Chapter 10 Introduction to Organic Chemistry Alkanes 10








- Slides: 58

Chapter 10 Introduction to Organic Chemistry: Alkanes 10. 1 Organic Compounds Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 1

Organic Chemistry An organic compound: • is a compound made from carbon atoms. • has one or more C atoms. • has many H atoms. • may also contain O, S, N, and halogens. 2

Organic Compounds Typical organic compounds: • have covalent bonds. • have low melting points. • have low boiling points. • are flammable. • are soluble in nonpolar solvents. • are not soluble in water. oil (organic) and water (inorganic) 3

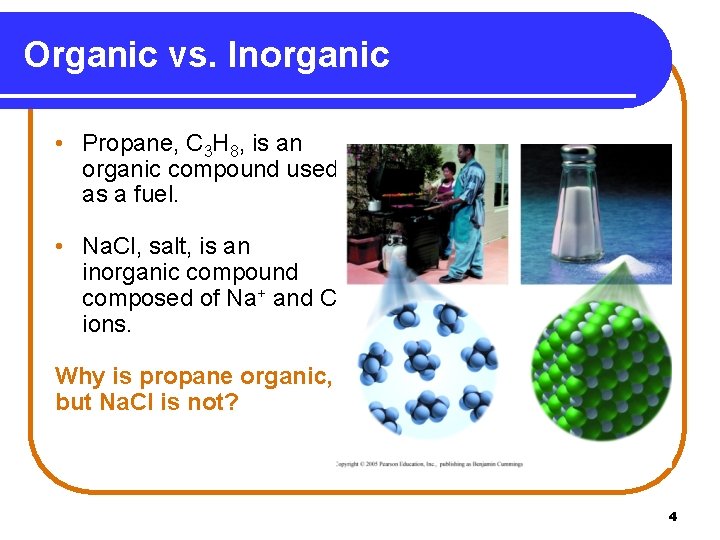
Organic vs. Inorganic • Propane, C 3 H 8, is an organic compound used as a fuel. • Na. Cl, salt, is an inorganic compound composed of Na+ and Clions. Why is propane organic, but Na. Cl is not? 4

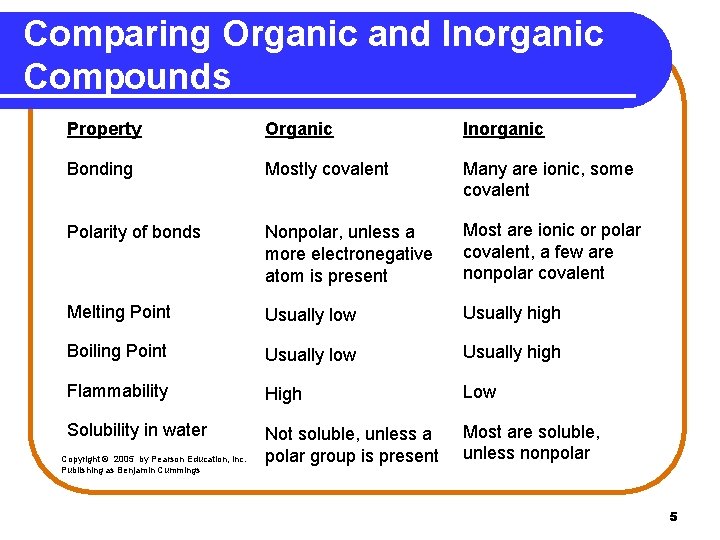
Comparing Organic and Inorganic Compounds Property Organic Inorganic Bonding Mostly covalent Many are ionic, some covalent Polarity of bonds Nonpolar, unless a more electronegative atom is present Most are ionic or polar covalent, a few are nonpolar covalent Melting Point Usually low Usually high Boiling Point Usually low Usually high Flammability High Low Solubility in water Not soluble, unless a polar group is present Most are soluble, unless nonpolar Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 5

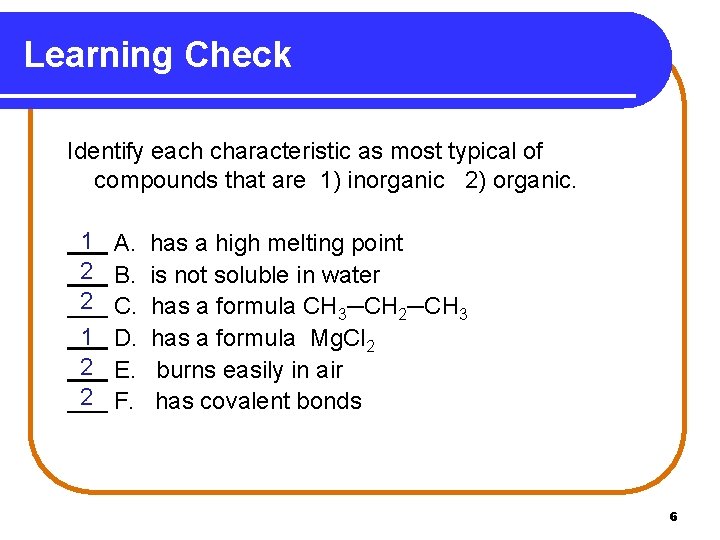
Learning Check Identify each characteristic as most typical of compounds that are 1) inorganic 2) organic. 1 2 2 A. B. C. D. E. F. has a high melting point is not soluble in water has a formula CH 3─CH 2─CH 3 has a formula Mg. Cl 2 burns easily in air has covalent bonds 6

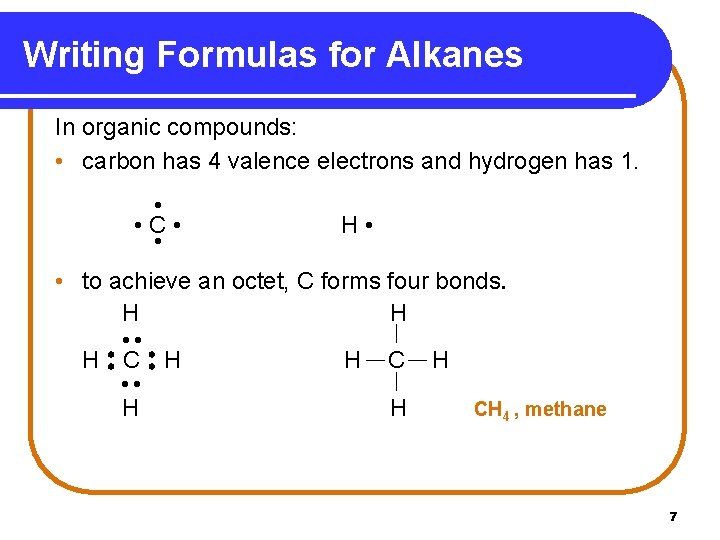
Writing Formulas for Alkanes In organic compounds: • carbon has 4 valence electrons and hydrogen has 1. • • C • • H • • to achieve an octet, C forms four bonds. H H H CH 4 , methane 7

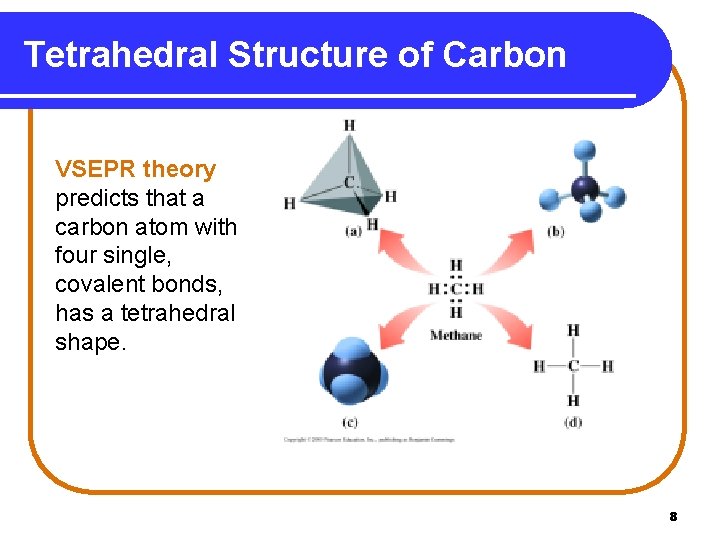
Tetrahedral Structure of Carbon VSEPR theory predicts that a carbon atom with four single, covalent bonds, has a tetrahedral shape. 8

