Chapter 10 Introduction to Organic Chemistry Alkanes 10

- Slides: 18

Chapter 10 Introduction to Organic Chemistry: Alkanes 10. 2 Alkanes Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 1

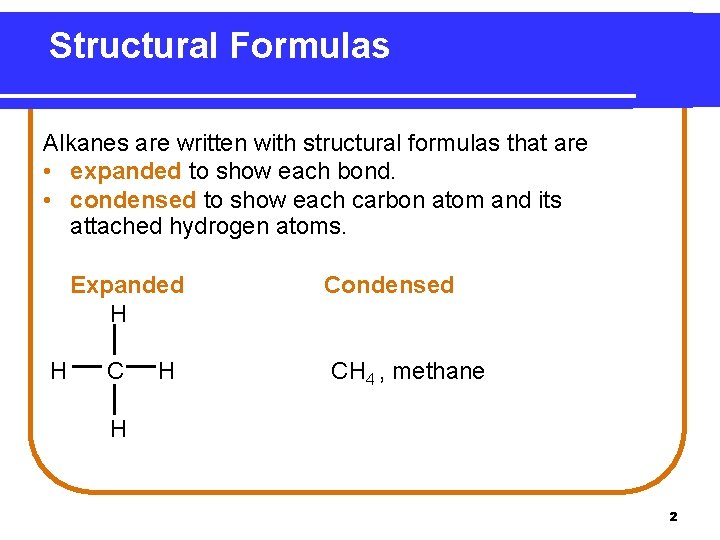
Structural Formulas Alkanes are written with structural formulas that are • expanded to show each bond. • condensed to show each carbon atom and its attached hydrogen atoms. Expanded H H Condensed CH 4 , methane H 2

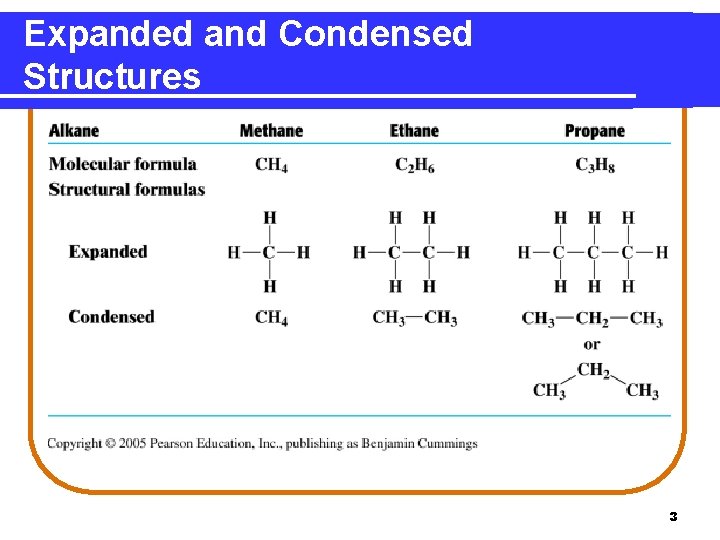
Expanded and Condensed Structures 3

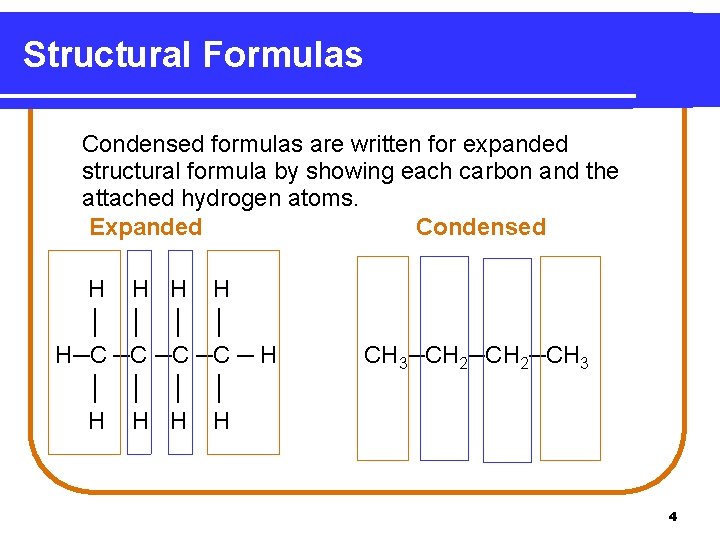
Structural Formulas Condensed formulas are written for expanded structural formula by showing each carbon and the attached hydrogen atoms. Expanded Condensed H H │ │ H─C ─C ─ H │ │ H H CH 3─CH 2─CH 3 4

