Chapter 10 Gases Respiratory therapists assess and treat








































- Slides: 149

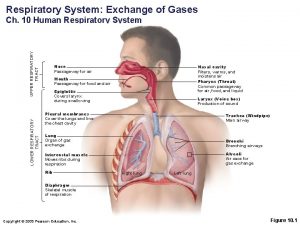
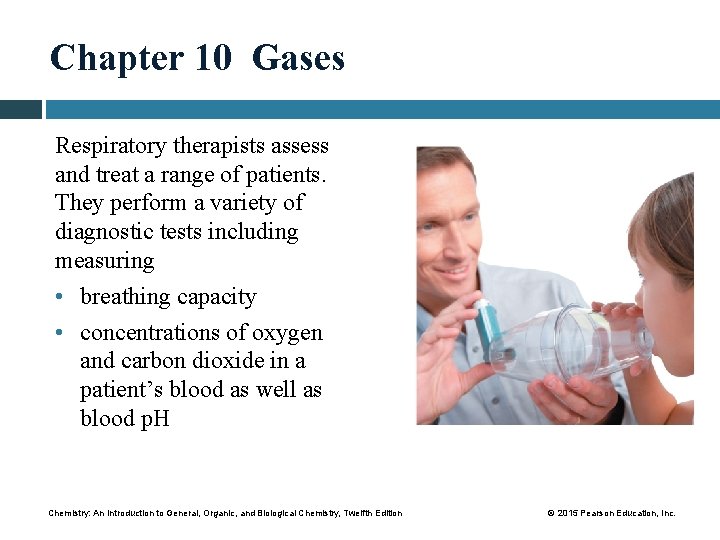
Chapter 10 Gases Respiratory therapists assess and treat a range of patients. They perform a variety of diagnostic tests including measuring • breathing capacity • concentrations of oxygen and carbon dioxide in a patient’s blood as well as blood p. H Chemistry: An Introduction to General, Organic, and Biological Chemistry, Twelfth Edition © 2015 Pearson Education, Inc.

Chapter 8 Readiness Key Math Skills • Solving Equations (1. 4 D) Core Chemistry Skills • Using Significant Figures in Calculations (2. 3) • Writing Conversion Factors from Equalities (2. 5) • Using Conversion Factors (2. 6) • Using Molar Mass as a Conversion Factor (7. 2) • Using Mole–Mole Factors (7. 6) Chemistry: An Introduction to General, Organic, and Biological Chemistry, Twelfth Edition © 2015 Pearson Education, Inc.

8. 1 Properties of Gases Generally, molecules with fewer than five atoms from the first two periods in the periodic table are gases at room temperature. In addition, the following are also gases: • H 2, N 2, O 2, F 2, and Cl 2 • oxides of the nonmetals on the upper right corner of the periodic table: CO, CO 2, NO 2, SO 2, and SO 3 • noble gases Learning Goal Describe the kinetic molecular theory of gases and the units of measurement used for gases. Chemistry: An Introduction to General, Organic, and Biological Chemistry, Twelfth Edition © 2015 Pearson Education, Inc.

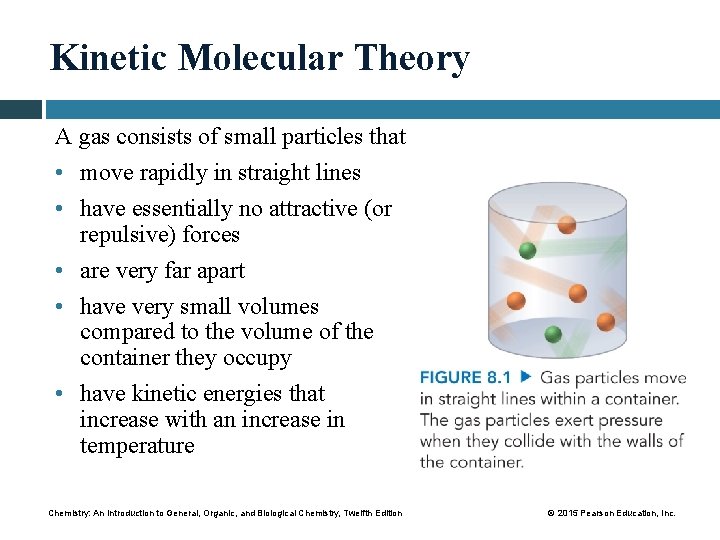
Kinetic Molecular Theory A gas consists of small particles that • move rapidly in straight lines • have essentially no attractive (or repulsive) forces • are very far apart • have very small volumes compared to the volume of the container they occupy • have kinetic energies that increase with an increase in temperature Chemistry: An Introduction to General, Organic, and Biological Chemistry, Twelfth Edition © 2015 Pearson Education, Inc.

Characteristics of Gases • Physical properties of gases are all similar. • Composed mainly of nonmetallic elements with simple formulas and low molar masses. • Unlike liquids and solids, gases Ø expand to fill their containers. Ø are highly compressible. Ø have extremely low densities. • Two or more gases form a homogeneous mixture. Chemistry: An Introduction to General, Organic, and Biological Chemistry, Twelfth Edition © 2015 Pearson Education, Inc.

Properties Which Define the State of a Gas Sample 1) 2) 3) 4) Temperature Pressure Volume Amount of gas, usually expressed as number of moles Ø Having already discussed three of these, we need to define pressure. Chemistry: An Introduction to General, Organic, and Biological Chemistry, Twelfth Edition © 2015 Pearson Education, Inc.

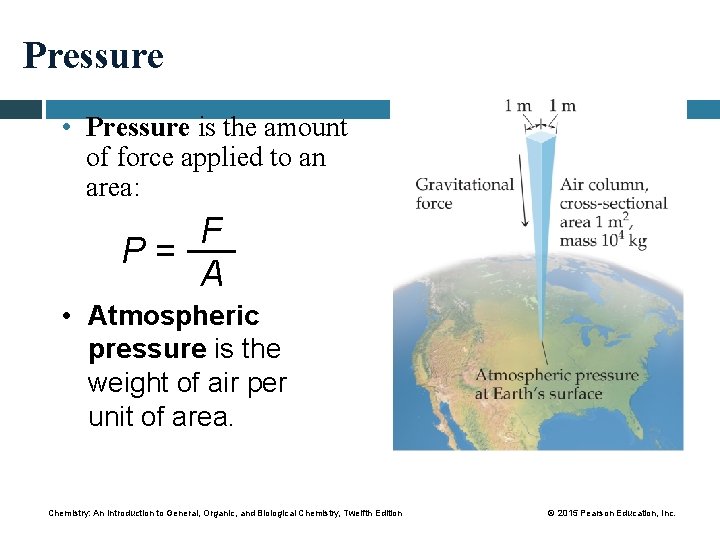
Pressure • Pressure is the amount of force applied to an area: F P= A • Atmospheric pressure is the weight of air per unit of area. Chemistry: An Introduction to General, Organic, and Biological Chemistry, Twelfth Edition © 2015 Pearson Education, Inc.

