CHAPTER 10 Hematocrit Packed cell volume PCV Determination









- Slides: 24

CHAPTER 10 Hematocrit / Packed cell volume (PCV) Determination

Acknowledgements n n n n Addisa Ababa University Jimma University Hawassa University Haramaya University of Gondar American Society for Clinical Pathology Center for Disease Control and Prevention-Ethiopia

Objectives At the end of this chapter students will be able to: n Define packed cell volume n Identify the methods used in hematocrit determination n List the materials required in PCV determination using the micro- and macro- hematocrit methods n Discuss the advantages of the microhematocrit method of PCV determination

Objectives cont’d n Discuss the clinical significance of PCV determination n Indicate the normal hematocrit values n Perform PCV determination on a sample of blood using the microhematocrit and Macrohematocrit method n List the sources of error in PCV determination n Perform QC measures for PCV determination

Outline n n Introduction Methods of PCV determination Clinical significance of PCV determination QC for PCV determination

10. 1. packed cell volume (PCV) i. Definition n Commonly referred to as hematocrit n Is a measure of the ratio of the volume occupied by the red cells to the volume of whole blood in a sample of capillary or venous blood n The ratio is measured after appropriate centrifugation in the manual methods n Expressed as a decimal fraction (in l/l) or as a percentage (%) to the nearest 0. 5%

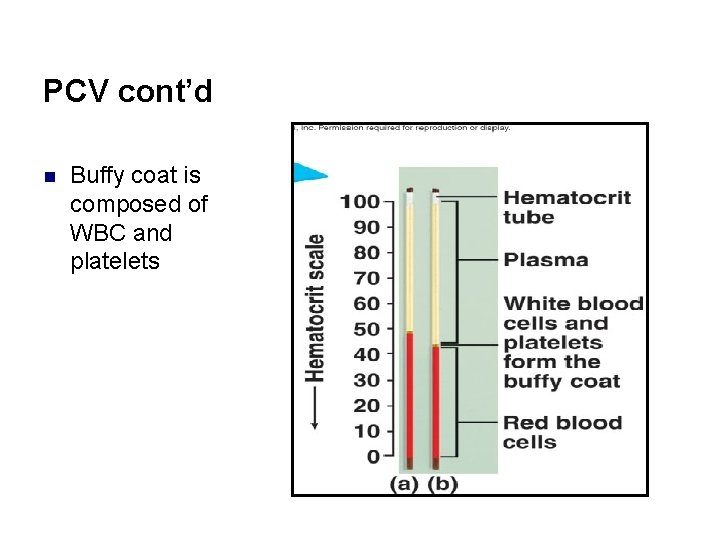
PCV cont’d n Buffy coat is composed of WBC and platelets

Cont’d Significance of the test n enables the calculation of the red cell indices that are widely used in the classification of anemias. These are: ¨ Mean cell volume (MCV), ¨ Mean cell Hb concentration (MCHC) and n to screen for anemia when it is not possible to measure hemoglobin, and n to diagnose polycythemia vera and to monitor its treatment.

10. 2. Hematocrit Determination Methods n n n Macro method ¨ Wintrobe Micro methods ¨ Adams microhematocrit method Electronic method ¨ Based on the principle that the average red cell volume is determined, the red cell count made , and the hematocrit found by calculation e. g. coulter counter

10. 2. 1. Microhematocrit Method Principle of test n Anticoagulated blood in a glass capillary of specified length, bore size, and wall-thickness is centrifuged in a microhematocrit centrifuge at 10000 -15000 RPM for 5 minutes to obtain complete packing of the red cells. The PCV value is read from the scale of a microhematocrit reader or calculated using a ruler by dividing the height of the red cell column by the height of the total column of blood. ¨ A small amount of plasma remains trapped between the packed red cells.

