Chapter 10 Mol Ratios Calculations with Equations Limiting









- Slides: 30

Chapter 10 Mol Ratios Calculations with Equations Limiting Reactions Percent Yield 1

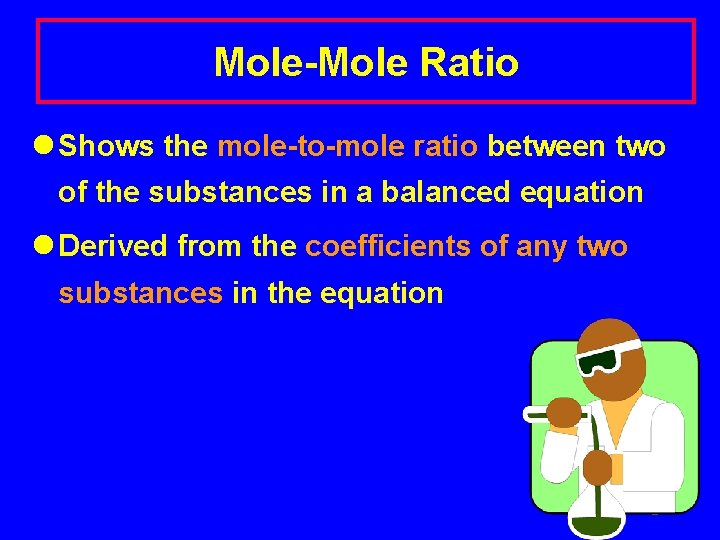
Mole-Mole Ratio l Shows the mole-to-mole ratio between two of the substances in a balanced equation l Derived from the coefficients of any two substances in the equation 2

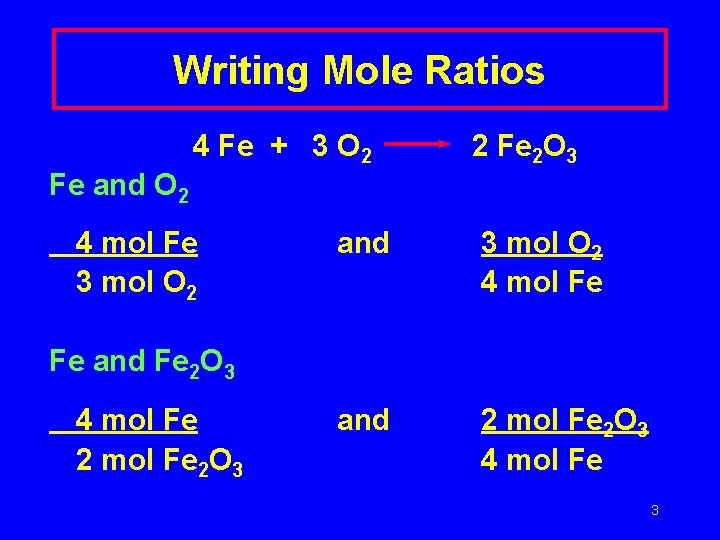
Writing Mole Ratios 4 Fe + 3 O 2 2 Fe 2 O 3 Fe and O 2 4 mol Fe 3 mol O 2 and 3 mol O 2 4 mol Fe and 2 mol Fe 2 O 3 4 mol Fe Fe and Fe 2 O 3 4 mol Fe 2 O 3 3

O 2 and Fe 2 O 3 3 mol O 2 2 mol Fe 2 O 3 and 2 mol Fe 2 O 3 3 mol O 2 4

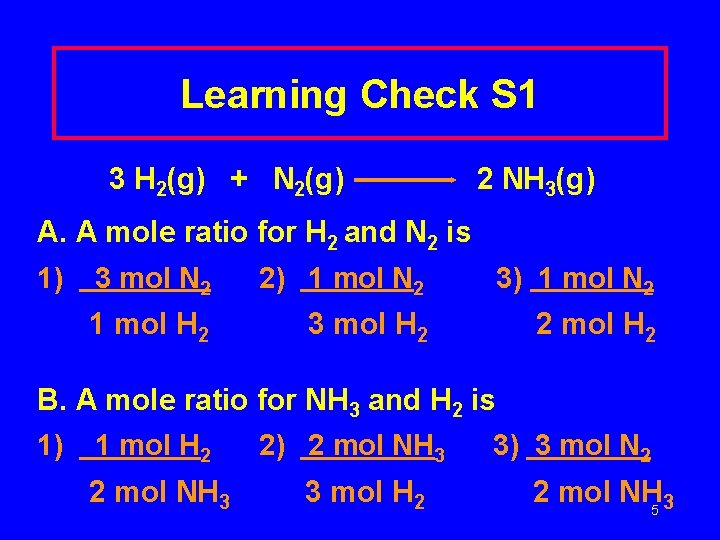
Learning Check S 1 3 H 2(g) + N 2(g) 2 NH 3(g) A. A mole ratio for H 2 and N 2 is 1) 3 mol N 2 2) 1 mol N 2 3) 1 mol N 2 1 mol H 2 3 mol H 2 2 mol H 2 B. A mole ratio for NH 3 and H 2 is 1) 1 mol H 2 2 mol NH 3 2) 2 mol NH 3 3 mol H 2 3) 3 mol N 2 2 mol NH 5 3