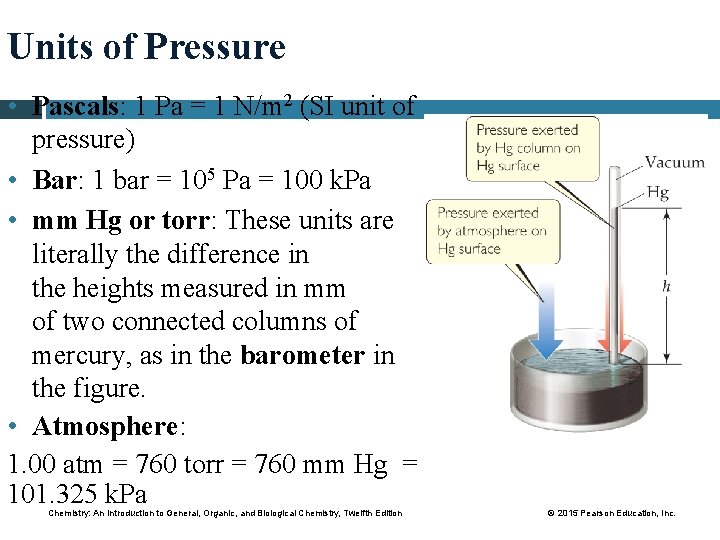
Units of Pressure • Pascals: 1 Pa = 1 N/m 2 (SI unit of pressure) • Bar: 1 bar = 105 Pa = 100 k. Pa • mm Hg or torr: These units are literally the difference in the heights measured in mm of two connected columns of mercury, as in the barometer in the figure. • Atmosphere: 1. 00 atm = 760 torr = 760 mm Hg = 101. 325 k. Pa Chemistry: An Introduction to General, Organic, and Biological Chemistry, Twelfth Edition © 2015 Pearson Education, Inc.

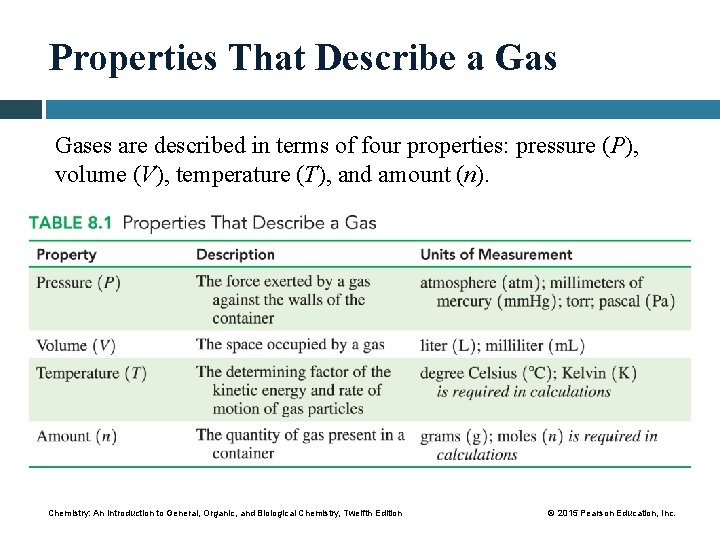
Properties That Describe a Gases are described in terms of four properties: pressure (P), volume (V), temperature (T), and amount (n). Chemistry: An Introduction to General, Organic, and Biological Chemistry, Twelfth Edition © 2015 Pearson Education, Inc.

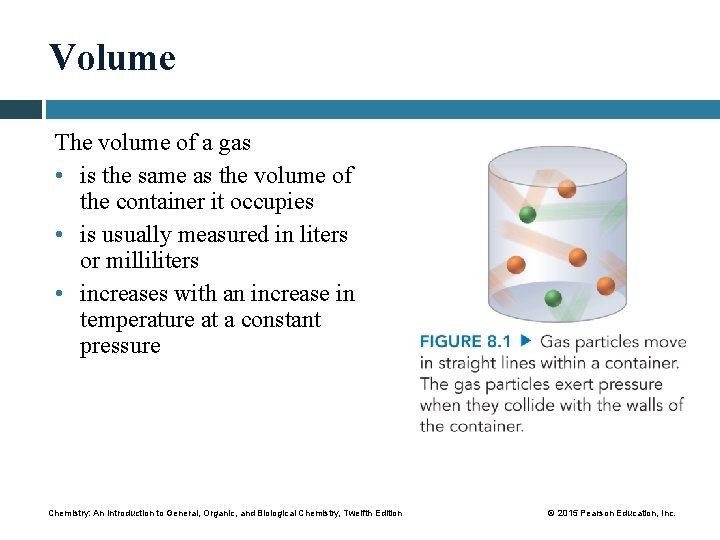
Volume The volume of a gas • is the same as the volume of the container it occupies • is usually measured in liters or milliliters • increases with an increase in temperature at a constant pressure Chemistry: An Introduction to General, Organic, and Biological Chemistry, Twelfth Edition © 2015 Pearson Education, Inc.

Temperature The temperature of a gas relates to the average kinetic energy of the molecules and is measured in the Kelvin (K) temperature scale. When the temperature of a gas is • decreased, the molecules have fewer collisions • increased, the molecules have more collisions Chemistry: An Introduction to General, Organic, and Biological Chemistry, Twelfth Edition © 2015 Pearson Education, Inc.