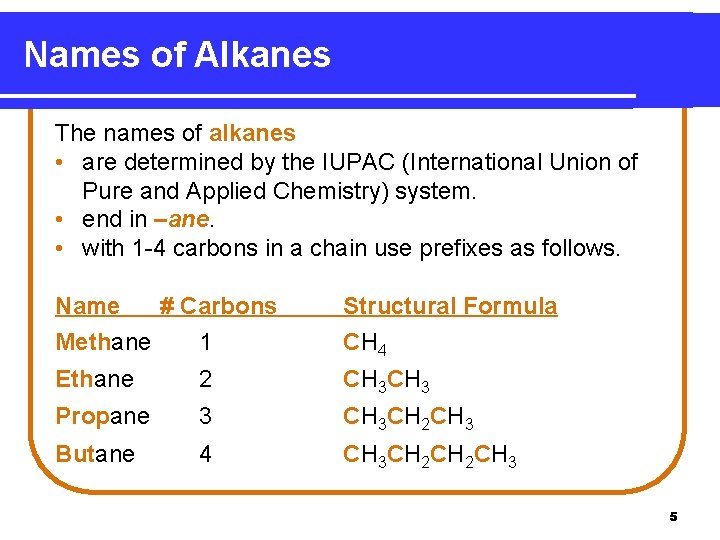
Names of Alkanes The names of alkanes • are determined by the IUPAC (International Union of Pure and Applied Chemistry) system. • end in –ane. • with 1 -4 carbons in a chain use prefixes as follows. Name # Carbons Methane 1 Structural Formula CH 4 Ethane 2 CH 3 Propane 3 CH 3 CH 2 CH 3 Butane 4 CH 3 CH 2 CH 3 5

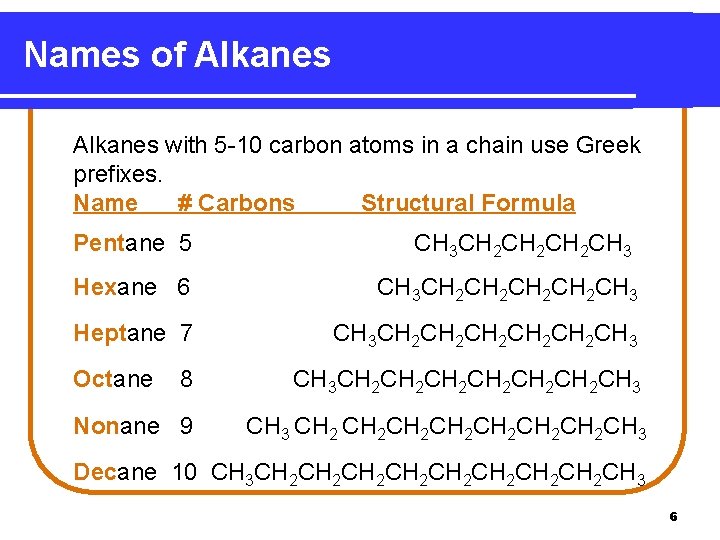
Names of Alkanes with 5 -10 carbon atoms in a chain use Greek prefixes. Name # Carbons Structural Formula Pentane 5 CH 3 CH 2 CH 2 CH 3 Hexane 6 CH 3 CH 2 CH 2 CH 3 Heptane 7 CH 3 CH 2 CH 2 CH 2 CH 3 Octane 8 CH 3 CH 2 CH 2 CH 2 CH 3 Nonane 9 CH 3 CH 2 CH 2 CH 3 Decane 10 CH 3 CH 2 CH 2 CH 3 6

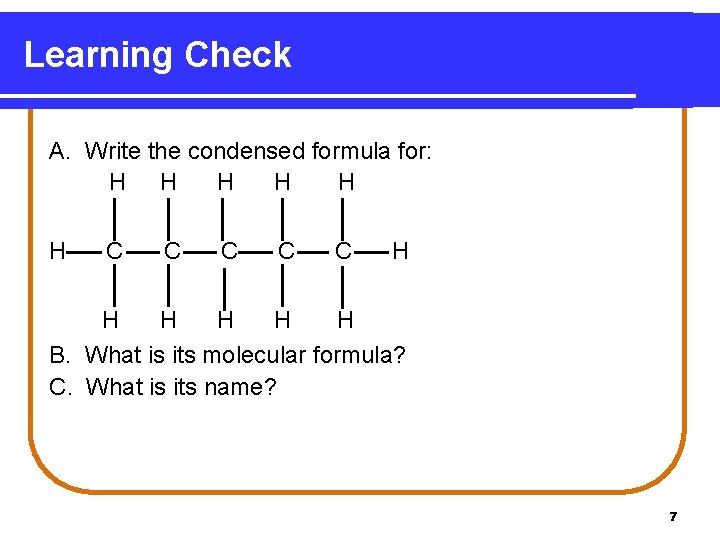
Learning Check A. Write the condensed formula for: H H H C C C H H H B. What is its molecular formula? C. What is its name? 7