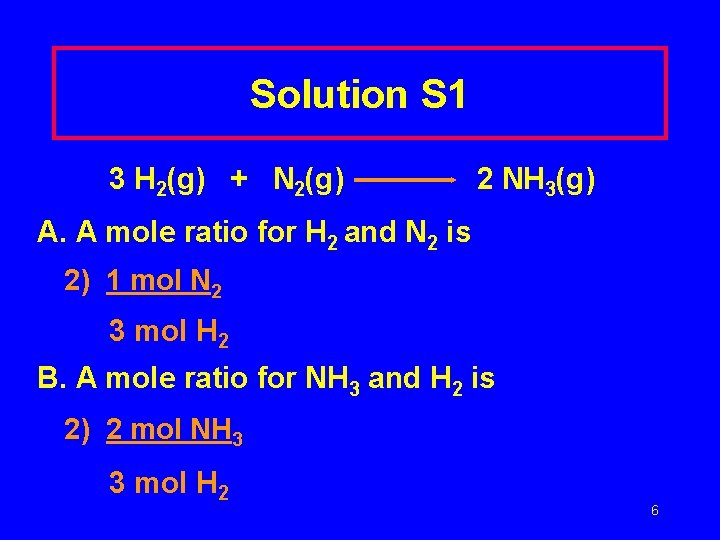
Solution S 1 3 H 2(g) + N 2(g) 2 NH 3(g) A. A mole ratio for H 2 and N 2 is 2) 1 mol N 2 3 mol H 2 B. A mole ratio for NH 3 and H 2 is 2) 2 mol NH 3 3 mol H 2 6

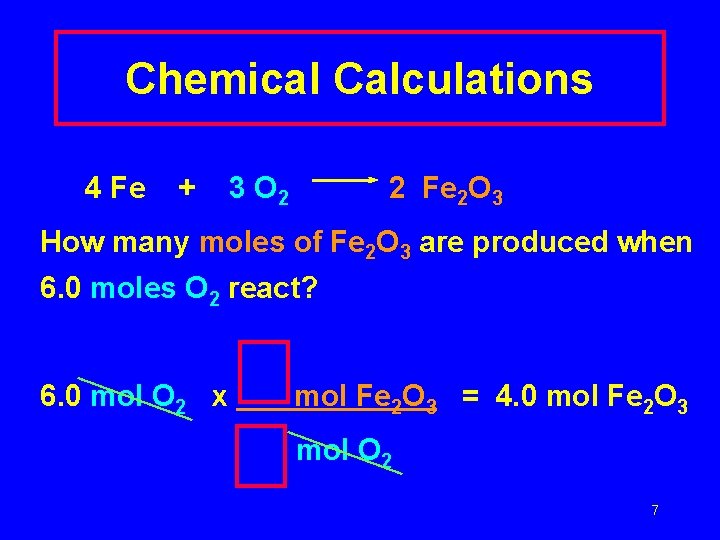
Chemical Calculations 4 Fe + 3 O 2 2 Fe 2 O 3 How many moles of Fe 2 O 3 are produced when 6. 0 moles O 2 react? 6. 0 mol O 2 x mol Fe 2 O 3 = 4. 0 mol Fe 2 O 3 mol O 2 7

