Chapter 10 Notes Atomic Structure The Periodic Table

Chapter 10 Notes Atomic Structure & The Periodic Table
Atomic Structure Element - only one kind of atom - Cannot be further broken down ex- Pb, Cu, Ne, H, O, C Periodic Table-Table of 114 elements know to man -90 naturally occurring Earth -24 artificially made in labs

The ATOM Smallest part of an element Still has properties of that element Greeks atomos = indivisible
4 models: Dalton 1803 -solid sphere/indestructible -element made of same atoms
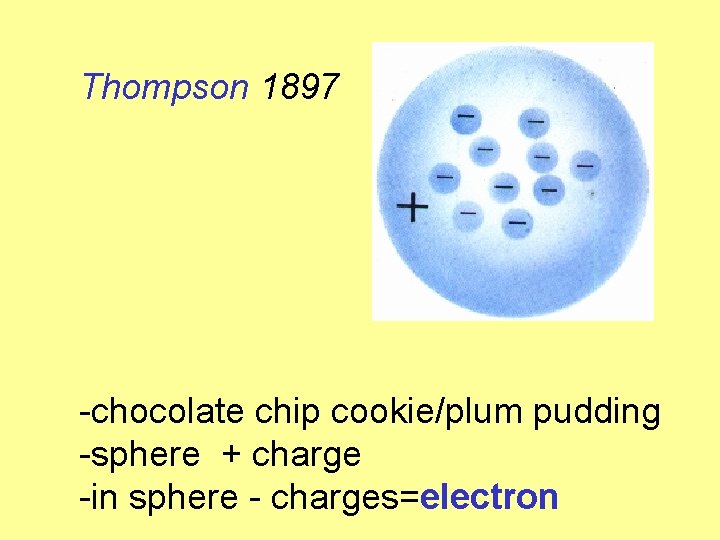
Thompson 1897 -chocolate chip cookie/plum pudding -sphere + charge -in sphere - charges=electron
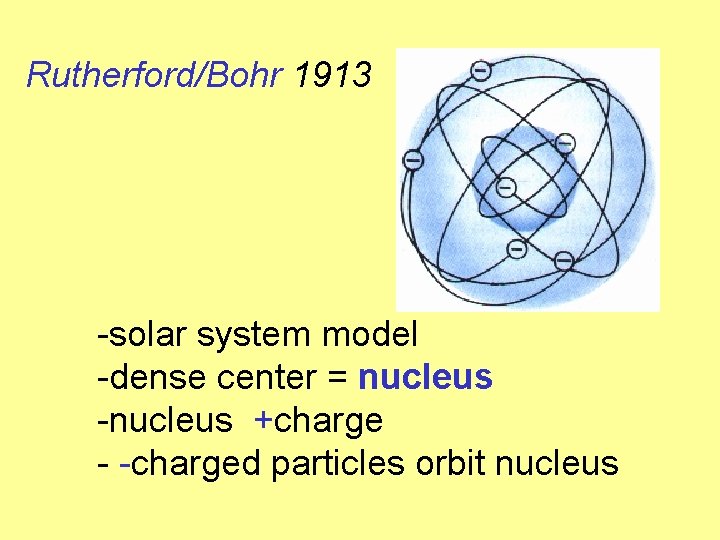
Rutherford/Bohr 1913 -solar system model -dense center = nucleus -nucleus +charge - -charged particles orbit nucleus
