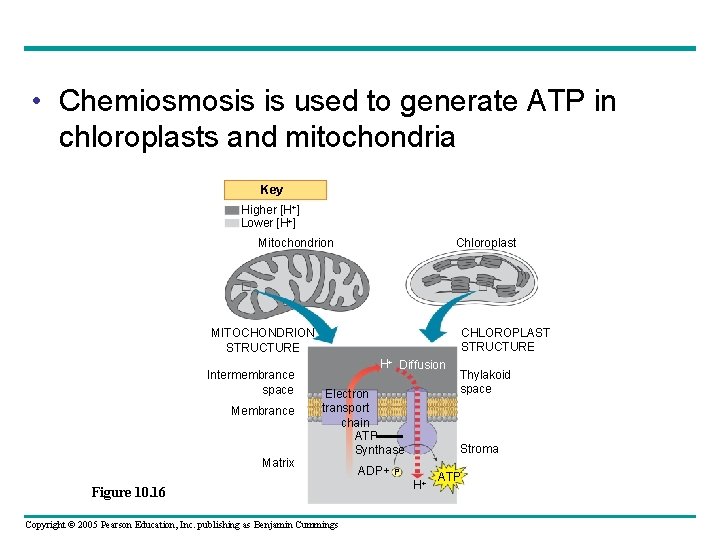
• Chemiosmosis is used to generate ATP in chloroplasts and mitochondria Key Higher [H+] Lower [H+] Chloroplast Mitochondrion CHLOROPLAST STRUCTURE MITOCHONDRION STRUCTURE Intermembrance space Membrance Matrix H+ Diffusion Electron transport chain ATP Synthase Figure 10. 16 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings ADP+ Thylakoid space Stroma P H+ ATP
The Light-Independent Reactions of Photosynthesis • Concept 10. 3: The Calvin cycle uses ATP and NADPH to convert CO 2 to sugar • The Calvin cycle – Is similar to the citric acid cycle (Krebs) – Occurs in the stroma Trivia: Cycle was deduced in 1960 by Calvin & Benson, using 14 C isotope tracer to label CO 2 path thru sugarcane 1961 Nobel Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
The Calvin Cycle • The Calvin cycle has three phases – Carbon fixation – Reduction – Regeneration of the CO 2 acceptor • The starting (and ending) compound is a 5 -C sugar with 3 phosphates attached = Ru. BP Ribulose biphosphate rubisco In the initial step, CO 2 binds to Ru. BP ------> Ru. BPCO 2 …which then splits into 2 molecules of PGAL (phosphoglyceraldehyde) 3 -C* each (enzyme: Ru. BP carboxylase) * = this is why it's called the "three carbon pathway" Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
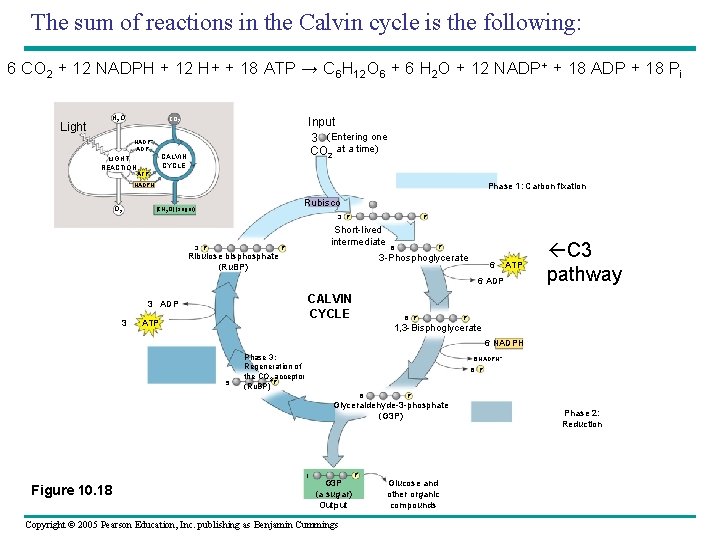
The sum of reactions in the Calvin cycle is the following: 6 CO 2 + 12 NADPH + 12 H+ + 18 ATP → C 6 H 12 O 6 + 6 H 2 O + 12 NADP+ + 18 ADP + 18 Pi Light H 2 O Input 3 (Entering one CO 2 at a time) CO 2 NADP+ ADP LIGHT REACTION CALVIN CYCLE ATP NADPH O 2 Phase 1: Carbon fixation Rubisco [CH 2 O] (sugar) 3 P Ribulose bisphosphate (Ru. BP) P Short-lived intermediate P P 6 3 -Phosphoglycerate 6 ATP 6 ADP CALVIN CYCLE 3 ADP 3 ATP 6 P C 3 pathway P 1, 3 -Bisphoglycerate 6 NADPH 5 Phase 3: Regeneration of the CO 2 acceptor P (Ru. BP) 6 NADPH+ 6 P (G 3 P) 6 P Glyceraldehyde-3 -phosphate (G 3 P) 1 Figure 10. 18 G 3 P (a sugar) Output Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings P Glucose and other organic compounds Phase 2: Reduction