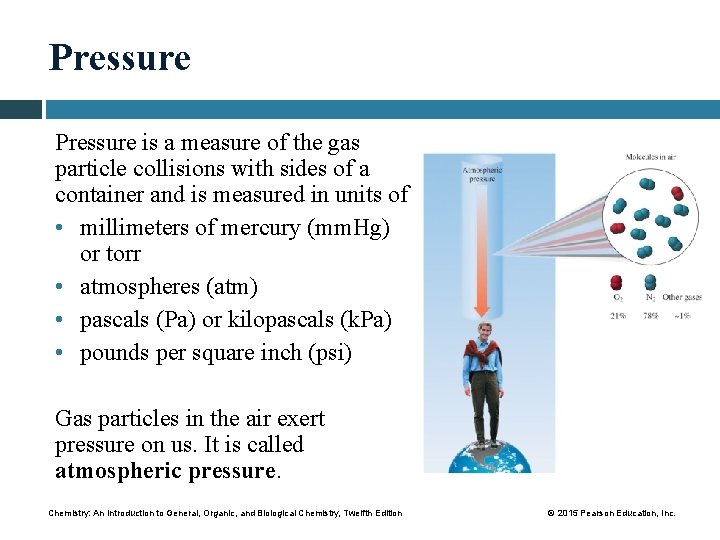
Pressure is a measure of the gas particle collisions with sides of a container and is measured in units of • millimeters of mercury (mm. Hg) or torr • atmospheres (atm) • pascals (Pa) or kilopascals (k. Pa) • pounds per square inch (psi) Gas particles in the air exert pressure on us. It is called atmospheric pressure. Chemistry: An Introduction to General, Organic, and Biological Chemistry, Twelfth Edition © 2015 Pearson Education, Inc.

Sample Problem 8. 1 Properties of Gases Identify the property of a gas that is described by each of the following: a. increases the kinetic energy of gas particles b. the force of the gas particles hitting the walls of the container c. the space that is occupied by a gas Solution a. temperature b. pressure c. volume Study Check 8. 1 As more helium gas is added to a balloon, the number of grams of helium increases. What property of a gas is described? Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

Barometers Measure Pressure A barometer • measures the pressure exerted by the gases in the atmosphere • indicates atmospheric pressure as the height in millimeters of the mercury column • 760 mm. Hg = 1 atm = 760 torr The barometer was invented by Evangelista Torricelli. At exactly 1 atm, the barometer tube is exactly 760 mm high. Chemistry: An Introduction to General, Organic, and Biological Chemistry, Twelfth Edition © 2015 Pearson Education, Inc.

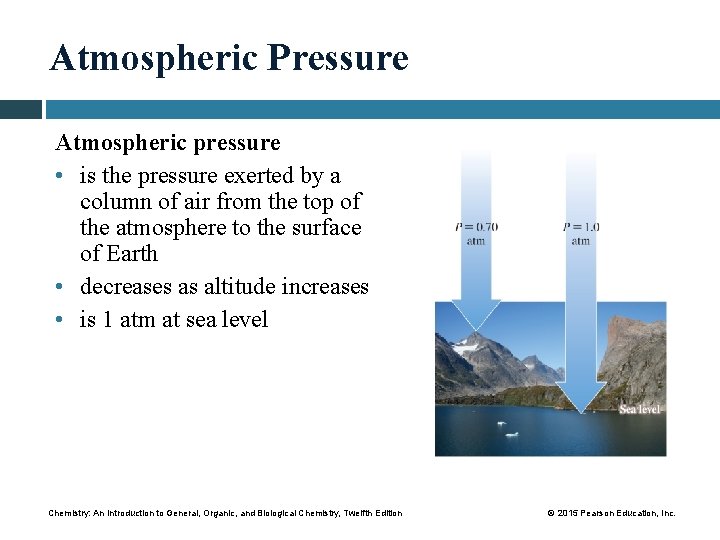
Atmospheric Pressure Atmospheric pressure • is the pressure exerted by a column of air from the top of the atmosphere to the surface of Earth • decreases as altitude increases • is 1 atm at sea level Chemistry: An Introduction to General, Organic, and Biological Chemistry, Twelfth Edition © 2015 Pearson Education, Inc.

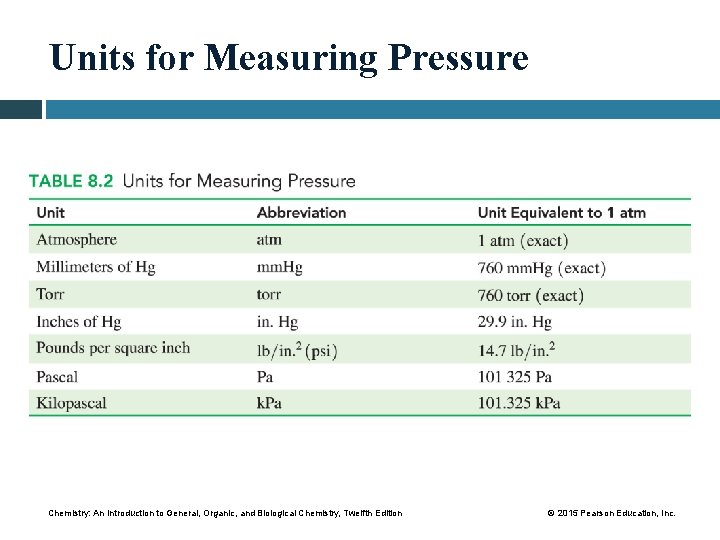
Units for Measuring Pressure Chemistry: An Introduction to General, Organic, and Biological Chemistry, Twelfth Edition © 2015 Pearson Education, Inc.

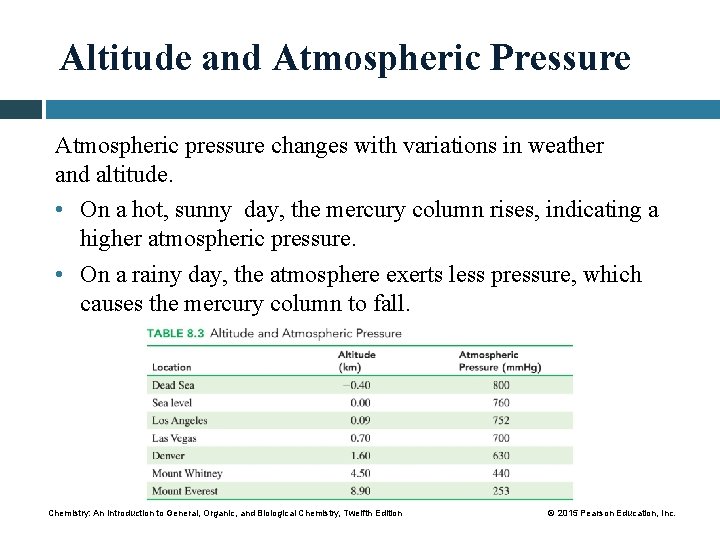
Altitude and Atmospheric Pressure Atmospheric pressure changes with variations in weather and altitude. • On a hot, sunny day, the mercury column rises, indicating a higher atmospheric pressure. • On a rainy day, the atmosphere exerts less pressure, which causes the mercury column to fall. Chemistry: An Introduction to General, Organic, and Biological Chemistry, Twelfth Edition © 2015 Pearson Education, Inc.