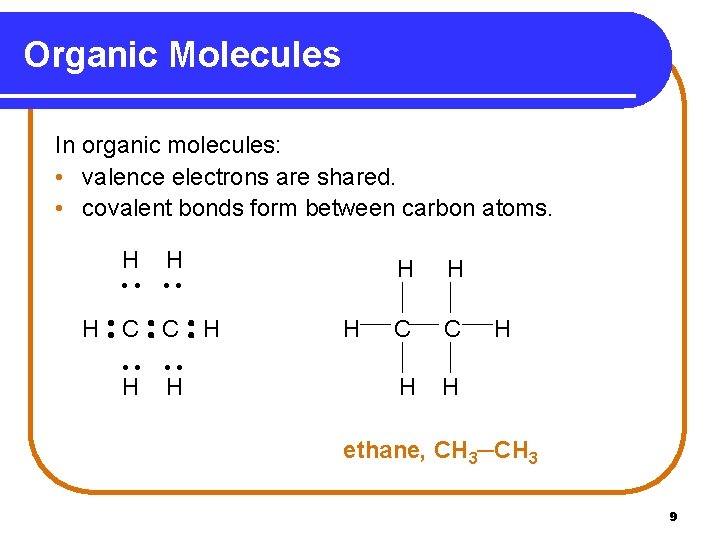
Organic Molecules In organic molecules: • valence electrons are shared. • covalent bonds form between carbon atoms. H H • • H C C H • • H H H H C C H H H ethane, CH 3─CH 3 9

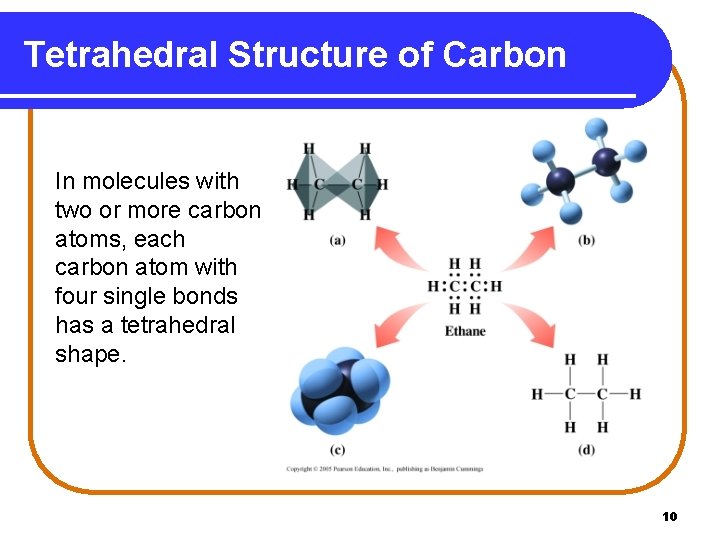
Tetrahedral Structure of Carbon In molecules with two or more carbon atoms, each carbon atom with four single bonds has a tetrahedral shape. 10

Chapter 10 Introduction to Organic Chemistry: Alkanes 10. 2 Alkanes Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 11

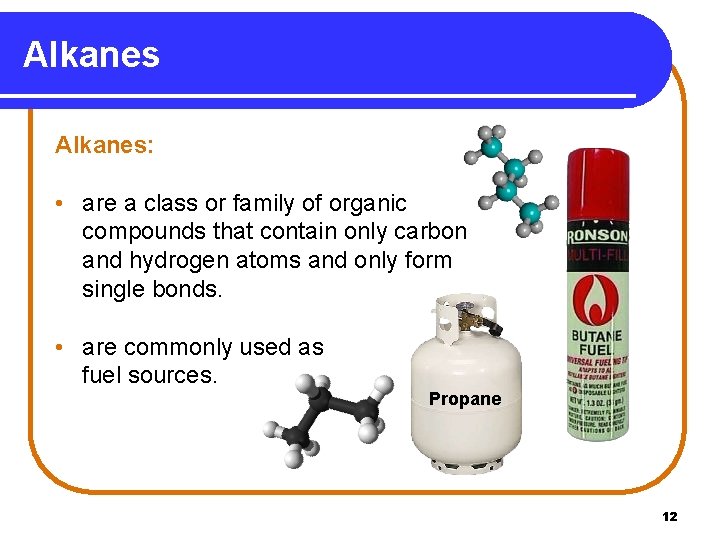
Alkanes: • are a class or family of organic compounds that contain only carbon and hydrogen atoms and only form single bonds. • are commonly used as fuel sources. Propane 12

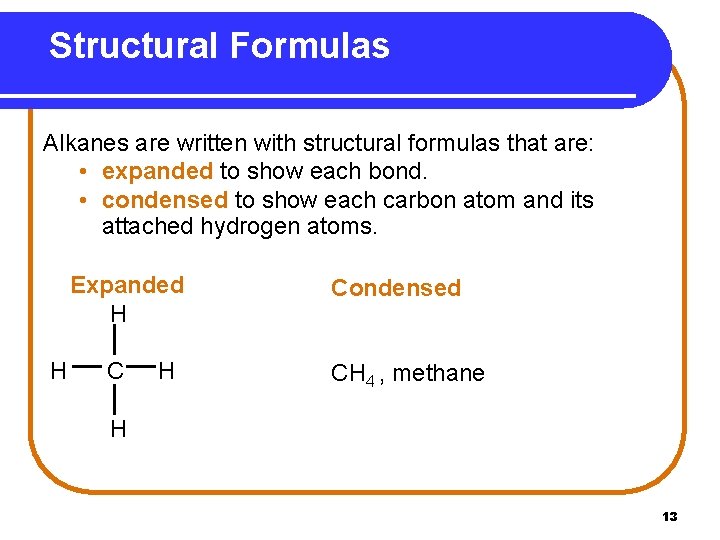
Structural Formulas Alkanes are written with structural formulas that are: • expanded to show each bond. • condensed to show each carbon atom and its attached hydrogen atoms. Expanded H H Condensed CH 4 , methane H 13

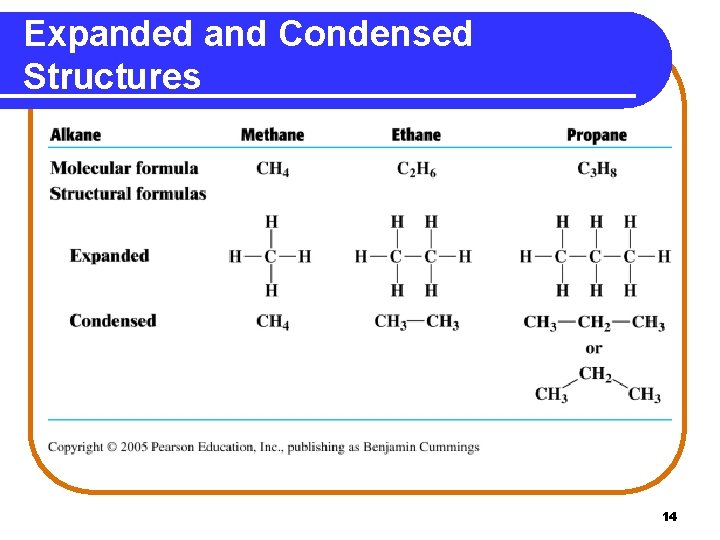
Expanded and Condensed Structures 14

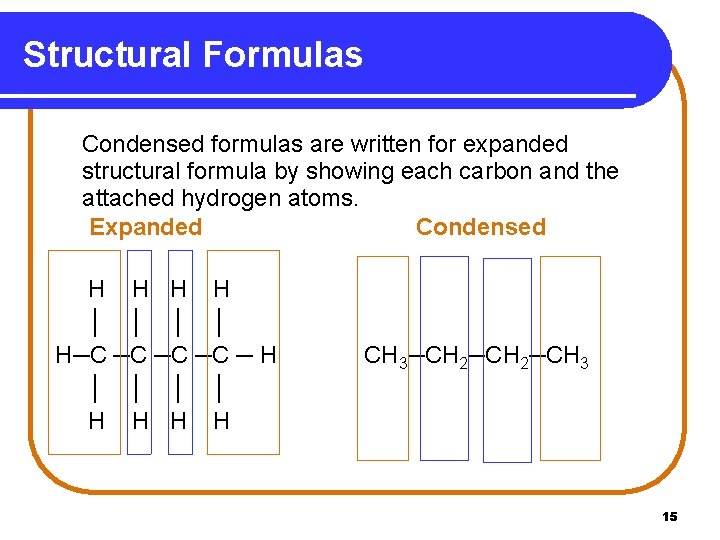
Structural Formulas Condensed formulas are written for expanded structural formula by showing each carbon and the attached hydrogen atoms. Expanded Condensed H H │ │ H─C ─C ─ H │ │ H H CH 3─CH 2─CH 3 15