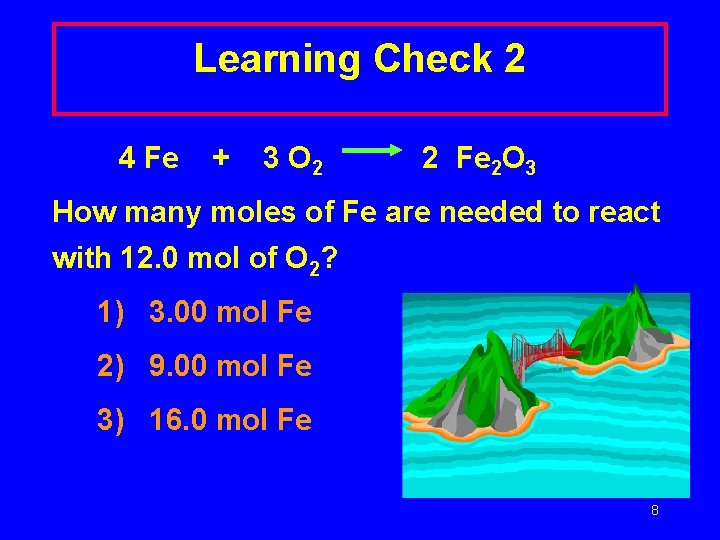
Learning Check 2 4 Fe + 3 O 2 2 Fe 2 O 3 How many moles of Fe are needed to react with 12. 0 mol of O 2? 1) 3. 00 mol Fe 2) 9. 00 mol Fe 3) 16. 0 mol Fe 8

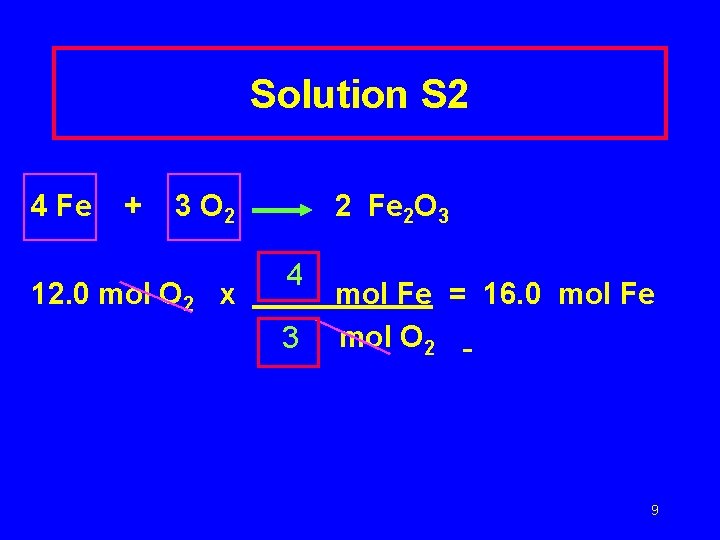
Solution S 2 4 Fe + 3 O 2 12. 0 mol O 2 x 2 Fe 2 O 3 4 3 mol Fe = 16. 0 mol Fe mol O 2 9

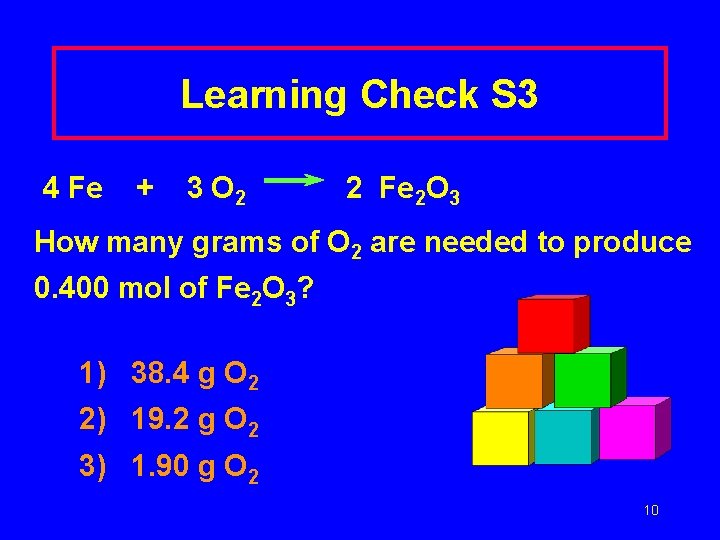
Learning Check S 3 4 Fe + 3 O 2 2 Fe 2 O 3 How many grams of O 2 are needed to produce 0. 400 mol of Fe 2 O 3? 1) 38. 4 g O 2 2) 19. 2 g O 2 3) 1. 90 g O 2 10