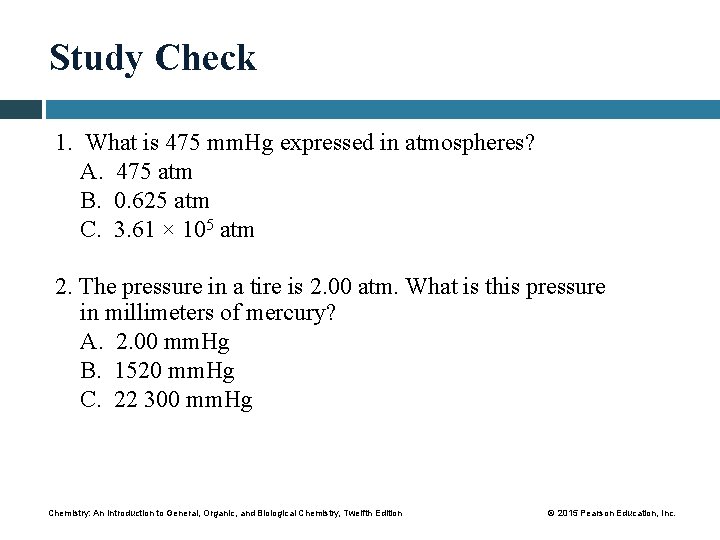
Study Check 1. What is 475 mm. Hg expressed in atmospheres? A. 475 atm B. 0. 625 atm C. 3. 61 × 105 atm 2. The pressure in a tire is 2. 00 atm. What is this pressure in millimeters of mercury? A. 2. 00 mm. Hg B. 1520 mm. Hg C. 22 300 mm. Hg Chemistry: An Introduction to General, Organic, and Biological Chemistry, Twelfth Edition © 2015 Pearson Education, Inc.

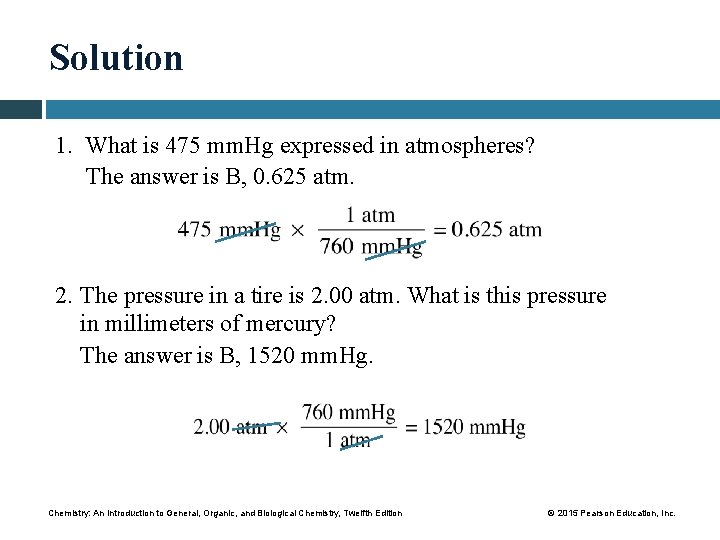
Solution 1. What is 475 mm. Hg expressed in atmospheres? The answer is B, 0. 625 atm. 2. The pressure in a tire is 2. 00 atm. What is this pressure in millimeters of mercury? The answer is B, 1520 mm. Hg. Chemistry: An Introduction to General, Organic, and Biological Chemistry, Twelfth Edition © 2015 Pearson Education, Inc.

Study Check 1. The downward pressure on the Hg in a barometer is _____ the pressure of the atmosphere. A. greater than B. less than C. the same as 2. A water barometer is 13. 6 times taller than a Hg barometer (d. Hg = 13. 6 g/m. L) because A. H 2 O is less dense than mercury B. H 2 O is heavier than mercury C. air is more dense than H 2 O Chemistry: An Introduction to General, Organic, and Biological Chemistry, Twelfth Edition © 2015 Pearson Education, Inc.

Solution 1. The downward pressure on the Hg in a barometer is _____ the pressure of the atmosphere. The answer is C, the same as. 2. A water barometer is 13. 6 times taller than a Hg barometer (d. Hg = 13. 6 g/m. L) because The answer is A, H 2 O is less dense than mercury. Chemistry: An Introduction to General, Organic, and Biological Chemistry, Twelfth Edition © 2015 Pearson Education, Inc.

CHAPTER 8: GASES 8. 1 Which of the following properties of gases can be explained by kinetic molecular theory? A. The volume of a gas is the same as the volume of the container the gas occupies. B. The attractive forces between gas molecules are very small. C. Gas pressure is defined as the force of the gas molecules striking the sides of the container. D. All of these are explained by kinetic molecular theory. © 2015 Pearson Education, Inc.

CHAPTER 8: GASES 8. 1 Which of the following properties of gases can be explained by kinetic molecular theory? A. The volume of a gas is the same as the volume of the container the gas occupies. B. The attractive forces between gas molecules are very small. C. Gas pressure is defined as the force of the gas molecules striking the sides of the container. D. All of these are explained by kinetic molecular theory. © 2015 Pearson Education, Inc.

CHAPTER 8: GASES 8. 2 A sample of butane gas (C 4 H 10) has a pressure of 0. 650 atm. What is this pressure in mm. Hg? A. 494 mm. Hg B. 1250 mm. Hg C. 380. mm. Hg D. 1170 mm. Hg © 2015 Pearson Education, Inc.

CHAPTER 8: GASES 8. 2 A sample of butane gas (C 4 H 10) has a pressure of 0. 650 atm. What is this pressure in mm. Hg? A. 494 mm. Hg B. 1250 mm. Hg C. 380. mm. Hg D. 1170 mm. Hg © 2015 Pearson Education, Inc.

