Chapter 10 Hybridization and Molecular Orbitals 1 Atomic

Chapter 10 Hybridization and Molecular Orbitals 1

Atomic Orbitals Don’t Work to explain some molecular geometry. n In methane, CH 4 , the shape s tetrahedral. n The valence electrons of carbon should be two in s, and two in p. n the p orbitals would have to be at right angles. n The atomic orbitals change when making a molecule n 2

Hybridization We blend the s and p orbitals of the valence electrons and end up with the tetrahedral geometry. n We combine one s orbital and 3 p orbitals. n n 3 sp 3 hybridization has tetrahedral geometry.
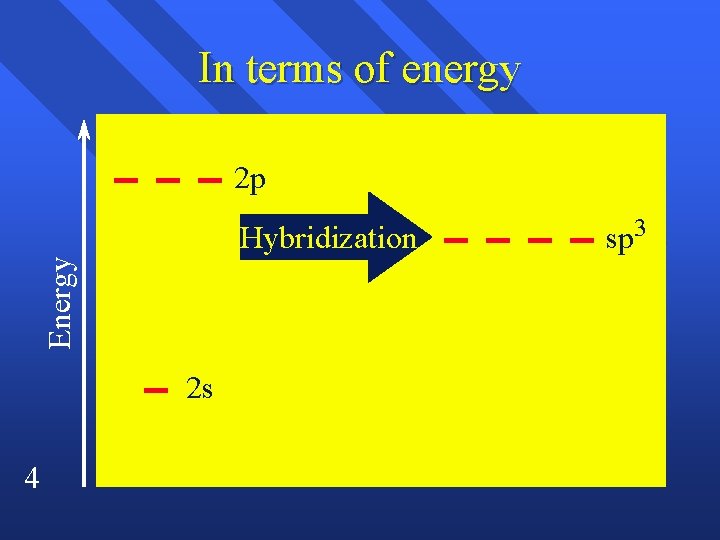
In terms of energy 2 p Energy Hybridization 2 s 4 sp 3

How we get to hybridization n n n 5 We know the geometry from experiment. We know the orbitals of the atom. hybridizing atomic orbitals can explain the geometry. So if the geometry requires a tetrahedral shape, it is sp 3 hybridized. If the molecule has four single bonds, it is sp 3 hybridized. This includes bent and trigonal pyramidal molecules because one of the sp 3 lobes holds the lone pair.

sp 2 hybridization C 2 H 4 n Double bond acts as one pair. n trigonal planar n Have to end up with three blended orbitals. n Use one s and two p orbitals to make sp 2 orbitals. n Leaves one p orbital perpendicular. n 6
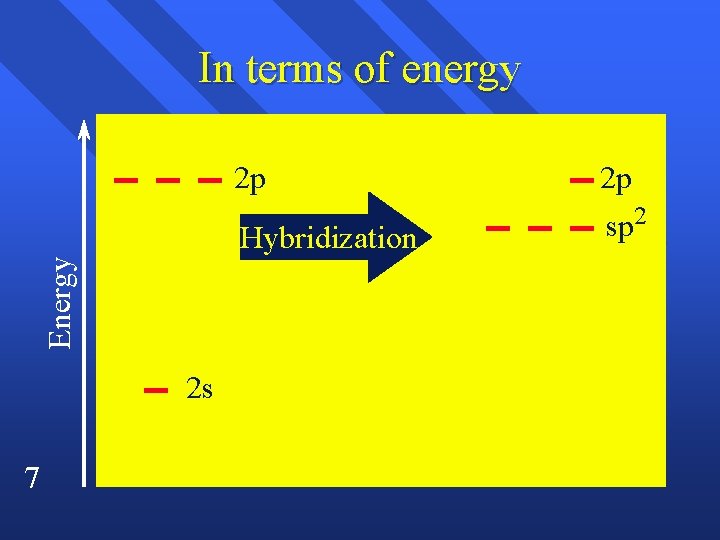
Energy In terms of energy 2 s 7 2 p 2 p Hybridization sp 2

Where is the P orbital? Perpendicular n The overlap of orbitals makes a sigma bond (s bond) n 8

Two types of Bonds Sigma bonds from overlap of orbitals. n Between the atoms. n Pi bond (p bond) above and below atoms n Between adjacent p orbitals. n The two bonds of a double bond. n 9

H H 10 H ∏ C C H

sp 2 hybridization When three things come off atom. n trigonal planar n 120º n on s one p bond n 11