Solution S 3 0. 400 mol Fe 2 O 3 x 3 mol O 2 x 32. 0 g O 2 2 mol Fe 2 O 3 1 mol O 2 = 19. 2 g O 2 11

Calculating Mass of A Substance n Balance equation n Convert starting amount to moles n Use coefficients to write a mol-mol factor n Convert moles of desired to grams 12

Calculation The reaction between H 2 and O 2 produces 13. 1 g of water. How many grams of O 2 reacted? Write the equation H 2 (g) + O 2 (g) H 2 O (g) Balance the equation 2 H 2 (g) + O 2 (g) 2 H 2 O (g) 13

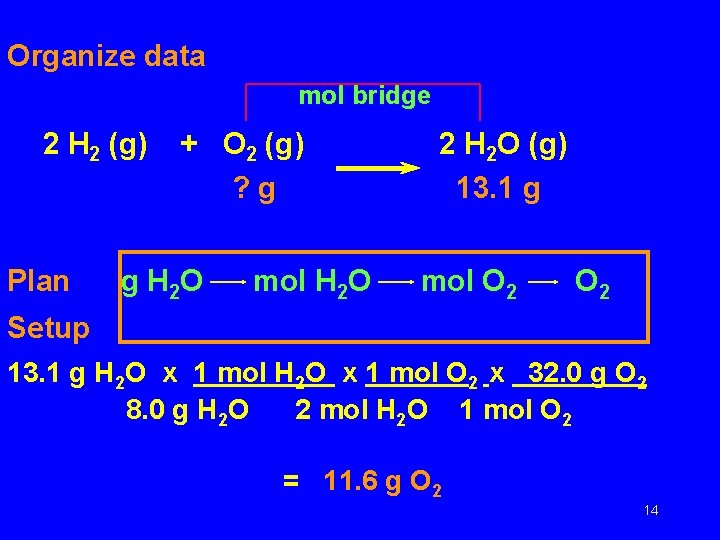
Organize data mol bridge 2 H 2 (g) Plan + O 2 (g) ? g g H 2 O mol H 2 O 2 H 2 O (g) 13. 1 g mol O 2 Setup 13. 1 g H 2 O x 1 mol O 2 x 32. 0 g O 2 8. 0 g H 2 O 2 mol H 2 O 1 mol O 2 = 11. 6 g O 2 14

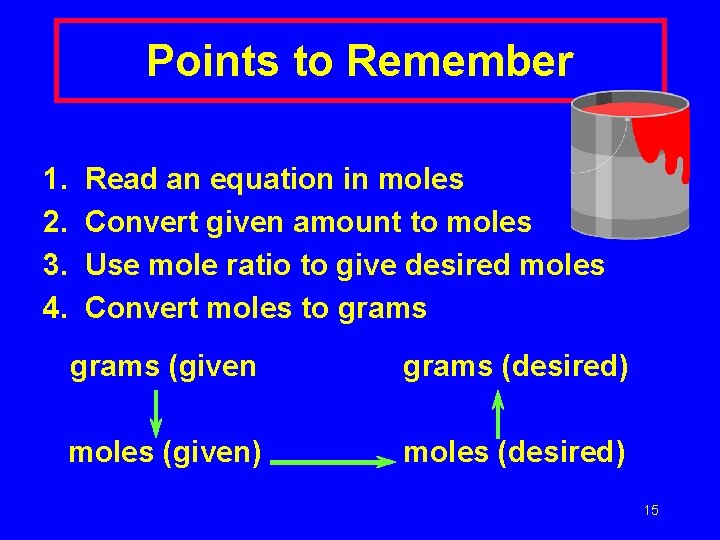
Points to Remember 1. 2. 3. 4. Read an equation in moles Convert given amount to moles Use mole ratio to give desired moles Convert moles to grams (given grams (desired) moles (given) moles (desired) 15