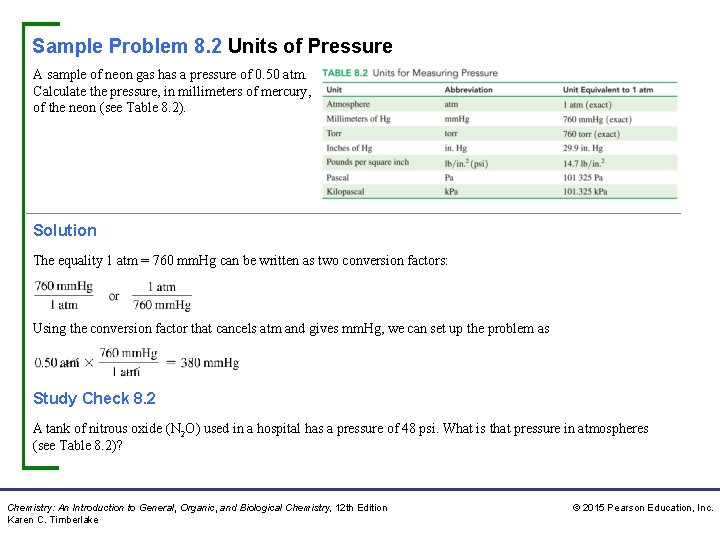
Sample Problem 8. 2 Units of Pressure A sample of neon gas has a pressure of 0. 50 atm. Calculate the pressure, in millimeters of mercury, of the neon (see Table 8. 2). Solution The equality 1 atm = 760 mm. Hg can be written as two conversion factors: Using the conversion factor that cancels atm and gives mm. Hg, we can set up the problem as Study Check 8. 2 A tank of nitrous oxide (N 2 O) used in a hospital has a pressure of 48 psi. What is that pressure in atmospheres (see Table 8. 2)? Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

8. 2 Pressure and Volume: Boyle’s Law The inverse relationship between the pressure and volume of a gas is known as Boyle’s law. Changes occur in opposite directions. When volume increases, the pressure decreases provided the temperature and moles of the gas remain constant. Learning Goal Use the pressure–volume relationship (Boyle’s law) to determine the final pressure or volume when the temperature and amount of gas are constant. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

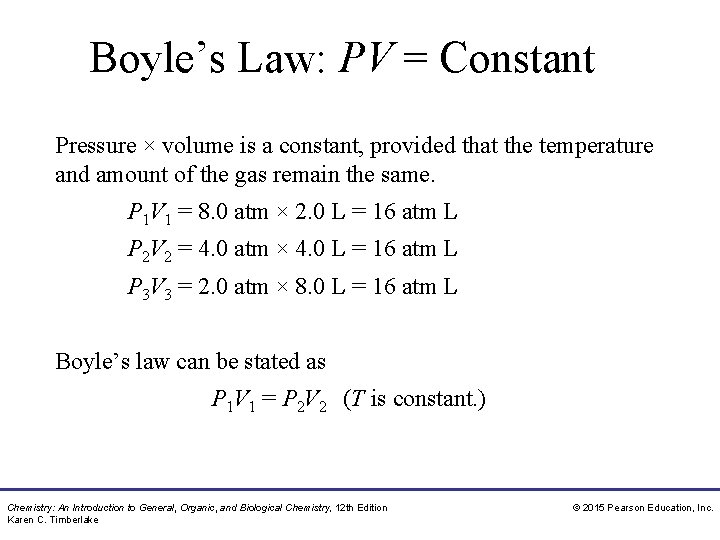
Boyle’s Law Boyle’s law states that • the pressure of a gas is inversely related to its volume when T is constant • the product P × V is constant when temperature and amount of a gas are held constant • if volume decreases, the pressure increases P 1 V 1 = P 2 V 2 Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

Boyle’s Law: PV = Constant Pressure × volume is a constant, provided that the temperature and amount of the gas remain the same. P 1 V 1 = 8. 0 atm × 2. 0 L = 16 atm L P 2 V 2 = 4. 0 atm × 4. 0 L = 16 atm L P 3 V 3 = 2. 0 atm × 8. 0 L = 16 atm L Boyle’s law can be stated as P 1 V 1 = P 2 V 2 (T is constant. ) Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

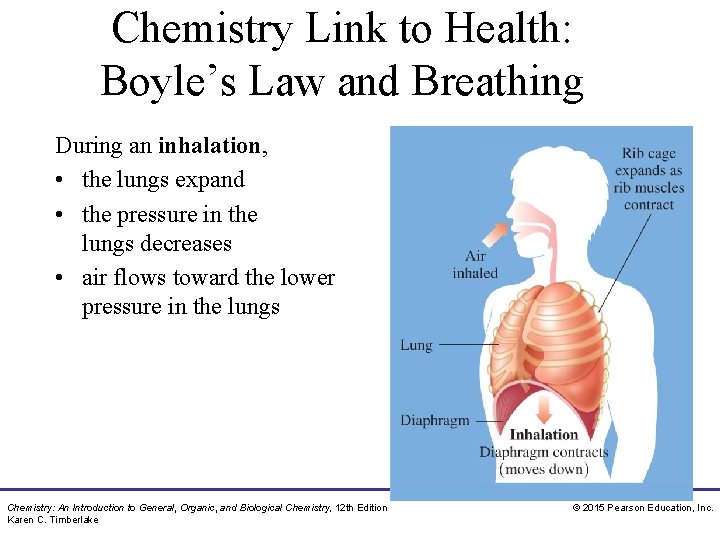
Chemistry Link to Health: Boyle’s Law and Breathing During an inhalation, • the lungs expand • the pressure in the lungs decreases • air flows toward the lower pressure in the lungs Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

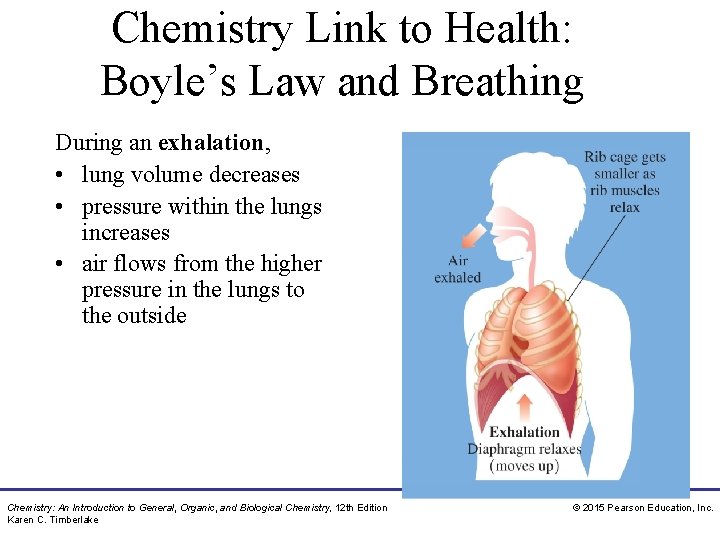
Chemistry Link to Health: Boyle’s Law and Breathing During an exhalation, • lung volume decreases • pressure within the lungs increases • air flows from the higher pressure in the lungs to the outside Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