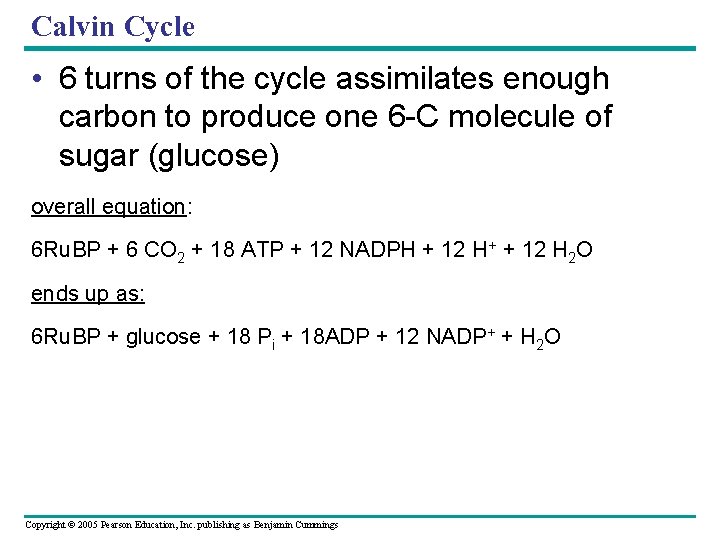
Calvin Cycle • 6 turns of the cycle assimilates enough carbon to produce one 6 -C molecule of sugar (glucose) overall equation: 6 Ru. BP + 6 CO 2 + 18 ATP + 12 NADPH + 12 H+ + 12 H 2 O ends up as: 6 Ru. BP + glucose + 18 Pi + 18 ADP + 12 NADP+ + H 2 O Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
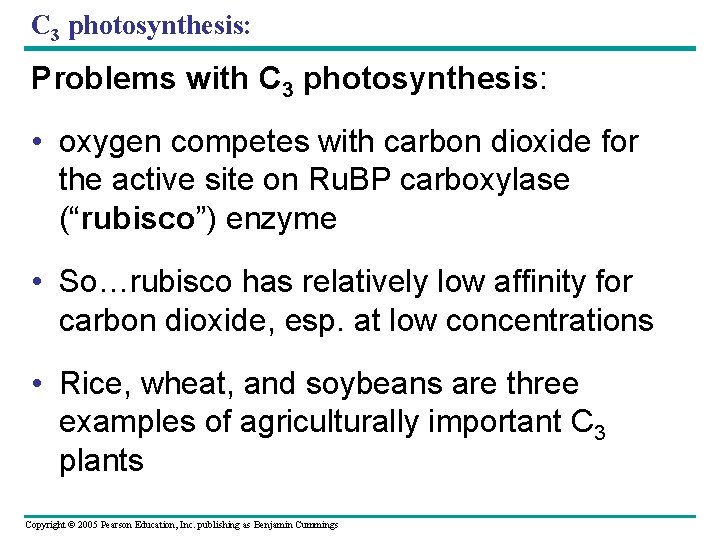
C 3 photosynthesis: Problems with C 3 photosynthesis: • oxygen competes with carbon dioxide for the active site on Ru. BP carboxylase (“rubisco”) enzyme • So…rubisco has relatively low affinity for carbon dioxide, esp. at low concentrations • Rice, wheat, and soybeans are three examples of agriculturally important C 3 plants Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
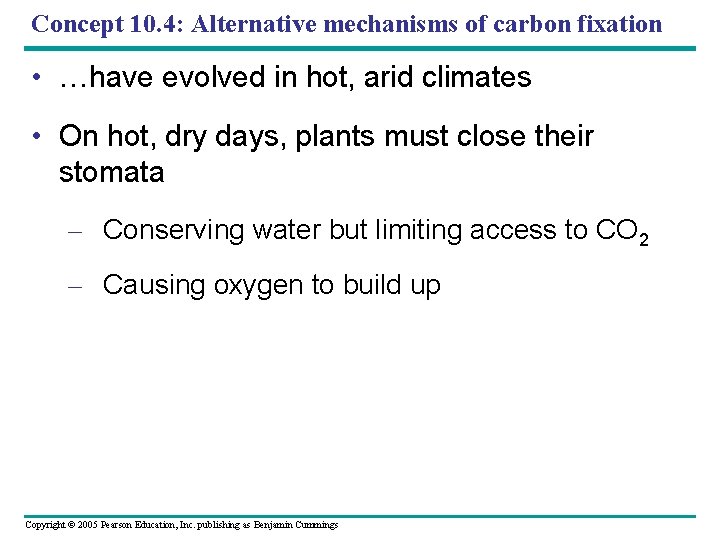
Concept 10. 4: Alternative mechanisms of carbon fixation • …have evolved in hot, arid climates • On hot, dry days, plants must close their stomata – Conserving water but limiting access to CO 2 – Causing oxygen to build up Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Photorespiration: An “Evolutionary Relic”*? • In photorespiration – O 2 substitutes for CO 2 in the active site of the enzyme rubisco – The photosynthetic rate is reduced • “photo” = occurs in light • “respiration” = consumes O 2 while producing CO 2 – However, unlike cellular respiration, it produces no ATP (in fact, consumes it) – Produces no sugar, actually inhibits Calvin Cycle *Remember- the young atmosphere contained little O 2, so rubisco was less-likely to be inhibited Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