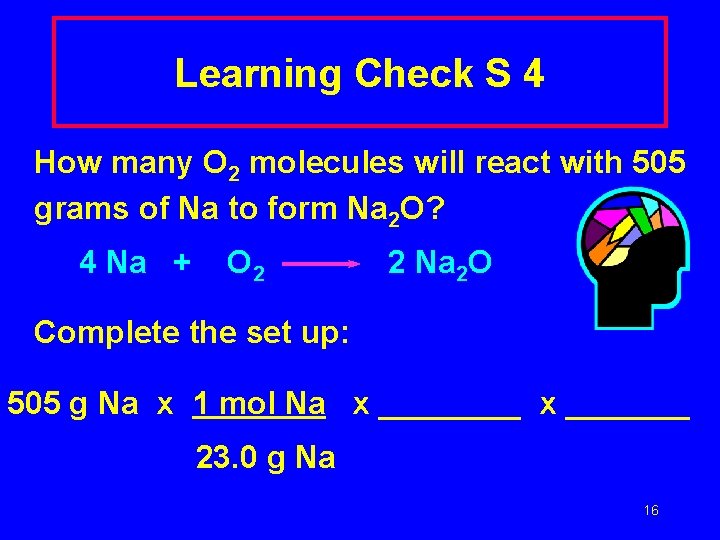
Learning Check S 4 How many O 2 molecules will react with 505 grams of Na to form Na 2 O? 4 Na + O 2 2 Na 2 O Complete the set up: 505 g Na x 1 mol Na x _______ 23. 0 g Na 16

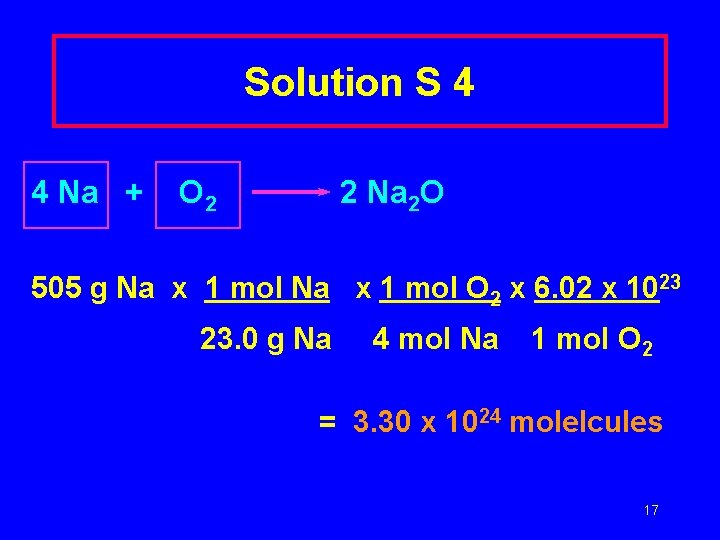
Solution S 4 4 Na + O 2 2 Na 2 O 505 g Na x 1 mol O 2 x 6. 02 x 1023 23. 0 g Na 4 mol Na 1 mol O 2 = 3. 30 x 1024 molelcules 17

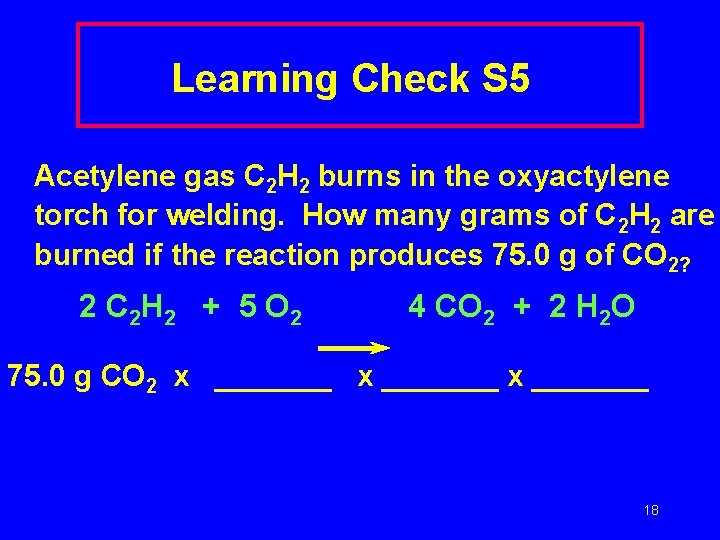
Learning Check S 5 Acetylene gas C 2 H 2 burns in the oxyactylene torch for welding. How many grams of C 2 H 2 are burned if the reaction produces 75. 0 g of CO 2? 2 C 2 H 2 + 5 O 2 4 CO 2 + 2 H 2 O 75. 0 g CO 2 x _______ 18