Guide to Using Gas Laws Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

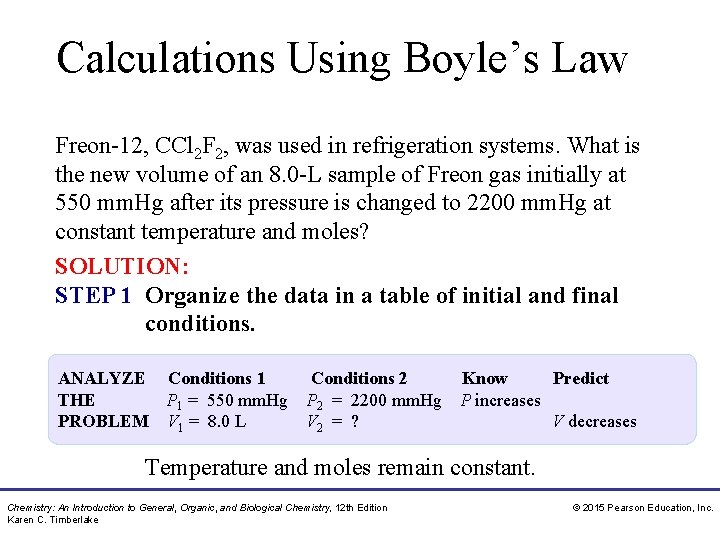
Calculations Using Boyle’s Law Freon-12, CCl 2 F 2, was used in refrigeration systems. What is the new volume of an 8. 0 -L sample of Freon gas initially at 550 mm. Hg after its pressure is changed to 2200 mm. Hg at constant temperature and moles? SOLUTION: STEP 1 Organize the data in a table of initial and final conditions. ANALYZE Conditions 1 Conditions 2 THE P 1 = 550 mm. Hg P 2 = 2200 mm. Hg PROBLEM V 1 = 8. 0 L V 2 = ? Know Predict P increases V decreases Temperature and moles remain constant. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

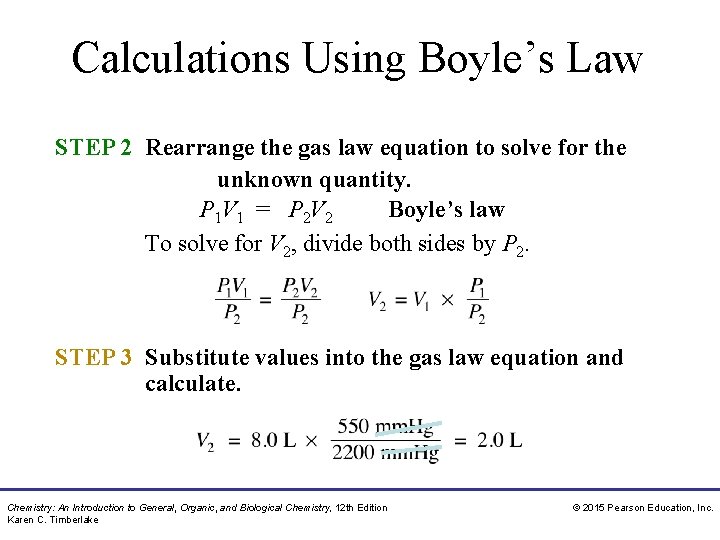
Calculations Using Boyle’s Law STEP 2 Rearrange the gas law equation to solve for the unknown quantity. P 1 V 1 = P 2 V 2 Boyle’s law To solve for V 2, divide both sides by P 2. STEP 3 Substitute values into the gas law equation and calculate. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

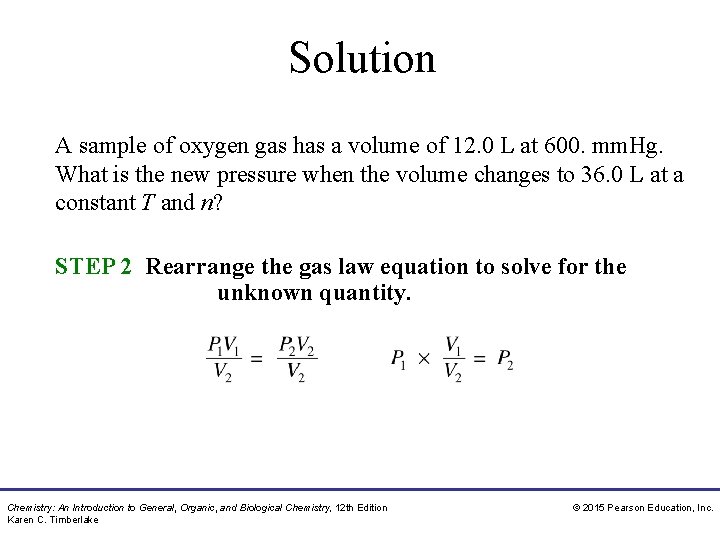
Study Check A sample of oxygen gas has a volume of 12. 0 L at 600. mm. Hg. What is the new pressure when the volume changes to 36. 0 L at a constant T and n? A. 200. mm. Hg B. 400. mm. Hg C. 1200 mm. Hg Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

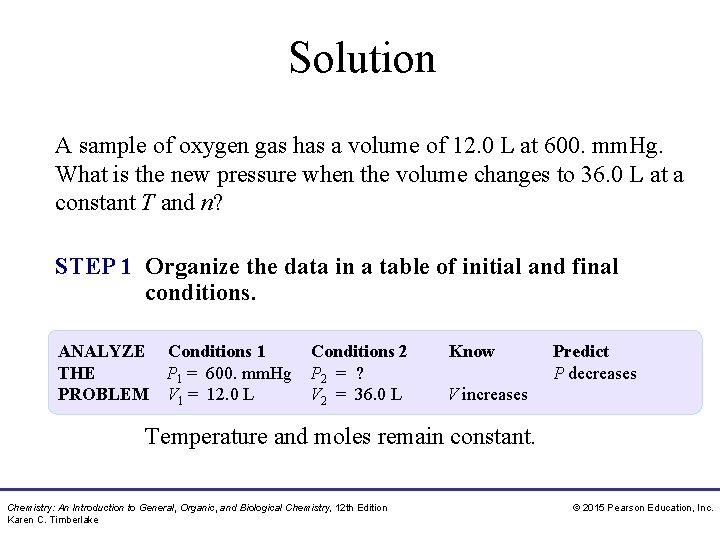
Solution A sample of oxygen gas has a volume of 12. 0 L at 600. mm. Hg. What is the new pressure when the volume changes to 36. 0 L at a constant T and n? STEP 1 Organize the data in a table of initial and final conditions. ANALYZE Conditions 1 Conditions 2 THE P 1 = 600. mm. Hg P 2 = ? PROBLEM V 1 = 12. 0 L V 2 = 36. 0 L Know Predict P decreases V increases Temperature and moles remain constant. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