What about two When two things come off. n One s and one p hybridize. n linear n 12

sp hybridization End up with two lobes 180º apart. n p orbitals are at right angles n Makes room for two p bonds and two sigma bonds. n A triple bond or two double bonds. n 13
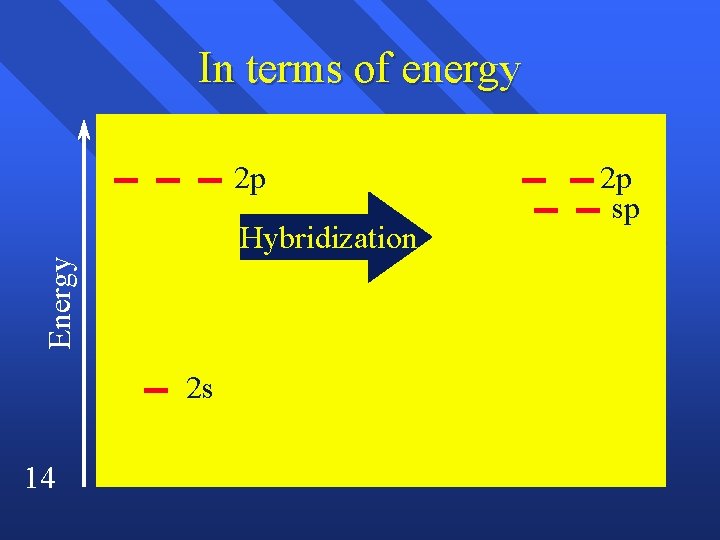
In terms of energy 2 p Energy Hybridization 2 s 14 2 p sp
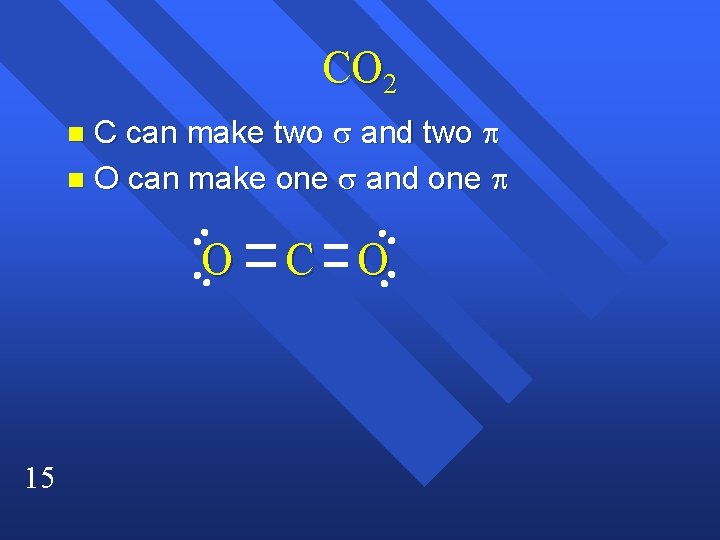
CO 2 C can make two s and two p n O can make one s and one p n O 15 C O
Breaking the octet PCl 5 n The model predicts that we must use the d orbitals. n dsp 3 hybridization n There is some controversy about how involved the d orbitals are. n 16

dsp 3 Trigonal bipyrimidal n can only s bond. n can’t p bond. n basic shape for five things. n 17

PCl 5 Can’t tell the hybridization of Cl Assume sp 3 to minimize repulsion of electron pairs. 18

d 2 sp 3 gets us to six things around n octahedral n 19
Molecular Orbital Model Localized Model we have learned explains much about bonding. n It doesn’t deal well with the ideal of resonance, unpaired electrons, and bond energy. n The MO model is a parallel of the atomic orbital, using quantum mechanics. n Each MO can hold two electrons with opposite spins 20 n Square of wave function tells probability n
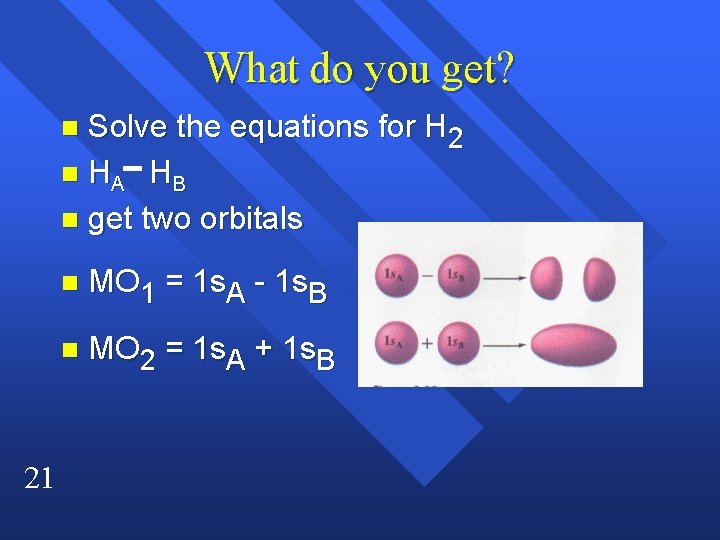
What do you get? Solve the equations for H 2 n HA H B n get two orbitals n 21 n MO 1 = 1 s. A - 1 s. B n MO 2 = 1 s. A + 1 s. B
The Molecular Orbital Model +The molecular orbitals are centered on a line through the nuclei – MO 1 the greatest probability is between the nuclei – MO 2 it is on either side of the nuclei – this shape is called a sigma molecular orbital 22
The Molecular Orbital Model +In the molecule only the molecular 23 orbitals exist, the atomic orbitals are gone +MO 1 is lower in energy than the 1 s orbitals they came from. – This favors molecule formation – Called an bonding orbital MO 2 is higher in energy – This goes against bonding – antibonding orbital
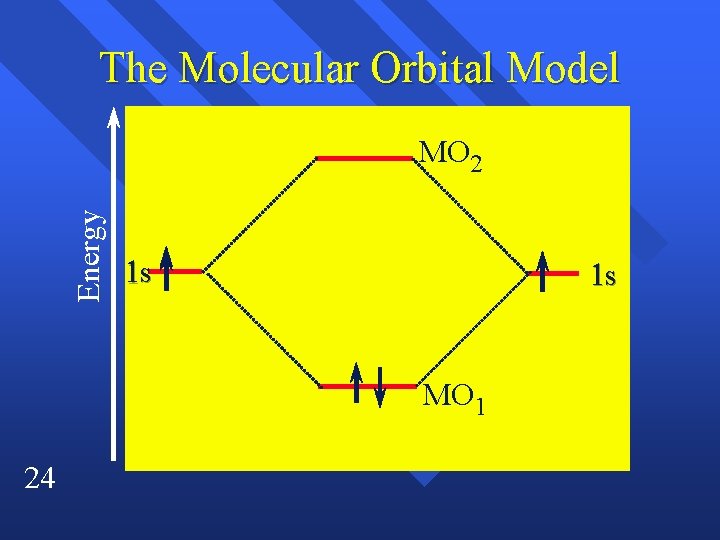
The Molecular Orbital Model Energy MO 2 1 s 1 s MO 1 24
The Molecular Orbital Model +We use labels to indicate shapes, and whether the MO’s are bonding or antibonding. – MO 1 = s 1 s – MO 2 = s 1 s* (* indicates antibonding) +Can write them the same way as atomic orbitals – H 2 = s 1 s 2 25