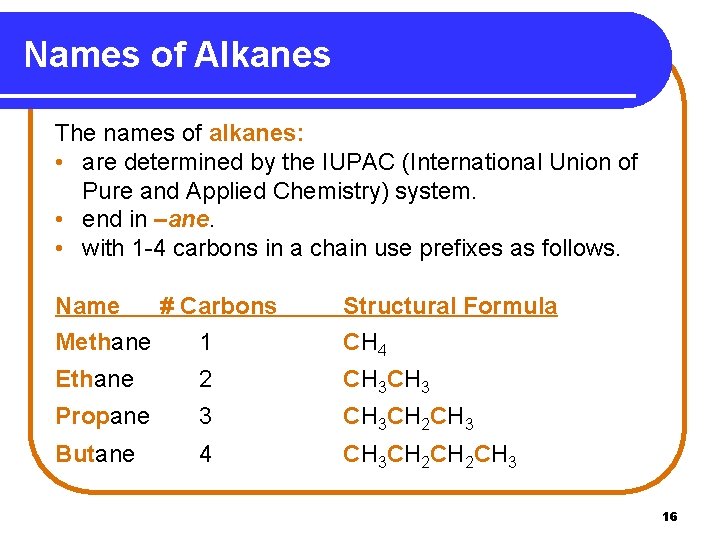
Names of Alkanes The names of alkanes: • are determined by the IUPAC (International Union of Pure and Applied Chemistry) system. • end in –ane. • with 1 -4 carbons in a chain use prefixes as follows. Name # Carbons Methane 1 Structural Formula CH 4 Ethane 2 CH 3 Propane 3 CH 3 CH 2 CH 3 Butane 4 CH 3 CH 2 CH 3 16

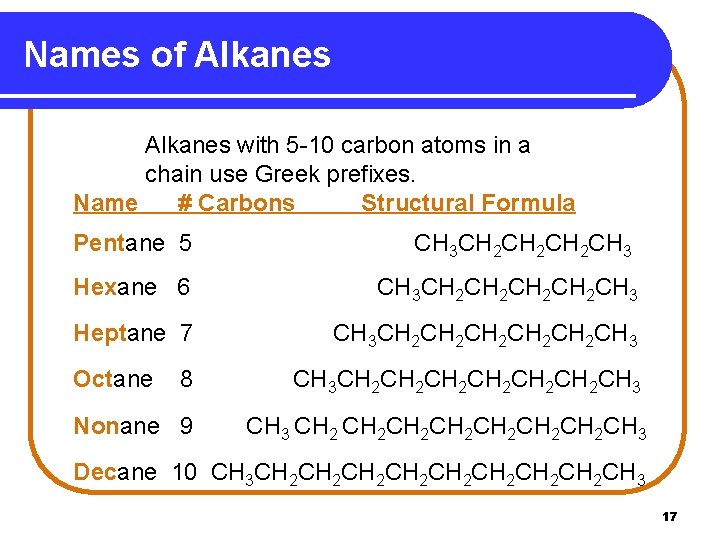
Names of Alkanes with 5 -10 carbon atoms in a chain use Greek prefixes. Name # Carbons Structural Formula Pentane 5 CH 3 CH 2 CH 2 CH 3 Hexane 6 CH 3 CH 2 CH 2 CH 3 Heptane 7 CH 3 CH 2 CH 2 CH 2 CH 3 Octane 8 CH 3 CH 2 CH 2 CH 2 CH 3 Nonane 9 CH 3 CH 2 CH 2 CH 3 Decane 10 CH 3 CH 2 CH 2 CH 3 17

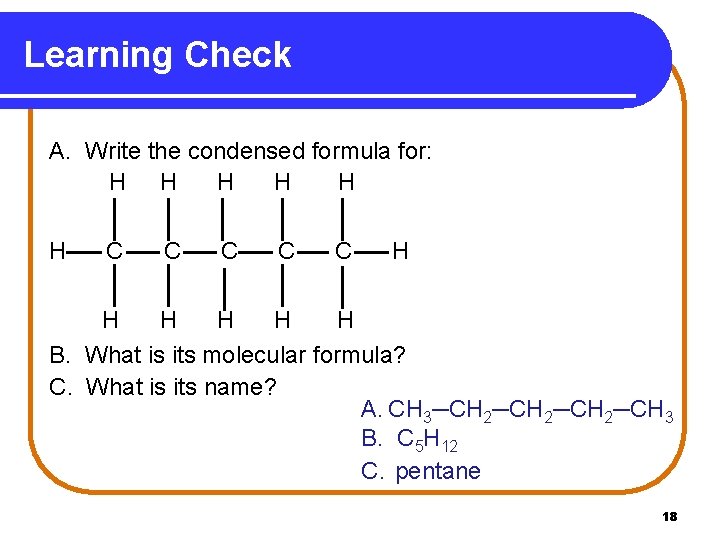
Learning Check A. Write the condensed formula for: H H H C C C H H H B. What is its molecular formula? C. What is its name? A. CH 3─CH 2─CH 3 B. C 5 H 12 C. pentane 18

Writing Structural Formulas Carbon atoms in a chain: • maintain tetrahedral shape. • are connected in a zig-zag pattern. • are drawn as 2 -dimensional. • can be written in several conformations. 19

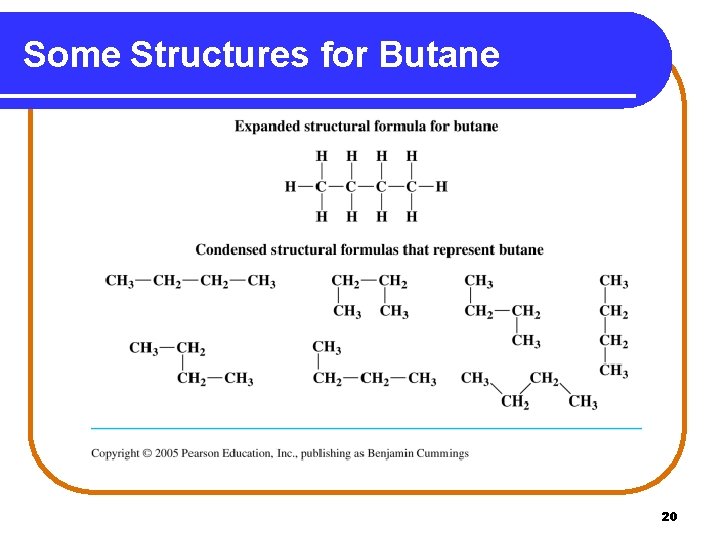
Some Structures for Butane 20

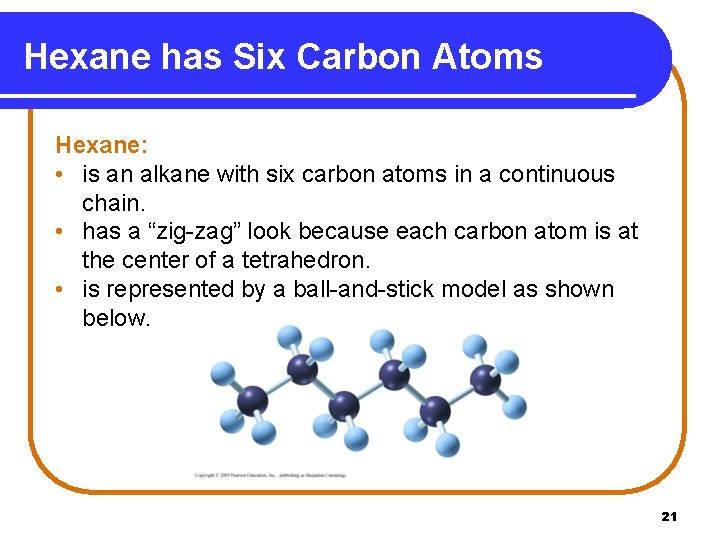
Hexane has Six Carbon Atoms Hexane: • is an alkane with six carbon atoms in a continuous chain. • has a “zig-zag” look because each carbon atom is at the center of a tetrahedron. • is represented by a ball-and-stick model as shown below. 21