Cont’d n Note: Due to trapped plasma, PCV values using a centrifugation technique are 1 -3% higher than those obtained from an electronic cell analyzer which computes the value from the MCV and red cell count (PCV = MCV x RBC). Specimen: n Either well mixed EDTA anticoagulated blood or n capillary blood collected into a heparinized capillary tube

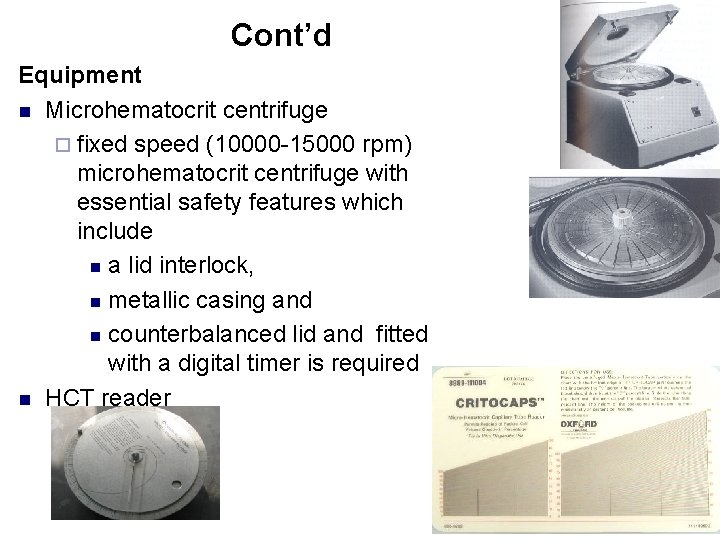
Cont’d Equipment n Microhematocrit centrifuge ¨ fixed speed (10000 -15000 rpm) microhematocrit centrifuge with essential safety features which include n a lid interlock, n metallic casing and n counterbalanced lid and fitted with a digital timer is required n HCT reader

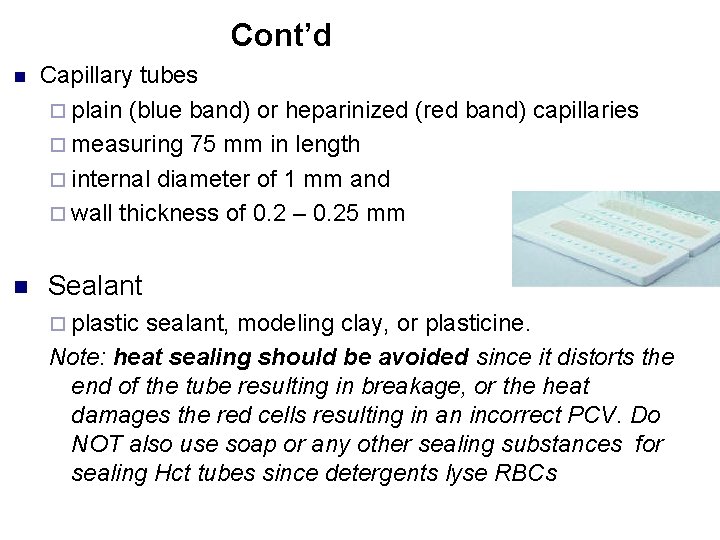
Cont’d n n Capillary tubes ¨ plain (blue band) or heparinized (red band) capillaries ¨ measuring 75 mm in length ¨ internal diameter of 1 mm and ¨ wall thickness of 0. 2 – 0. 25 mm Sealant ¨ plastic sealant, modeling clay, or plasticine. Note: heat sealing should be avoided since it distorts the end of the tube resulting in breakage, or the heat damages the red cells resulting in an incorrect PCV. Do NOT also use soap or any other sealing substances for sealing Hct tubes since detergents lyse RBCs

Method Take either capillary or venous blood from the patient using SOPs Ø If anticoagulated venous blood, mix specimen well 2. Allow the blood to enter the tube by capillarity, until 3/4 th of the tube is filled ; do in duplicate 3. Seal the capillary tubes by vertically placing the dry end into a tray of sealing compound (wax or plasticin) 4. Rotate the capillary tube slightly and remove it from the tray. The sealant plug should be 4 -6 mm long. Inspect the seal for a flat bottom. 5. Place the filled, sealed capillary tube in the groove (slots) of the centrifuge with the sealed end toward the periphery. 1.