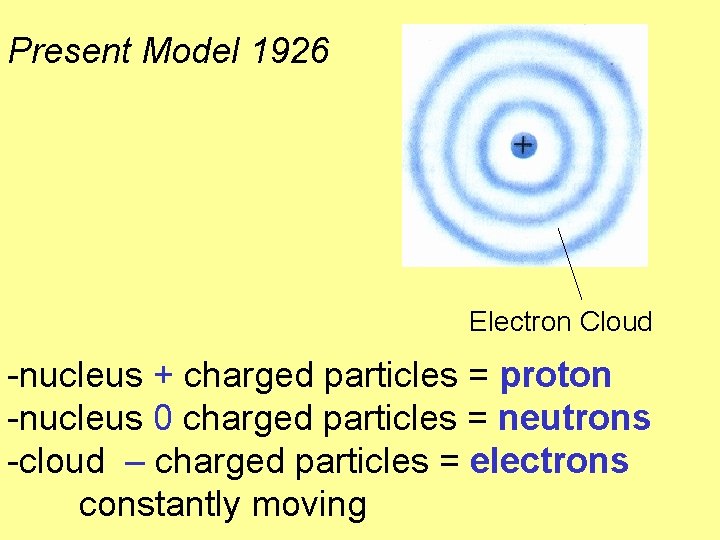
Present Model 1926 Electron Cloud -nucleus + charged particles = proton -nucleus 0 charged particles = neutrons -cloud – charged particles = electrons constantly moving
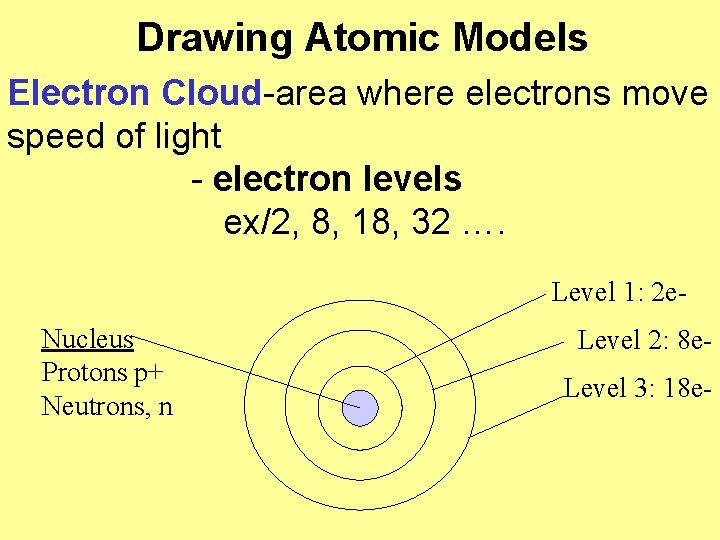
Drawing Atomic Models Electron Cloud-area where electrons move speed of light - electron levels ex/2, 8, 18, 32 …. Level 1: 2 e. Nucleus Protons p+ Neutrons, n Level 2: 8 e. Level 3: 18 e-
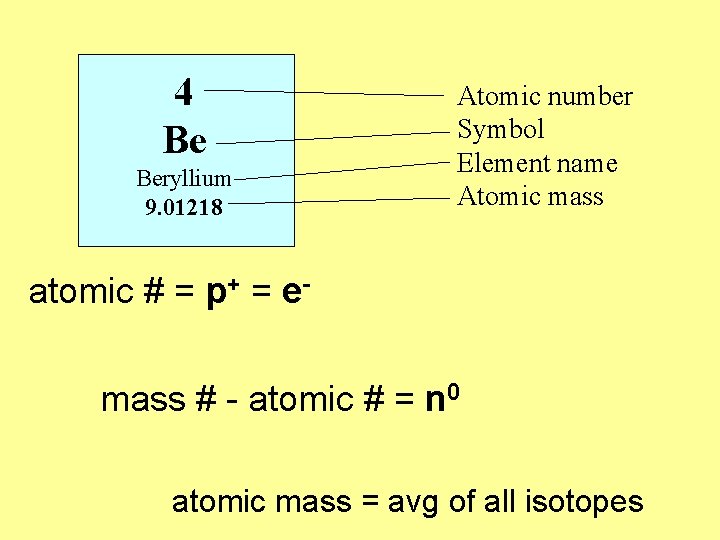
4 Be Beryllium 9. 01218 Atomic number Symbol Element name Atomic mass atomic # = p+ = emass # - atomic # = n 0 atomic mass = avg of all isotopes
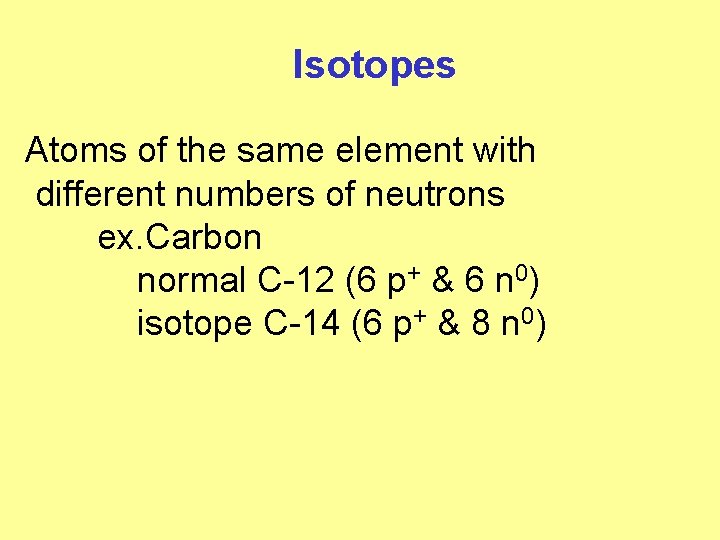
Isotopes Atoms of the same element with different numbers of neutrons ex. Carbon normal C-12 (6 p+ & 6 n 0) isotope C-14 (6 p+ & 8 n 0)