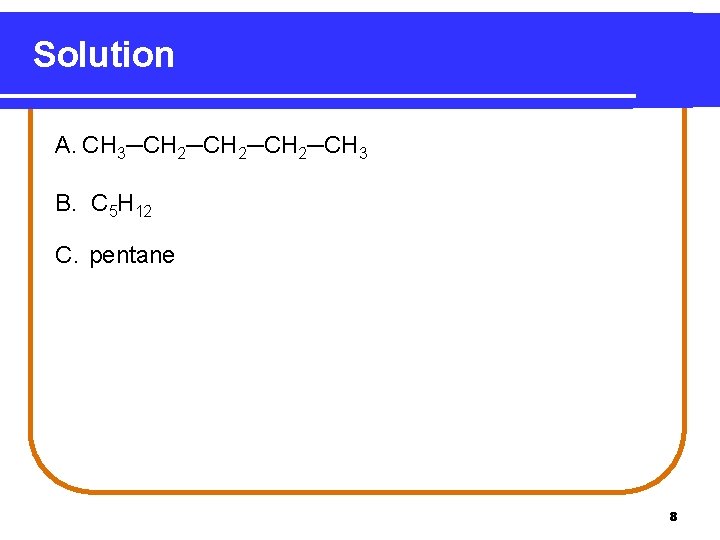
Solution A. CH 3─CH 2─CH 3 B. C 5 H 12 C. pentane 8

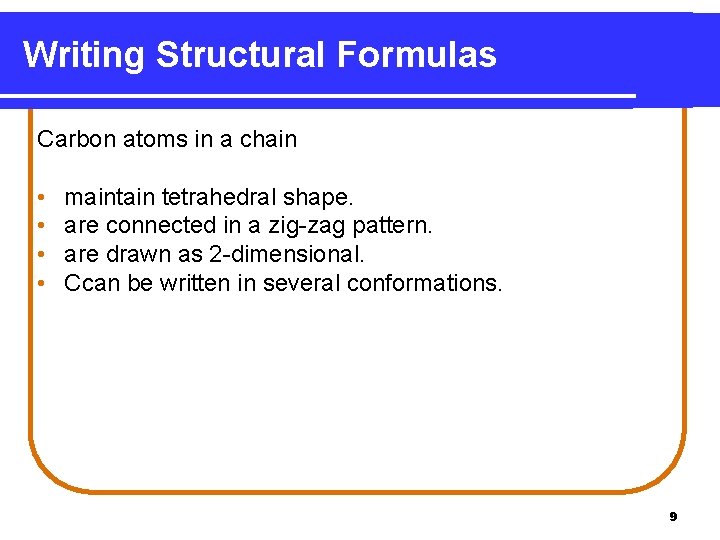
Writing Structural Formulas Carbon atoms in a chain • • maintain tetrahedral shape. are connected in a zig-zag pattern. are drawn as 2 -dimensional. Ccan be written in several conformations. 9