Cont’d 6. Set the timer of the centrifuge at 5 minute and spin at 10, 000 -15, 000 g. 7. Read the PCV using a reading device that is either part of the centrifuge or separate from it ¨ Alternatively, the ratio of the red cell column to whole column (i. e. , plasma and red cells) can be calculated from measurements obtained by placing the tube against arithmetic graph paper ¨ If no reader at all, use a ruler Example: height of red cell column = 19 mm height of total blood column = 49 mm PCV = 19 mm/49 mm = 0. 388 (l/l) or 38. 8%

vii. Quality control n For precision check ¨ Perform in duplicates must agree within 1. 5 % n For accuracy check ¨ Run a whole blood control with known results n Note: Only for QC purpose check “Rule of 3” ¨ Hb x 3= Hct 3%

Sources of Error in Hct Procedures n n n n Specimen collection errors Inadequate mixing of specimen (for EDTA blood) Improper use of the hematocrit reader or including the buffy coat layer Improper centrifugation ¨ Wrong RPMs (speed) of the centrifuge ¨ Incorrect centrifuge time ¨ Manually stopping the centrifuge wrong sealing method used (e. g use of soap, heating) Incorrect reading due to uneven clay plug Excess anticoagulant

Cont’d n Incomplete packing due to insufficient centrifugation. Centrifuges should be regularly checked for properation (use tachometer to check rpm & document) n Incorrect reading of results. n Hemolysis or clotting of samples: ¨ Factors that cause hemolysis and clotting of samples should be controlled. n Occasionally, the red cell – plasma interface is not clearcut and the hematocrit is difficult to read. In such cases repeat the test ensuring proper filling and centrifugation. n Variation of the bore of the tubes cause serious errors if they are not manufactured within the narrow limits of precision that conform to defined standards

Interpretation Reference range varies between age, gender and altitude Children at birth 44 -54% Children 2 -5 years 34 -40% Children 6 -12 years 35 -45% Adult men 40 -54% Adult women 36 -46% n Ethiopia: Adult Males 41. 6 – 55. 1% Adult Females 35. 3 – 48. 8%

Cont’d n n Decreased in anemia Increased in polycythemia Advantages of the Microhematocrit Method n It enables higher centrifugation speeds with consequent shorter centrifugation times and superior packing. n The amount of trapped plasma is less than that in the Wintrobe method by virtue of the higher centrifugation speed employed. Limitation n Plasma trapping results in higher values (1 -3% higher than those obtained from an electronic cell analyzer)

Additional Note n n n The technician should cultivate the habit of inspecting both the buffy coat and the supernatant plasma when reading the hematocrit value. A note should be made on the patient’s report if an abnormal plasma or buffy coat is seen as this is often an important clue for the clinician. ¨ Example: n very thick buffy coat: may mean cases of leukemia n very thin buffy coat: may show marked leucopenia n yellowish plasma: may show jaundice (if pre-analytic error can be ruled out) When a thick buffy coat is seen, perform total and differential WBC count

10. 2. 2. Macrohematocrit method n n Procedure is no longer in routine use Wintrobe tube is filled with well mixed EDTA anticoagulated venous blood to the mark "0" on top using a long stem Pasteur pipette making sure that no air bubbles are trapped. ¨ The ratio of EDTA to volume of blood should be 1. 5 mg/ml or 0. 1 ml 10%w/v K 3 EDTA/ml. ¨ EDTA in excess of this proportion may cause a falsely low PCV as a consequence of cell shrinkage.

Cont’d n n The preparation is then spun at 2300 g for 30 minutes. The hematocrit is read from the scale on the right hand side of the tube taking the top of the black band of reduced erythrocytes immediately beneath the reddish gray leukocyte layer.

Review Questions 1. Define PCV. What is the significance of measuring PCV in a sample of blood? 2. List the possible sources of error in PCV determination using the micro-hematocrit method. 3. List the items that are required in PCV determination using the micro- and macro-hematocrit methods. 4. What is the advantage of the micro-hematocrit method of PCV determination? 5. How do you relate measured PCV and Hb values of a sample of blood? 6. What quality control measures do you apply to ensure the reliability of PCV values?