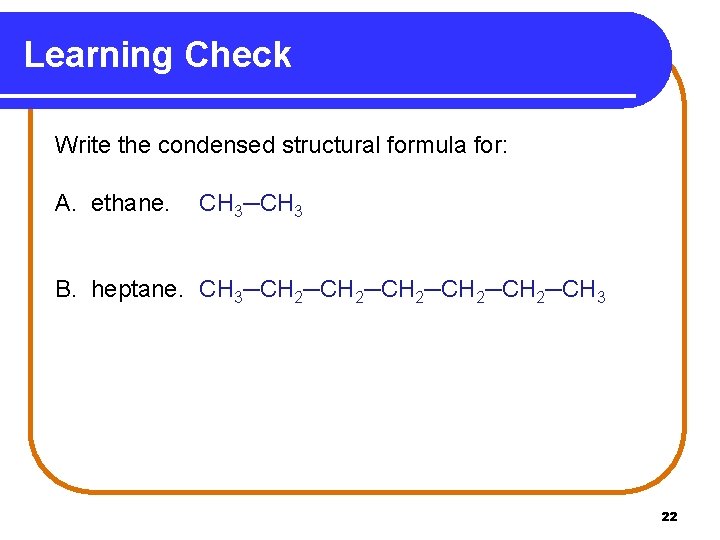
Learning Check Write the condensed structural formula for: A. ethane. CH 3─CH 3 B. heptane. CH 3─CH 2─CH 2─CH 3 22

Cycloalkanes: • are cyclic alkanes. • have two hydrogen atoms fewer than the open chain. • are named by using the prefix cyclo- before the name of the alkane chain with the same number of carbon atoms. 23

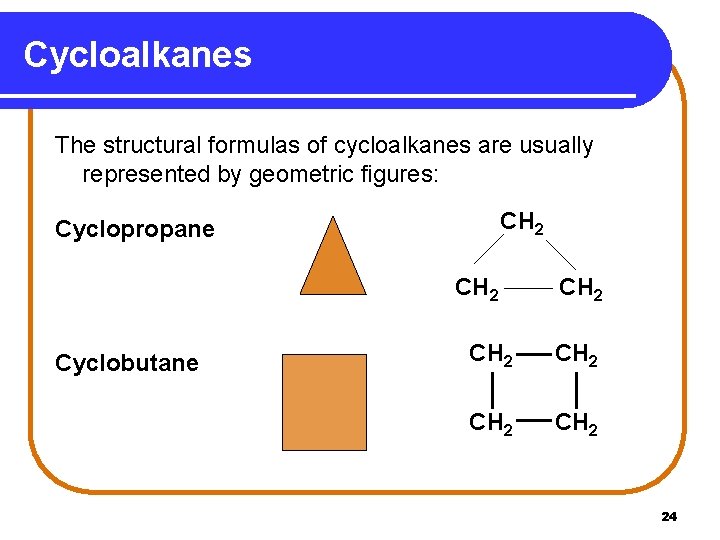
Cycloalkanes The structural formulas of cycloalkanes are usually represented by geometric figures: CH 2 Cyclopropane CH 2 Cyclobutane CH 2 CH 2 24

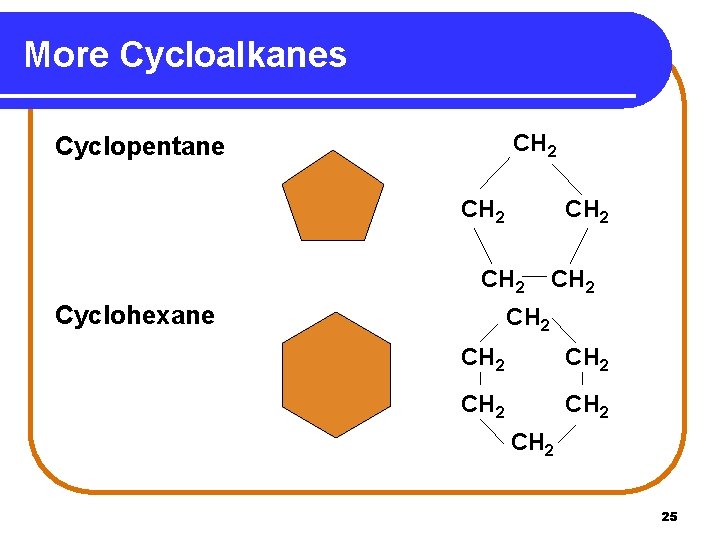
More Cycloalkanes CH 2 Cyclopentane CH 2 Cyclohexane CH 2 CH 2 25

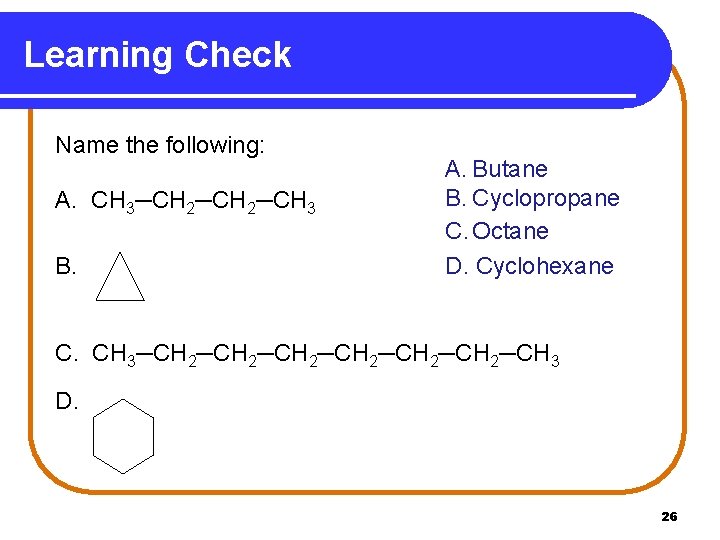
Learning Check Name the following: A. CH 3─CH 2─CH 3 B. A. Butane B. Cyclopropane C. Octane D. Cyclohexane C. CH 3─CH 2─CH 2─CH 3 D. 26

Chapter 10 Introduction to Organic Chemistry: Alkanes 10. 3 Alkanes with Substituents 27

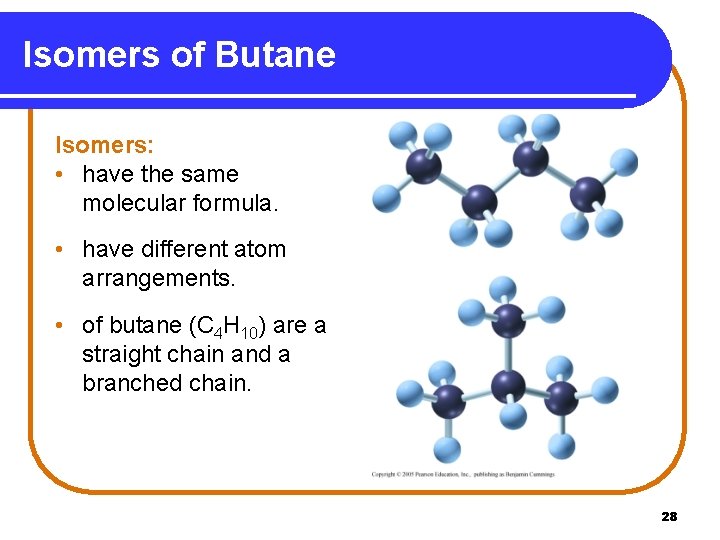
Isomers of Butane Isomers: • have the same molecular formula. • have different atom arrangements. • of butane (C 4 H 10) are a straight chain and a branched chain. 28