C 4 Plants have evolved a better “means to an end” • C 4 plants minimize the cost of photorespiration – By incorporating CO 2 into four-carbon compounds in mesophyll cells • These four carbon compounds – Are exported to bundle sheath cells, where they release CO 2 used in the Calvin cycle Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
C 4 -adapted plants While most plants bind CO 2 to Ru. BP in 1 st step of the light-independent rxns (3 -C pathway), some plants can go through a 4 -C pathway • …but not until it goes through an additional series of reactions called the "Hatch-Slack Pathway“ • catalyzed by enzyme PEP carboxylase; has higher CO 2 affinity; keeps CO 2 gradient in leaf • 1 st step binds CO 2 to PEP (phosphoenolpyruvate) to form a 4 -carbon compound called oxaloacetic acid (like Krebs!) • These four-carbon compounds are then exported to bundle sheath cells, where the CO 2 is then transferred to Ru. BP and enters the Calvin Cycle. • C-4 is better in drought-ridden areas or with "crowded" leaves (little gas exchange) – Maximizes the minimal CO 2 PEP binds CO 2 faster at lower conc. – Examples: sugarcane, corn, many grasses Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
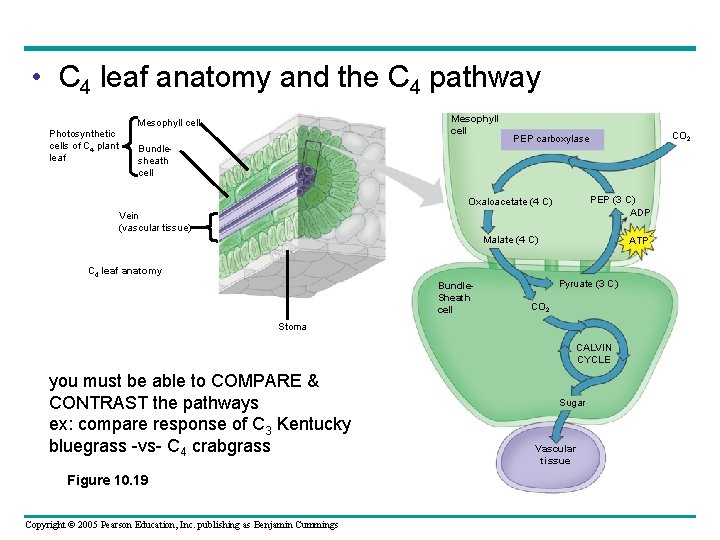
• C 4 leaf anatomy and the C 4 pathway Mesophyll cell Photosynthetic cells of C 4 plant leaf Bundlesheath cell CO CO 2 2 PEP carboxylase PEP (3 C) ADP Oxaloacetate (4 C) Vein (vascular tissue) Malate (4 C) C 4 leaf anatomy Bundle. Sheath cell ATP Pyruate (3 C) CO 2 Stoma CALVIN CYCLE you must be able to COMPARE & CONTRAST the pathways ex: compare response of C 3 Kentucky bluegrass -vs- C 4 crabgrass Figure 10. 19 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Sugar Vascular tissue