Solution A sample of oxygen gas has a volume of 12. 0 L at 600. mm. Hg. What is the new pressure when the volume changes to 36. 0 L at a constant T and n? STEP 2 Rearrange the gas law equation to solve for the unknown quantity. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

Solution A sample of oxygen gas has a volume of 12. 0 L at 600. mm. Hg. What is the new pressure when the volume changes to 36. 0 L at a constant T and n? STEP 3 Substitute values into the gas law equation and calculate. The answer is A, 200. mm. Hg. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

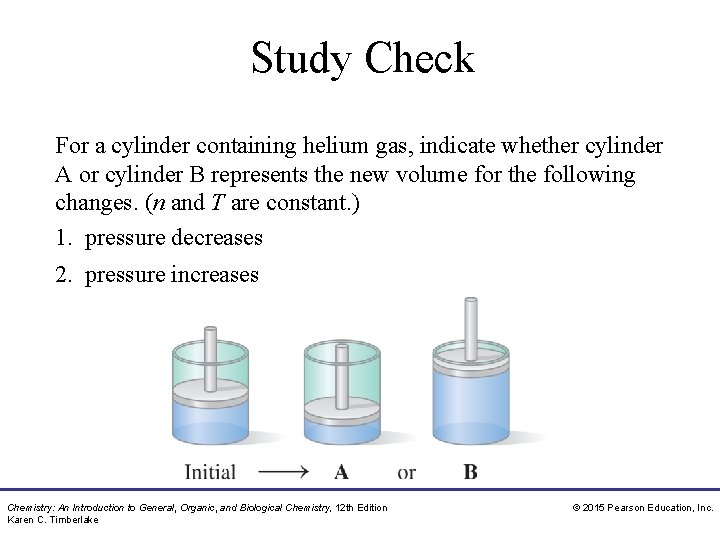
Study Check For a cylinder containing helium gas, indicate whether cylinder A or cylinder B represents the new volume for the following changes. (n and T are constant. ) 1. pressure decreases 2. pressure increases Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

Solution For a cylinder containing helium gas, indicate whether cylinder A or cylinder B represents the new volume for the following changes. (n and T are constant. ) 1. pressure decreases cylinder B 2. pressure increases cylinder A Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

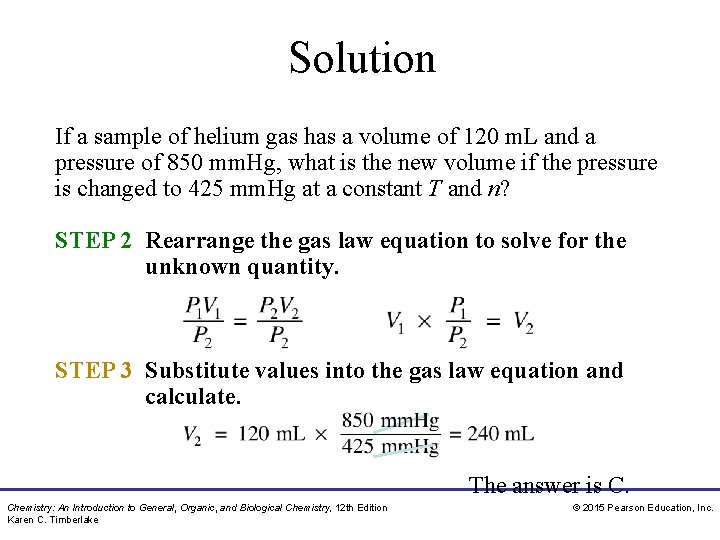
Study Check If a sample of helium gas has a volume of 120 m. L and a pressure of 850 mm. Hg, what is the new volume if the pressure is changed to 425 mm. Hg at a constant T and n? A. 60 m. L B. 120 m. L C. 240 m. L Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

Solution If a sample of helium gas has a volume of 120 m. L and a pressure of 850 mm. Hg, what is the new volume if the pressure is changed to 425 mm. Hg at a constant T and n? STEP 1 Organize the data in a table of initial and final conditions. ANALYZE Conditions 1 Conditions 2 THE P 1 = 850 mm. Hg P 2 = 425 mm. Hg PROBLEM V 1 = 120 m. L V 2 = ? Know P decreases Predict V increases Temperature and moles remain constant. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

Solution If a sample of helium gas has a volume of 120 m. L and a pressure of 850 mm. Hg, what is the new volume if the pressure is changed to 425 mm. Hg at a constant T and n? STEP 2 Rearrange the gas law equation to solve for the unknown quantity. STEP 3 Substitute values into the gas law equation and calculate. The answer is C. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

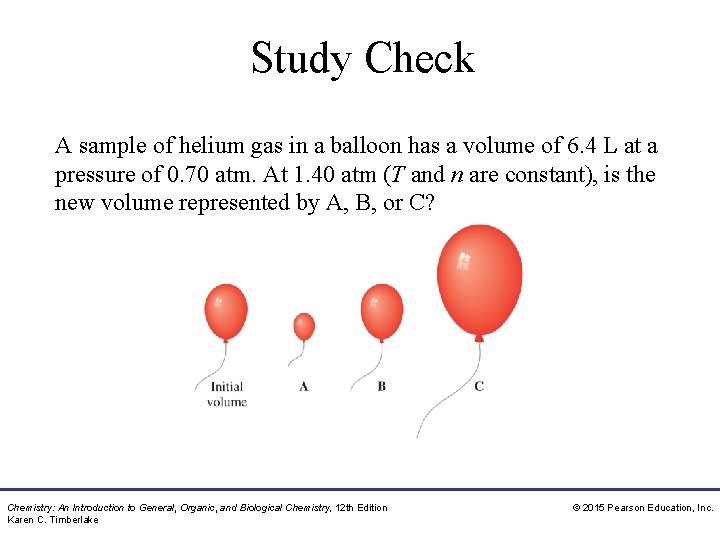
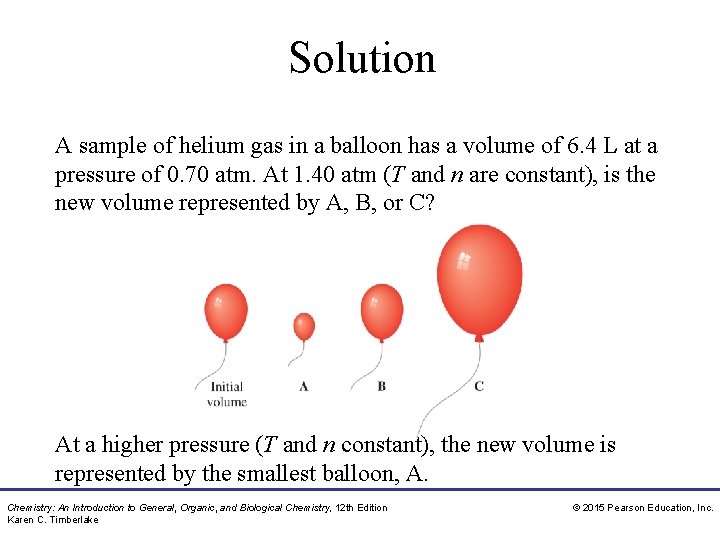
Study Check A sample of helium gas in a balloon has a volume of 6. 4 L at a pressure of 0. 70 atm. At 1. 40 atm (T and n are constant), is the new volume represented by A, B, or C? Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