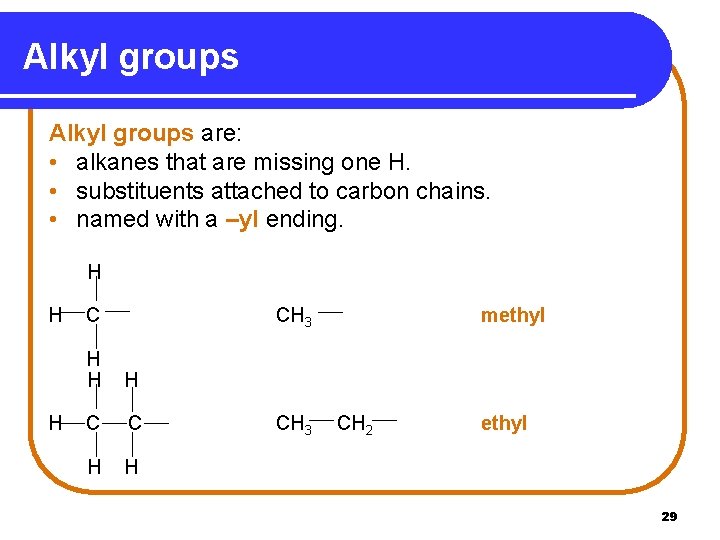
Alkyl groups are: • alkanes that are missing one H. • substituents attached to carbon chains. • named with a –yl ending. H H H C CH 3 H H H C C H H CH 3 methyl CH 2 ethyl 29

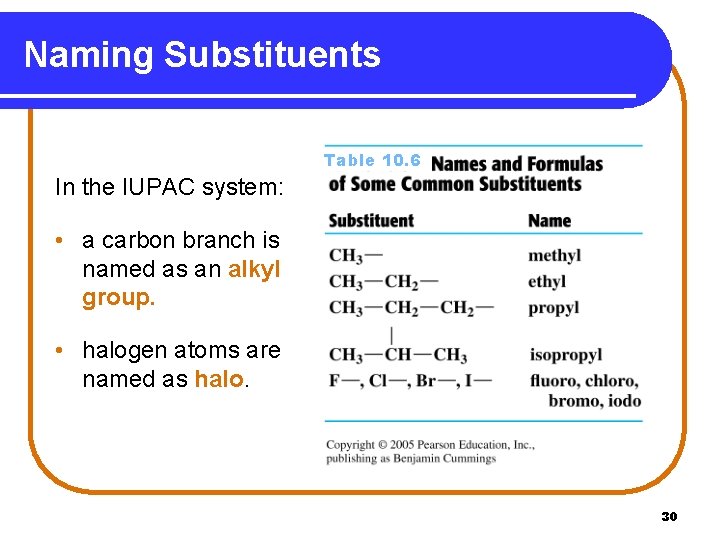
Naming Substituents Table 10. 6 In the IUPAC system: • a carbon branch is named as an alkyl group. • halogen atoms are named as halo. 30

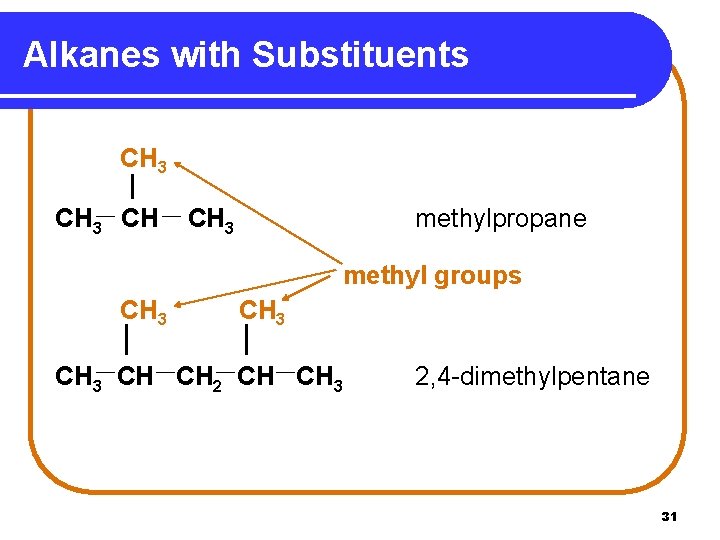
Alkanes with Substituents CH 3 CH CH 3 methylpropane methyl groups CH 3 CH CH 2 CH CH 3 2, 4 -dimethylpentane 31

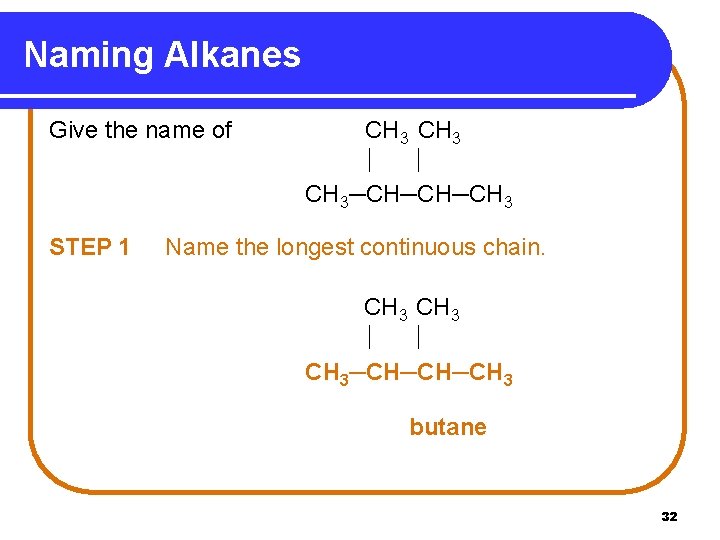
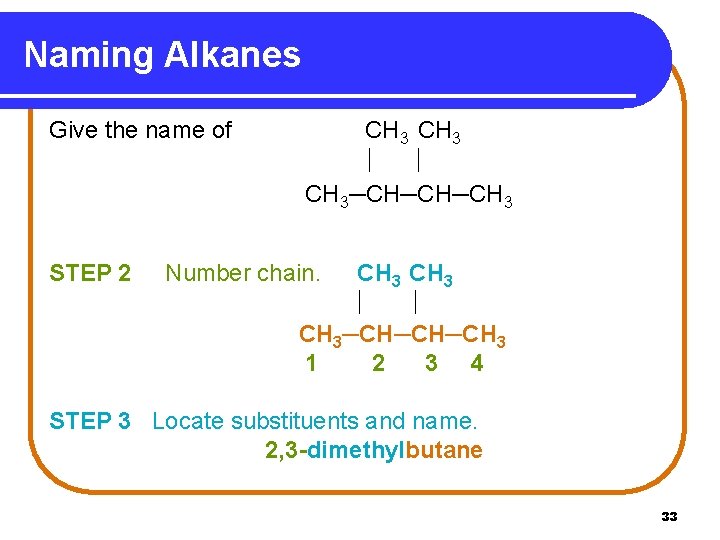
Naming Alkanes Give the name of STEP 1 CH 3─CH─CH─CH 3 Name the longest continuous chain. CH 3─CH─CH─CH 3 butane 32

Naming Alkanes Give the name of CH 3─CH─CH─CH 3 STEP 2 CH 3─CH─CH─CH 3 1 2 3 4 Number chain. STEP 3 Locate substituents and name. 2, 3 -dimethylbutane 33

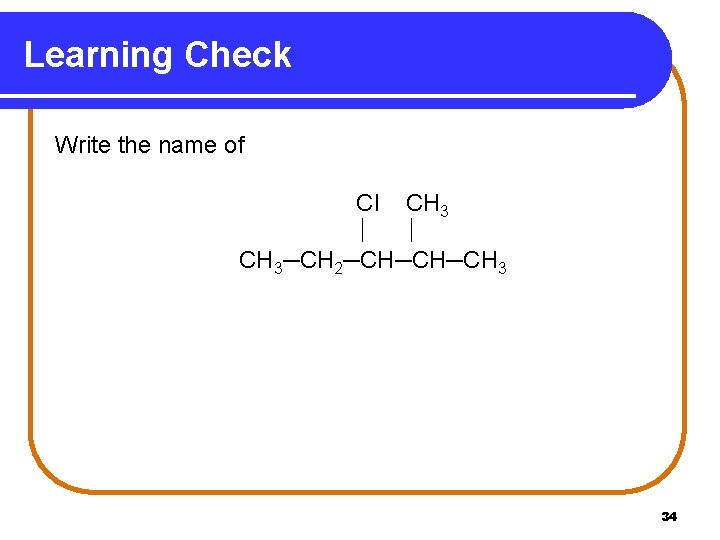
Learning Check Write the name of Cl CH 3─CH 2─CH─CH─CH 3 34