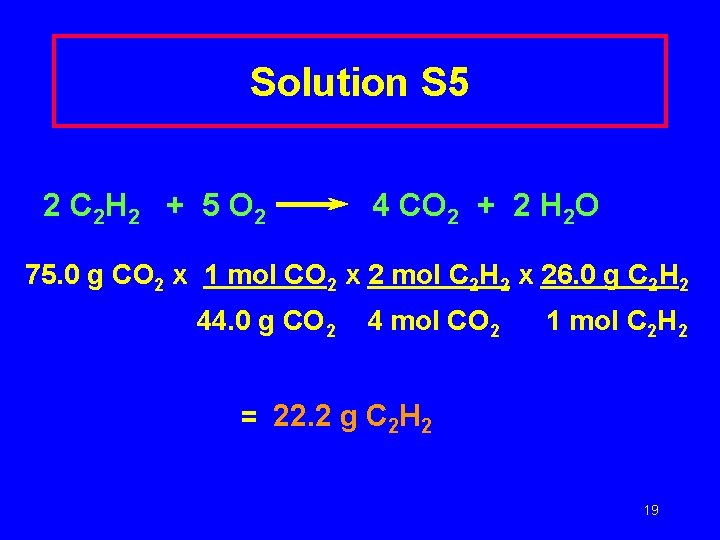
Solution S 5 2 C 2 H 2 + 5 O 2 4 CO 2 + 2 H 2 O 75. 0 g CO 2 x 1 mol CO 2 x 2 mol C 2 H 2 x 26. 0 g C 2 H 2 44. 0 g CO 2 4 mol CO 2 1 mol C 2 H 2 = 22. 2 g C 2 H 2 19

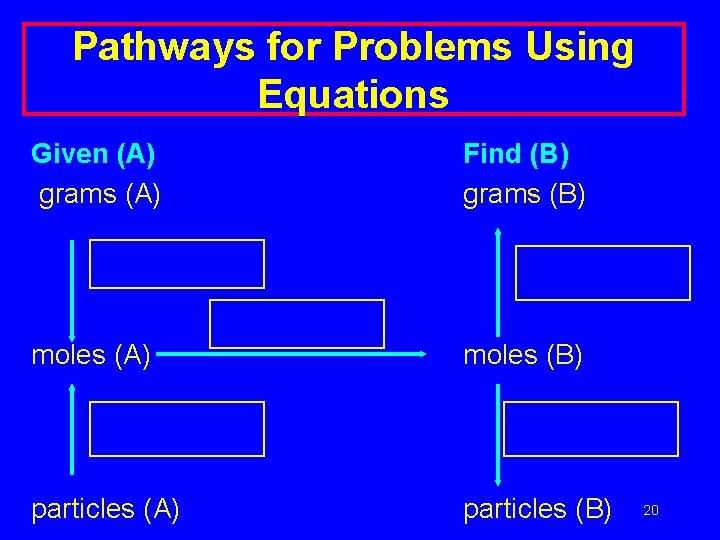
Pathways for Problems Using Equations Given (A) grams (A) Find (B) grams (B) moles (A) moles (B) particles (A) particles (B) 20

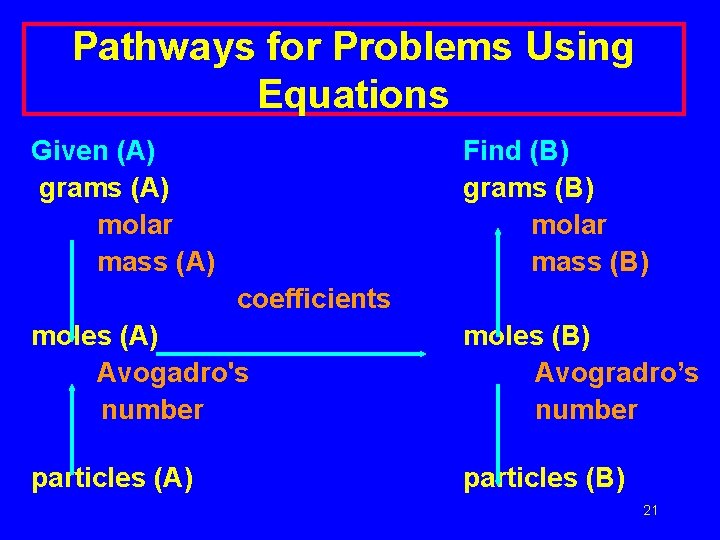
Pathways for Problems Using Equations Given (A) grams (A) molar mass (A) Find (B) grams (B) molar mass (B) coefficients moles (A) Avogadro's number moles (B) Avogradro’s number particles (A) particles (B) 21

Limiting Reactants l If the amounts of two reactants are given, the reactant used up first determines the amount of product formed. 22