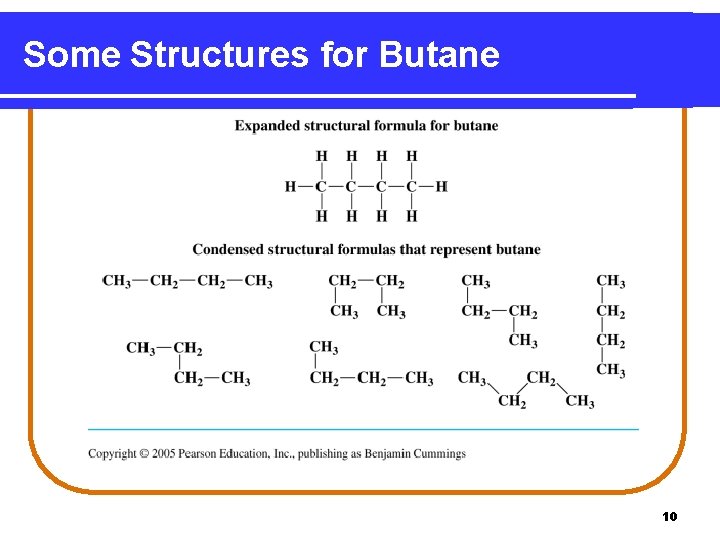
Some Structures for Butane 10

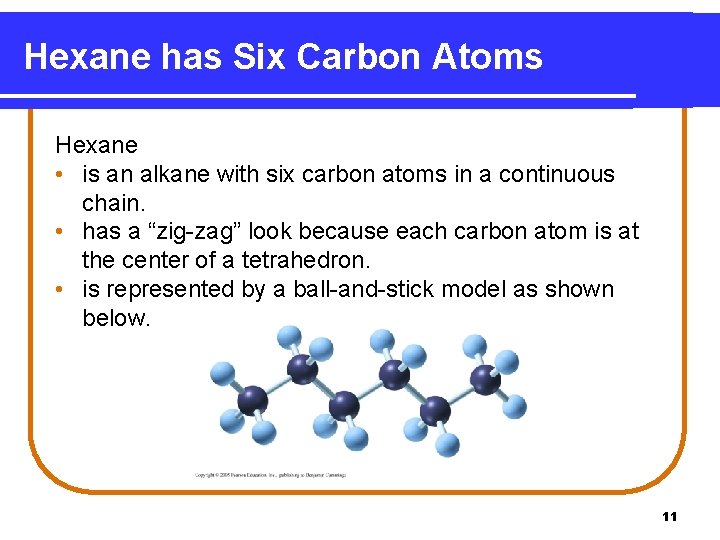
Hexane has Six Carbon Atoms Hexane • is an alkane with six carbon atoms in a continuous chain. • has a “zig-zag” look because each carbon atom is at the center of a tetrahedron. • is represented by a ball-and-stick model as shown below. 11

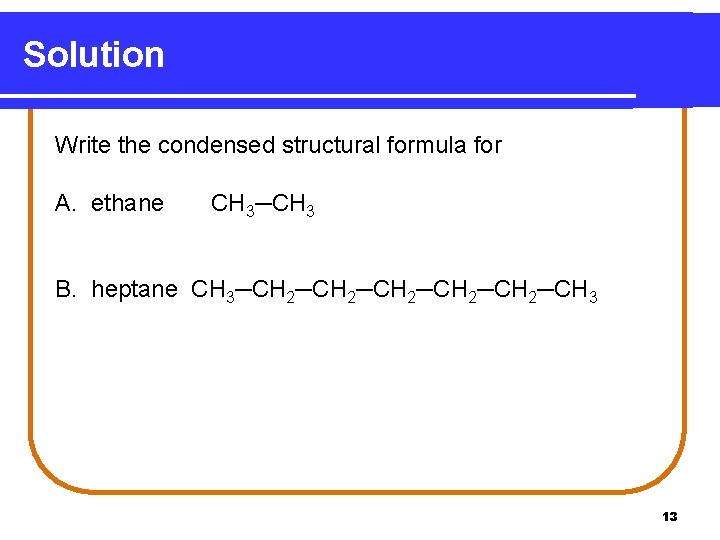
Learning Check Write the condensed structural formula for A. ethane. B. heptane. 12

Solution Write the condensed structural formula for A. ethane CH 3─CH 3 B. heptane CH 3─CH 2─CH 2─CH 3 13

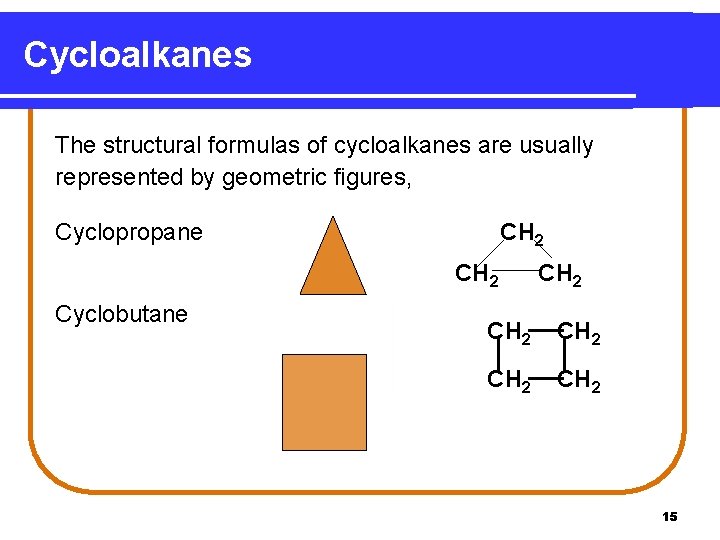
Cycloalkanes • are cyclic alkanes. • have two hydrogen atoms fewer than the open chain. • are named by using the prefix cyclo- before the name of the alkane chain with the same number of carbon atoms. 14