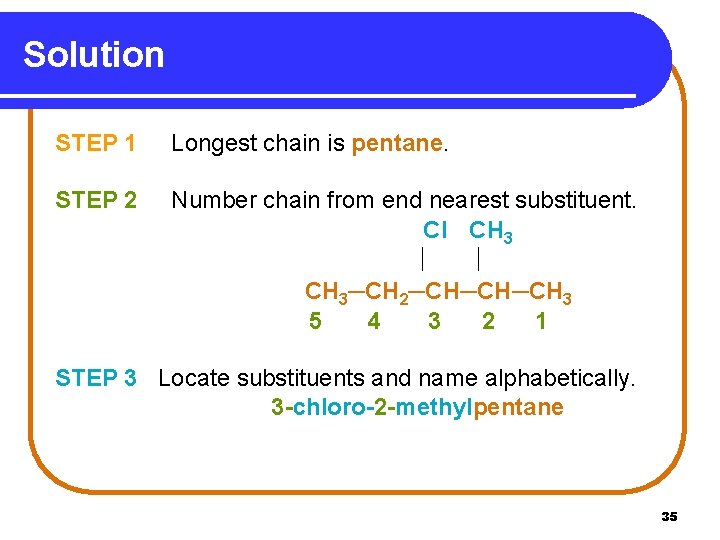
Solution STEP 1 Longest chain is pentane. STEP 2 Number chain from end nearest substituent. Cl CH 3─CH 2─CH─CH─CH 3 5 4 3 2 1 STEP 3 Locate substituents and name alphabetically. 3 -chloro-2 -methylpentane 35

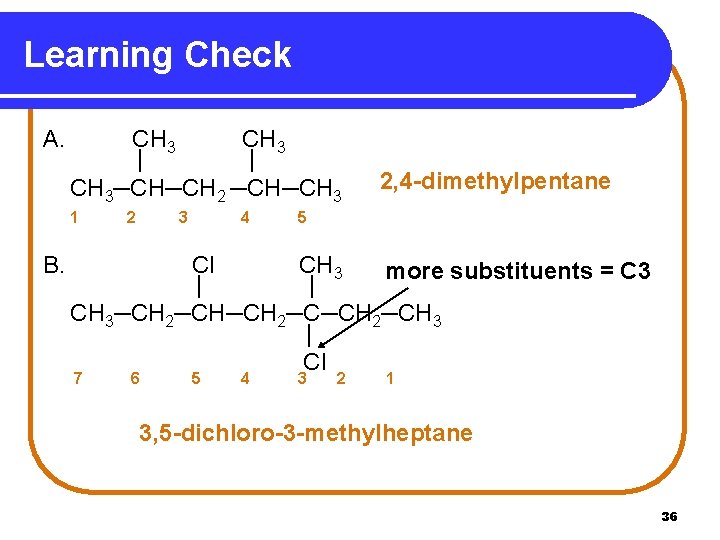
Learning Check A. CH 3 | | CH 3─CH─CH 2 ─CH─CH 3 1 B. 2 3 4 2, 4 -dimethylpentane 5 Cl CH 3 more substituents = C 3 | | CH 3─CH 2─CH─CH 2─CH 3 | Cl 7 6 5 4 3 2 1 3, 5 -dichloro-3 -methylheptane 36

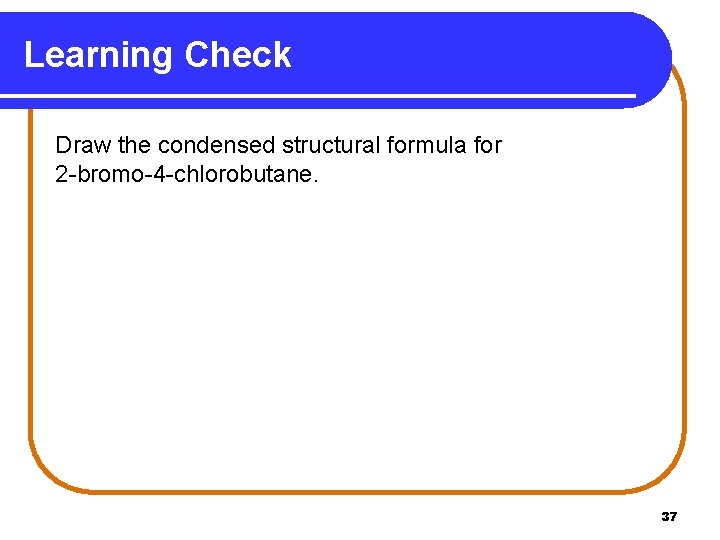
Learning Check Draw the condensed structural formula for 2 -bromo-4 -chlorobutane. 37

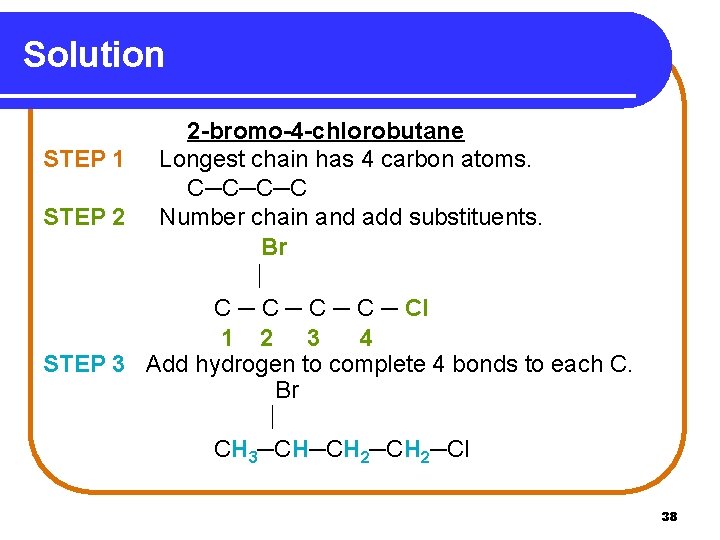
Solution 2 -bromo-4 -chlorobutane STEP 1 Longest chain has 4 carbon atoms. C─C─C─C STEP 2 Number chain and add substituents. Br C ─ C ─ Cl 1 2 3 4 STEP 3 Add hydrogen to complete 4 bonds to each C. Br CH 3─CH─CH 2─Cl 38

Naming Cycloalkanes with Substituents The name of a substituent is placed in front of the cycloalkane name. methylcyclobutane chlorocyclopentane CH 3 Cl 39

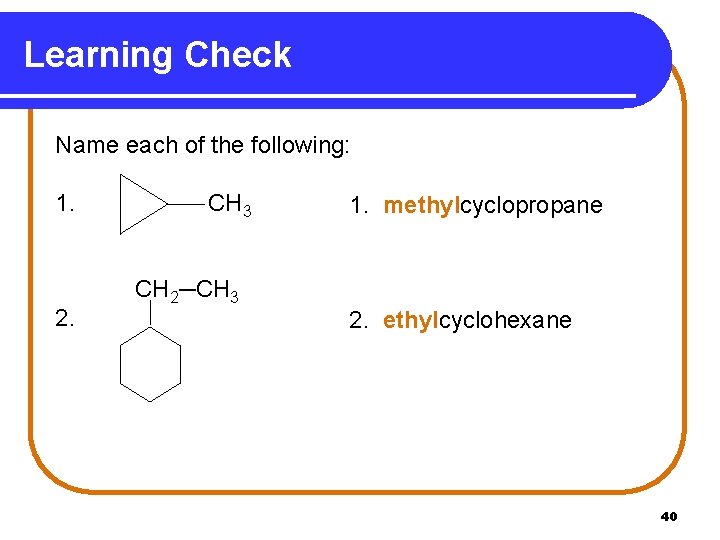
Learning Check Name each of the following: 1. 2. CH 3 1. methylcyclopropane CH 2─CH 3 2. ethylcyclohexane 40