CAM Plants • CAM plants – Open their stomata at night, incorporating CO 2 into organic acids – CAM = Crassulacean Acid Metabolism • During the day, the stomata close – And the CO 2 is released from the organic acids for use in the Calvin cycle Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
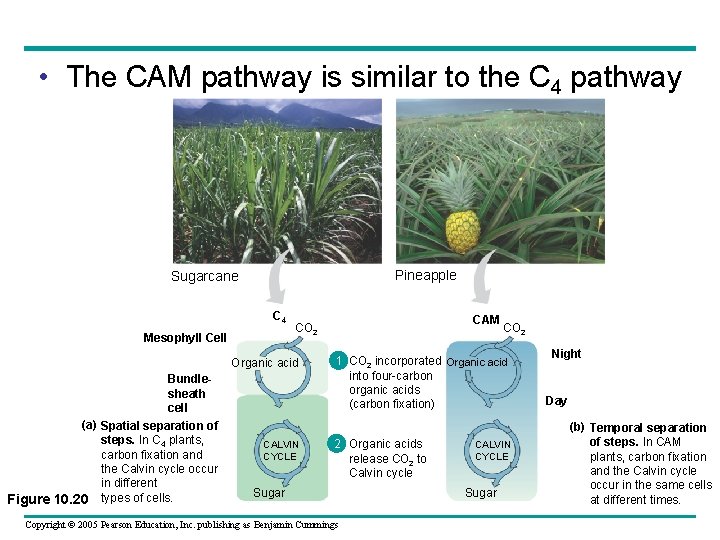
• The CAM pathway is similar to the C 4 pathway Pineapple Sugarcane C 4 Mesophyll Cell Organic acid Bundlesheath cell (a) Spatial separation of steps. In C 4 plants, carbon fixation and the Calvin cycle occur in different Figure 10. 20 types of cells. CAM CO 2 CALVIN CYCLE CO 2 1 CO 2 incorporated Organic acid into four-carbon organic acids (carbon fixation) 2 Organic acids release CO 2 to Calvin cycle Sugar Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings CALVIN CYCLE Sugar Night Day (b) Temporal separation of steps. In CAM plants, carbon fixation and the Calvin cycle occur in the same cells at different times.
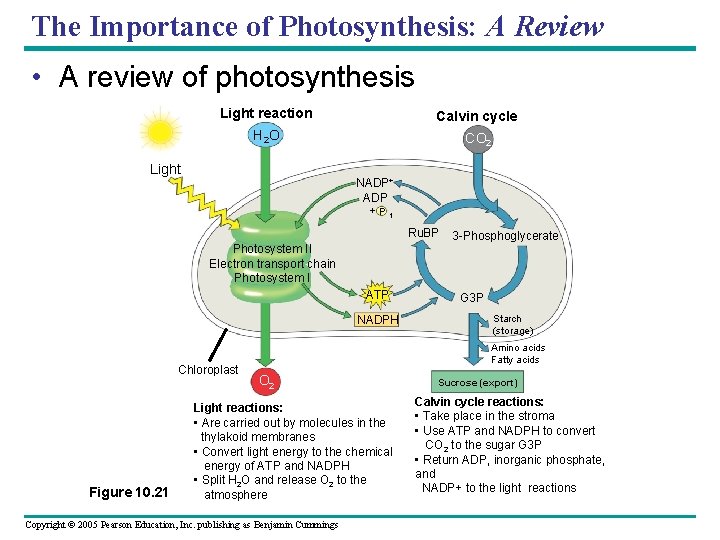
The Importance of Photosynthesis: A Review • A review of photosynthesis Light reaction Calvin cycle H 2 O CO 2 Light NADP+ ADP + P 1 Ru. BP Photosystem II Electron transport chain Photosystem I ATP NADPH Chloroplast Figure 10. 21 3 -Phosphoglycerate G 3 P Starch (storage) Amino acids Fatty acids O 2 Light reactions: • Are carried out by molecules in the thylakoid membranes • Convert light energy to the chemical energy of ATP and NADPH • Split H 2 O and release O 2 to the atmosphere Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Sucrose (export) Calvin cycle reactions: • Take place in the stroma • Use ATP and NADPH to convert CO 2 to the sugar G 3 P • Return ADP, inorganic phosphate, and NADP+ to the light reactions
Summary • Organic compounds produced by photosynthesis – Provide the energy and building material for ecosystems STILL CONFUSED? Tutorials: check out some photosynthesis animations Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
- Slides: 47