Periodic Table • 1834 – 1907 Russian chemist Mendeleev arranged all elements known 1900 by mass • Found patterns • Predicted mass & properties of undiscovered elements • Problems? atomic #s didn’t match up when arranged by properties • New table – atomic #s match up
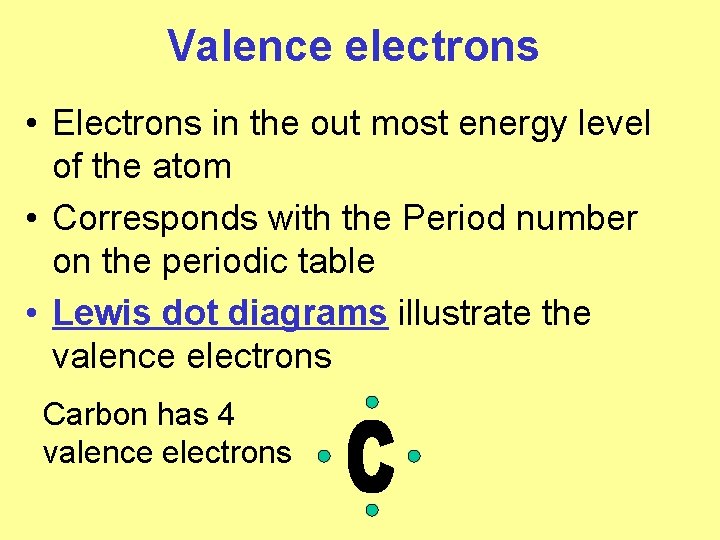
Valence electrons • Electrons in the out most energy level of the atom • Corresponds with the Period number on the periodic table • Lewis dot diagrams illustrate the valence electrons Carbon has 4 valence electrons
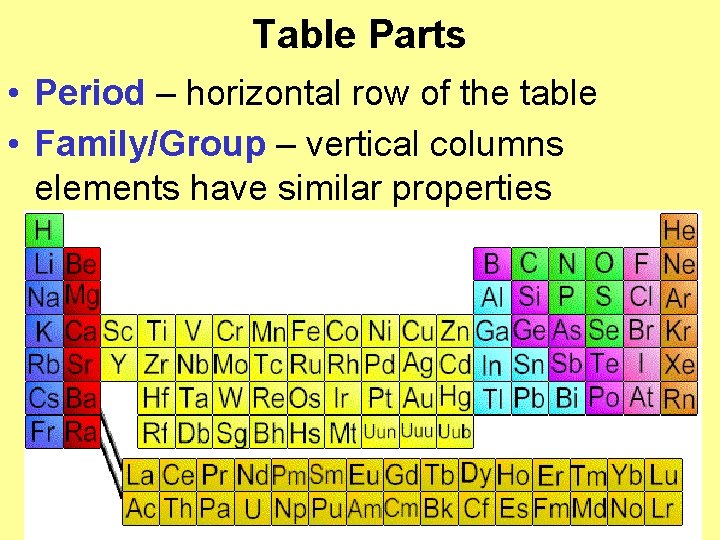
Table Parts • Period – horizontal row of the table • Family/Group – vertical columns elements have similar properties