Chapter 10 Introduction to Organic Chemistry: Alkanes 10. 4 Properties of Alkanes Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 41

Some Properties of Alkanes are: • nonpolar. • insoluble in water. • less dense than water. • flammable in air. 42

Some Properties of Alkanes with 1 -4 carbon atoms are • methane, propane, and butane. • gases at room temperature. • used as heating fuels. 43

Some Properties of Alkanes with 5 -8 carbon atoms are • liquids at room temperature. • pentane, hexane, heptane, and octane. • very volatile. • used to make gasoline. Alkanes with 9 -17 carbon atoms • are liquids at room temperature • have higher boiling points. • are found in kerosene, diesel, and jet fuels. 44

Some Properties of Alkanes with 18 or more carbon atoms • have high molar masses. • are waxy solids at room temperature. • used in waxy coatings of fruits and vegetables. 45

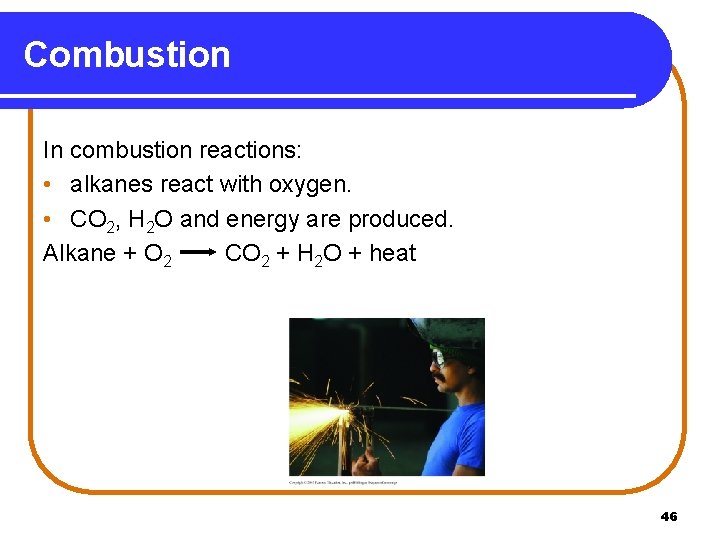
Combustion In combustion reactions: • alkanes react with oxygen. • CO 2, H 2 O and energy are produced. Alkane + O 2 CO 2 + H 2 O + heat 46

Learning Check Write a balanced equation for the complete combustion of propane. 47

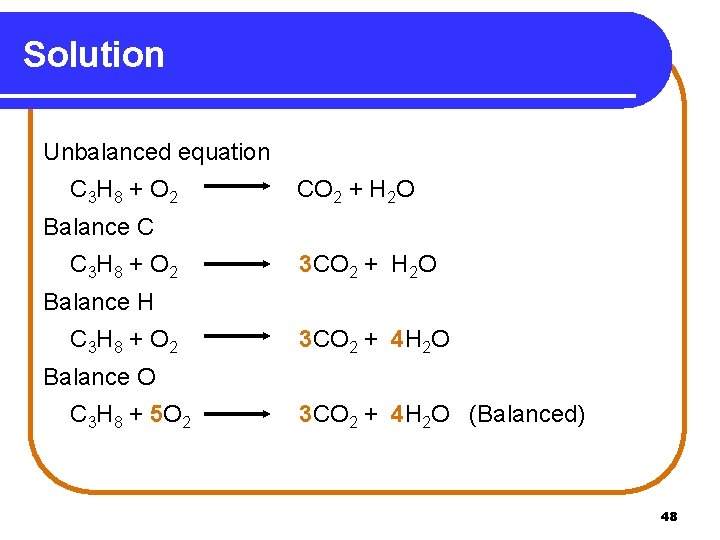
Solution Unbalanced equation C 3 H 8 + O 2 CO 2 + H 2 O Balance C C 3 H 8 + O 2 3 CO 2 + H 2 O Balance H C 3 H 8 + O 2 3 CO 2 + 4 H 2 O Balance O C 3 H 8 + 5 O 2 3 CO 2 + 4 H 2 O (Balanced) 48

Chapter 10 Introduction to Organic Chemistry: Alkanes 10. 5 Functional Groups Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 49

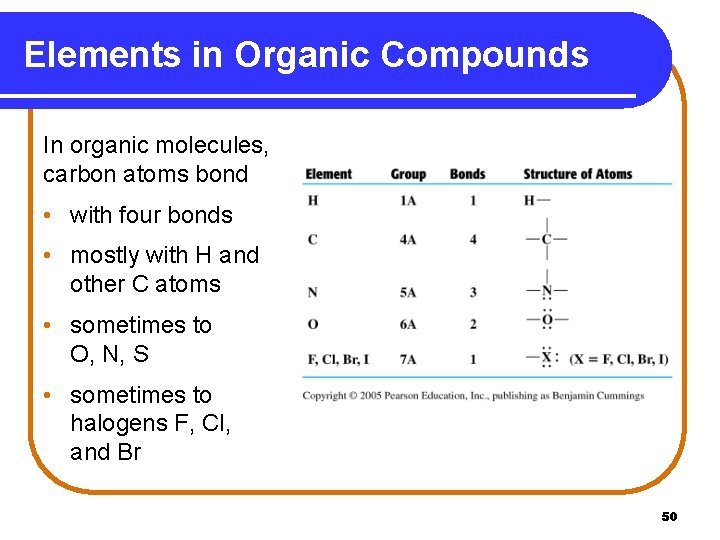
Elements in Organic Compounds In organic molecules, carbon atoms bond • with four bonds • mostly with H and other C atoms • sometimes to O, N, S • sometimes to halogens F, Cl, and Br 50

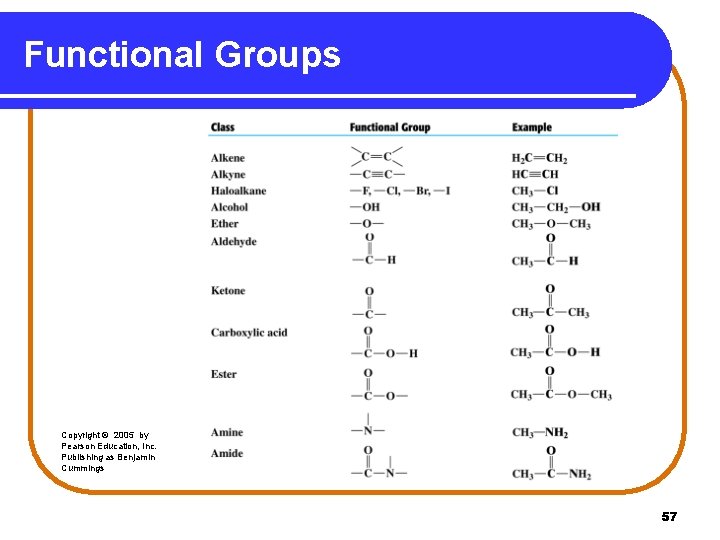
Functional Groups Functional groups are: • a characteristic feature of organic molecules that behave in a predictable way. • composed of an atom or group of atoms. • groups that replace a hydrogen atom in the corresponding alkane. • a way to classify families of organic compounds. 51

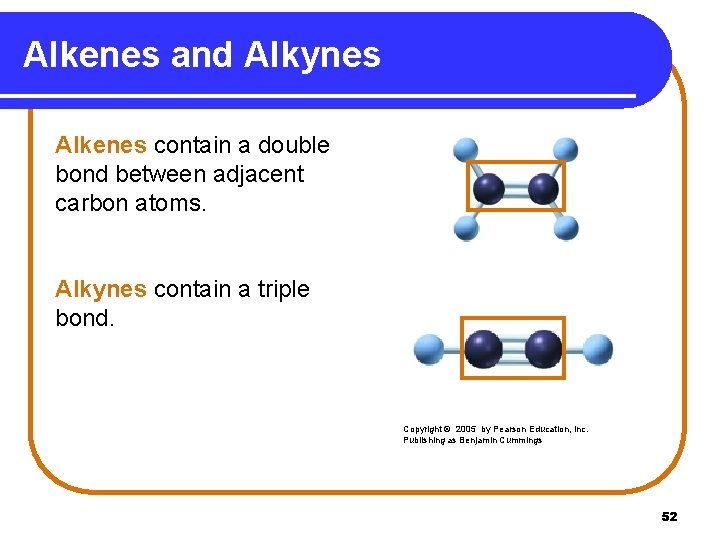
Alkenes and Alkynes Alkenes contain a double bond between adjacent carbon atoms. Alkynes contain a triple bond. Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 52