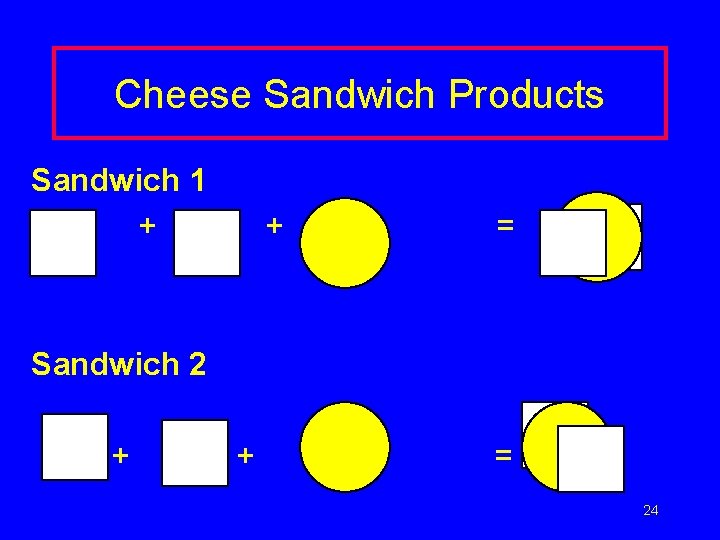
Analogy Suppose you are preparing cheese sandwiches. Each sandwich requires 2 pieces of bread and 1 slice of cheese. If you have 4 slices of cheese and 10 pieces of bread, how many cheese sandwiches can you make? 23

Cheese Sandwich Products Sandwich 1 + + = Sandwich 2 + + = 24

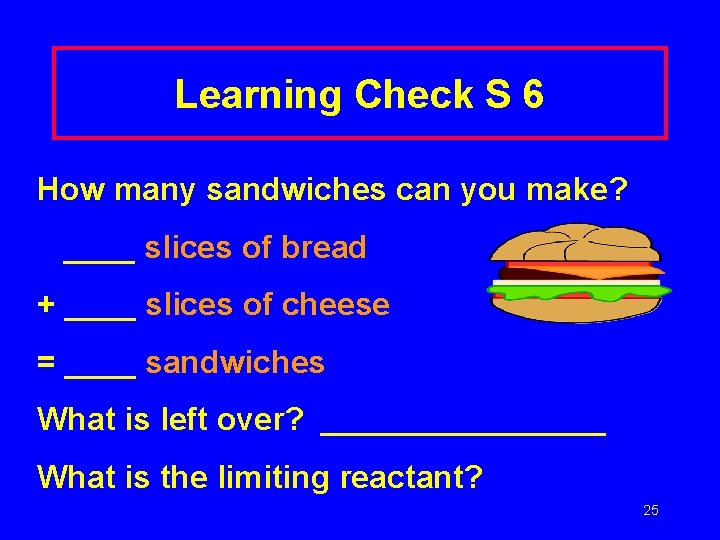
Learning Check S 6 How many sandwiches can you make? ____ slices of bread + ____ slices of cheese = ____ sandwiches What is left over? ________ What is the limiting reactant? 25

Solution S 6 How many sandwiches can you make? __10__ slices of bread + __4__ slices of cheese = __4__ sandwiches What is left over? _2 slices of bread What is the limiting reactant? cheese 26

Hints for LR Problems 1. For each reactant amount given, calculate the moles (or grams) of a product it could produce. 2. The reactant that produces the smaller amount of product is the limiting reactant. 3. The number of moles of product produced by the limiting reactant is ALL the product possible. There is no more limiting reactant left. 27

Percent Yield You prepared cookie dough to make 5 dozen cookies. The phone rings while a sheet of 12 cookies is baking. You talk too long and the cookies burn. You throw them out (or give them to your dog. ) The rest of the cookies are okay. How many cookies could you have made (theoretical yield)? How many cookies did you actually make to eat? (Actual yield) 28

Vocabulary Actual yield is the amount of product actually recovered from an experiment Theoretical (possible) yield is the maximum amount of product that could be produced from the reactant. Percent Yield is the actual yield compared to the maximum (theoretical yield) possible. 29

Percent Yield Calculation What is the percent yield of cookies? Percent Yield = Actual Yield (g) recovered X 100 Possible Yield (g) % cookie yield = 48 cookies x 100 = 80% yield 60 cookies 30