Group 1 – Alkali Metals • • Conduct heat & electricity Soft, low m. p. & low density React with acids Very reactive, never found as free elements • Malleable, ductile, luster • 1 e- in outer energy level (+1 cation) • Identified by flame test

Group 2 – Alkaline Earth Metals • Highly reactive, never found free in nature • Low density • React with acids • 2 e- in outer energy level (+2 cation) • Identified by flame test

Group 3 – 12: Transition Metals • • Common metal, properties vary High m. p. - react with acids Brittle, soft, strong, hard, malleable, ductile Alloys – mixture of 2 or more metals to gain desired characteristics • +2 - +4 cations • Ores – mineral or other natural material from which one or more metals can be obtained

Groups 13 – 17: Nonmetals • • Insulators for electricity, dull, brittle Not reactive w/ acids Solids, liquids & gases React with metals to form compounds – Group 17: Halogens • Form salts, all 3 states @ room temp • Toxic, different colors – Metalloids • Properties of metal & nonmetals • stairstep

Group 18 : Noble Gases • Perfect elements • 8 valence electrons • Inert- not reactive

Diatomic Seven • 7 elements that exist as 2 atoms combined together in nature – Hydrogen – Nitrogen – Oxygen – Fluorine – Chlorine – Bromine – Iodine

Chapter 12 Notes Elements & Properties

Metallic Bonding • Not ionic or covalent • Positively charged ions surrounded by “sea of electrons” • Outer electrons move freely

Flame Tests • Certain elements can be identified by the color of flame they produce

Transiton Elements • • • Bright colors of gemstones Iron, cobalt & nickel = magnetic fields Copper, gold & silver = coins Mercurcy – liquid @ room temp. Ores – metals in the Earths Crust
Synthetic Elements • Made by combining existing elements w/ fast moving particles • More than 92 protons = transuranium • Unstable breakdown quickly

Radioactive Elements • Nucleus breaks down giving off particles & energy • Becomes other elements as the number of protons change

Nonmetals • Diatomic 7 • Less reactive further down the column • Halogens & Noble gases • Sublimation – solids change into vapors - ex. Iodine

Hydrogen • Smallest element • Uniquie properties – in a group of its own
Mixed Groups • Semiconductors – conduct electric current under certain conditions • Allotropes – different forms of the same element – Graphite & diamonds = carbon – O 2 & O 3 both are oxygen
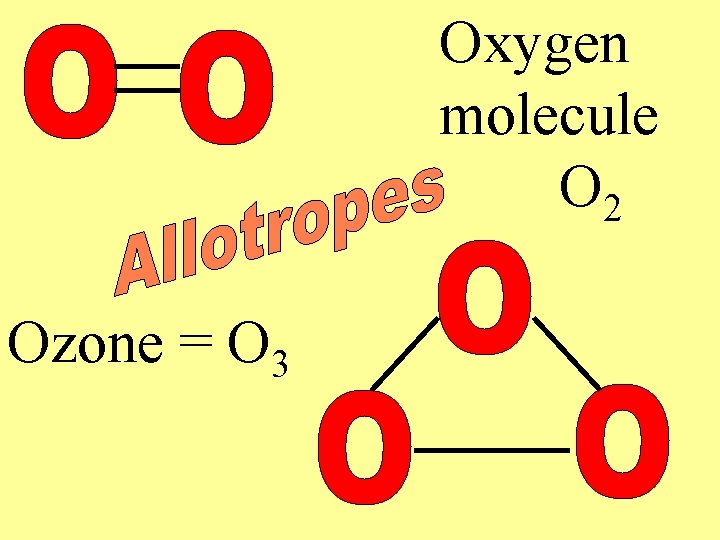
Oxygen molecule O 2 Ozone = O 3
- Slides: 29