Cycloalkanes The structural formulas of cycloalkanes are usually represented by geometric figures, Cyclopropane CH 2 Cyclobutane CH 2 CH 2 15

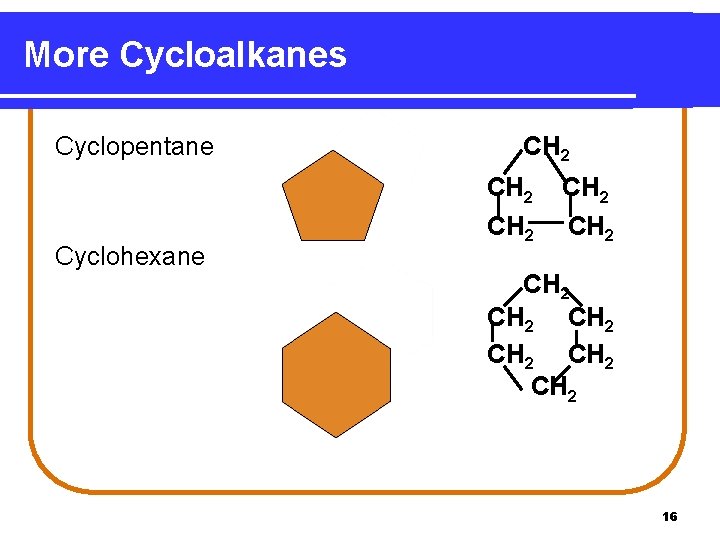
More Cycloalkanes Cyclopentane Cyclohexane CH 2 CH 2 CH 2 16

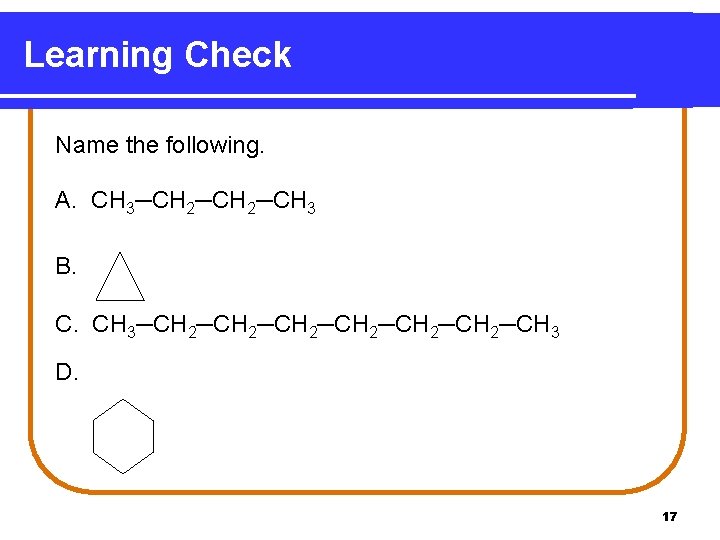
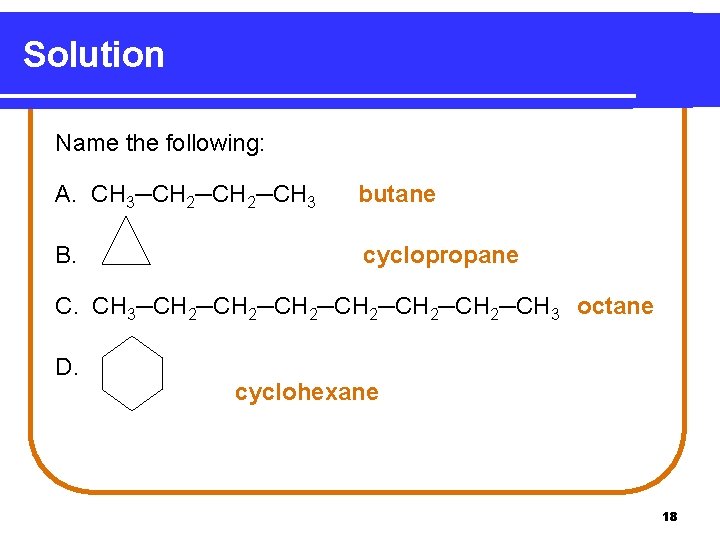
Learning Check Name the following. A. CH 3─CH 2─CH 3 B. C. CH 3─CH 2─CH 2─CH 3 D. 17

Solution Name the following: A. CH 3─CH 2─CH 3 butane B. cyclopropane C. CH 3─CH 2─CH 2─CH 3 octane D. cyclohexane 18