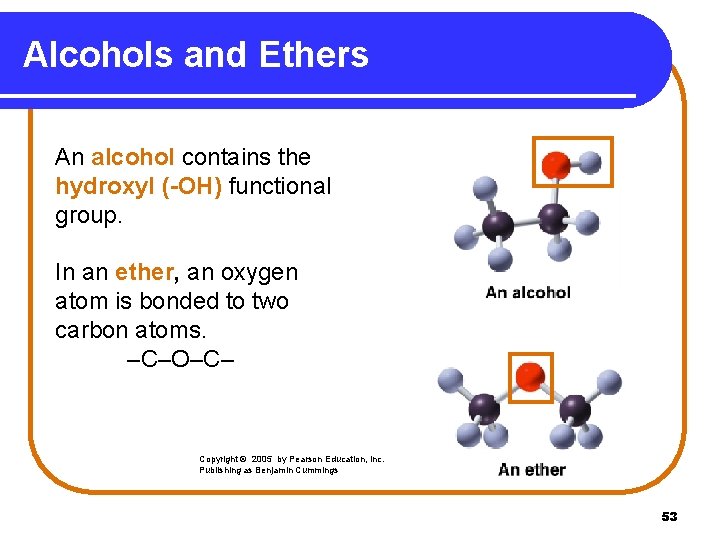
Alcohols and Ethers An alcohol contains the hydroxyl (-OH) functional group. In an ether, an oxygen atom is bonded to two carbon atoms. –C–O–C– Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 53

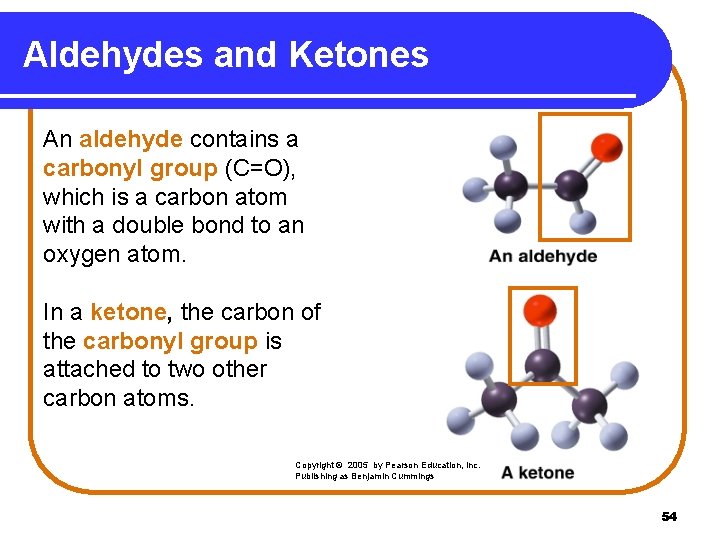
Aldehydes and Ketones An aldehyde contains a carbonyl group (C=O), which is a carbon atom with a double bond to an oxygen atom. In a ketone, the carbon of the carbonyl group is attached to two other carbon atoms. Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 54

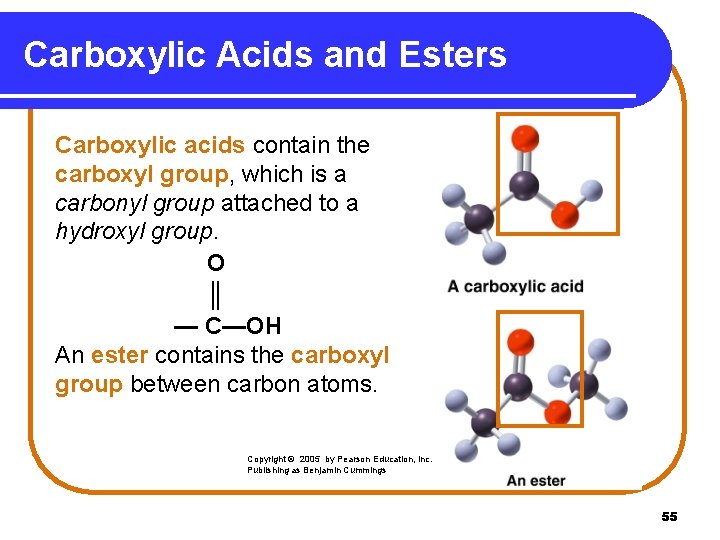
Carboxylic Acids and Esters Carboxylic acids contain the carboxyl group, which is a carbonyl group attached to a hydroxyl group. O ║ — C—OH An ester contains the carboxyl group between carbon atoms. Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 55

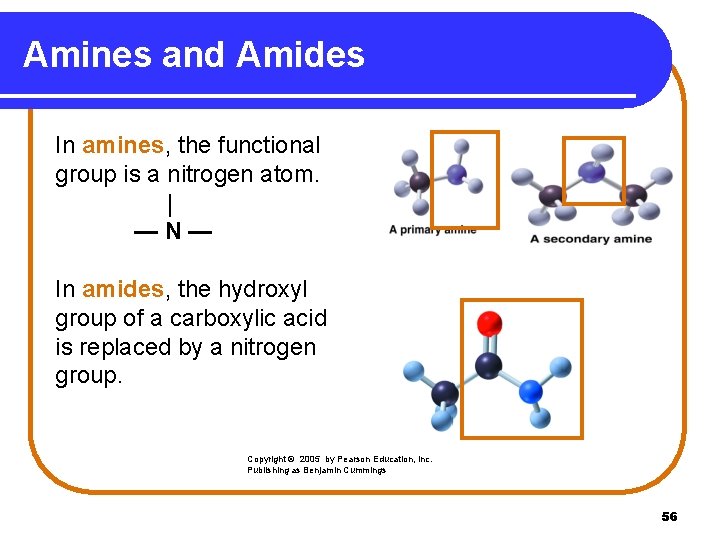
Amines and Amides In amines, the functional group is a nitrogen atom. | —N— In amides, the hydroxyl group of a carboxylic acid is replaced by a nitrogen group. Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 56

Functional Groups Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 57

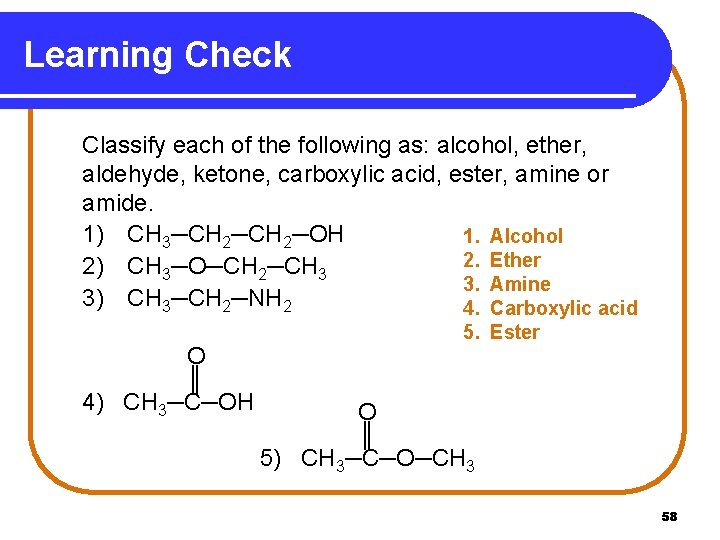
Learning Check Classify each of the following as: alcohol, ether, aldehyde, ketone, carboxylic acid, ester, amine or amide. 1) CH 3─CH 2─OH 1. Alcohol 2. Ether 2) CH 3─O─CH 2─CH 3 3. Amine 3) CH 3─CH 2─NH 2 4. Carboxylic acid O ║ 4) CH 3─C─OH 5. Ester O ║ 5) CH 3─C─O─CH 3 58