The Molecular Orbital Model +Each MO can hold two electrons, but they must have opposite spins +Orbitals are conserved. The number of molecular orbitals must equal the number atomic orbitals that are used to make them. 26
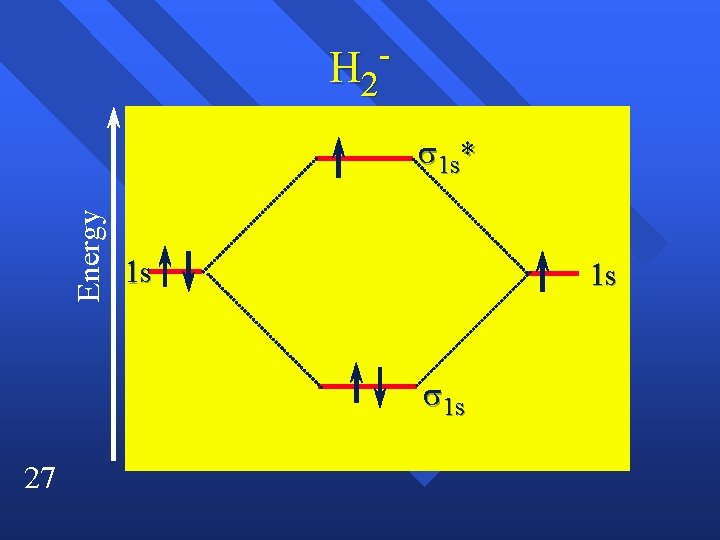
H 2 Energy s 1 s* 1 s 1 s 27
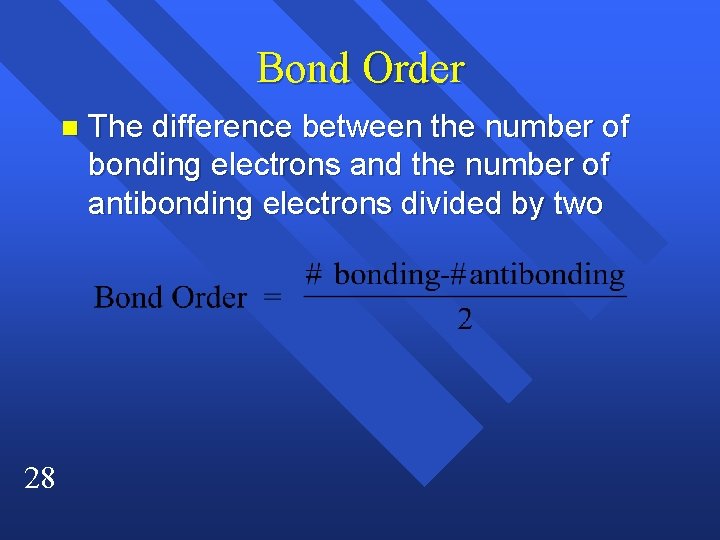
Bond Order n 28 The difference between the number of bonding electrons and the number of antibonding electrons divided by two
- Slides: 28