Solution A sample of helium gas in a balloon has a volume of 6. 4 L at a pressure of 0. 70 atm. At 1. 40 atm (T and n are constant), is the new volume represented by A, B, or C? At a higher pressure (T and n constant), the new volume is represented by the smallest balloon, A. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

CHAPTER 8: GASES 8. 3 Cyclopropane (C 3 H 6) is a general anesthetic. A 5. 0 -L sample has a pressure of 5. 0 atm. What is the volume in liters of the anesthetic given to a patient at a pressure of 1. 0 atm if the temperature and moles are constant? A. 13 L B. 25 L C. 37 L D. 50. L Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

CHAPTER 8: GASES 8. 3 Cyclopropane (C 3 H 6) is a general anesthetic. A 5. 0 -L sample has a pressure of 5. 0 atm. What is the volume in liters of the anesthetic given to a patient at a pressure of 1. 0 atm if the temperature and moles are constant? A. 13 L B. 25 L C. 37 L D. 50. L Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

CHAPTER 8: GASES 8. 7 Which statement best describes that part of the breathing cycle called expiration? A. The diaphragm contracts. B. The pressure within the lungs is less than the atmospheric pressure. C. The volume of the lungs decreases. D. The volume of the lungs expands. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

CHAPTER 8: GASES 8. 7 Which statement best describes that part of the breathing cycle called expiration? A. The diaphragm contracts. B. The pressure within the lungs is less than the atmospheric pressure. C. The volume of the lungs decreases. D. The volume of the lungs expands. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

Sample Problem 8. 3 Calculating Volume When Pressure Changes Oxygen gas is delivered through a face mask during oxygen therapy. The gauge on a 12 -L tank of compressed oxygen reads 3800 mm. Hg. How many liters would this same gas occupy at a pressure of 0. 75 atm when temperature and amount of gas do not change? Solution STEP 1. Organize the data in a table of initial and final conditions. To match the units for the initial and final pressures, we can convert either atm to mm. Hg or mm. Hg to atm. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

Sample Problem 8. 3 Calculating Volume When Pressure Changes Continued or We place the gas data using units of mm. Hg for pressure and liters for volume in a table. (We could have both pressures in units of atm as well. ) The properties that do not change, which are temperature and amount of gas, are shown below the table. We know that pressure decreases, and can predict that the volume increases. Factors that remain constant: T and n Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

Sample Problem 8. 3 Calculating Volume When Pressure Changes Continued STEP 2. Rearrange the gas law equation to solve for the unknown quantity. For a PV relationship, we use Boyle’s law and solve for V 2 by dividing both sides by P 2. According to Boyle’s law, a decrease in the pressure will cause an increase in the volume when T and n remain constant. STEP 3. Substitute values into the gas law equation and calculate. When we substitute in the values with pressures in units of mm. Hg or atm, the ratio of pressures (pressure factor) is greater than 1, which increases the volume as predicted in Step 1. or Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

Sample Problem 8. 3 Calculating Volume When Pressure Changes Continued Study Check 8. 3 In an underground natural gas reserve, a bubble of methane gas, CH 4, has a volume of 45. 0 m. L at 1. 60 atm. What volume, in milliliters, will the gas bubble occupy when it reaches the surface where the atmospheric pressure is 744 mm. Hg, if there is no change in the temperature or amount of gas? Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

8. 3 Temperature and Volume: Charles’s Law If we increase the temperature of a gas sample, kinetic molecular theory states that the motion (kinetic energy) of the gas particles will also increase. If the amount and pressure of the gas is held constant, the volume of the container will increase. Learning Goal Use the temperature–volume relationship (Charles’s law) to determine the final temperature or volume when the pressure and amount of gas are constant. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

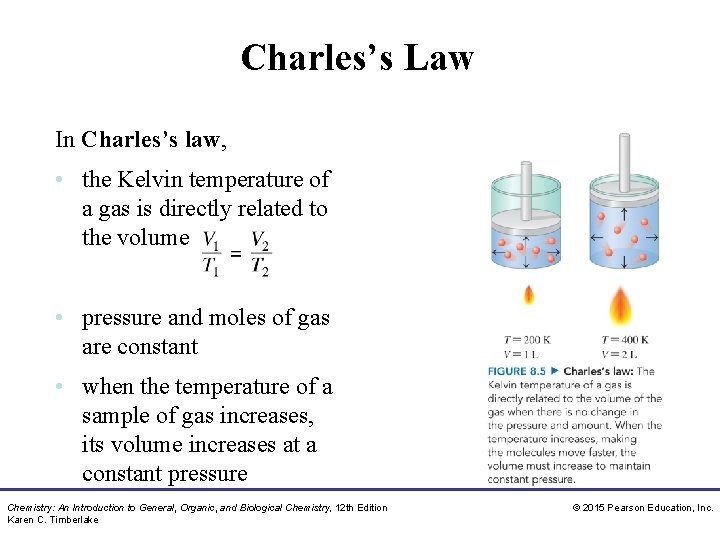
Charles’s Law In Charles’s law, • the Kelvin temperature of a gas is directly related to the volume • pressure and moles of gas are constant • when the temperature of a sample of gas increases, its volume increases at a constant pressure Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

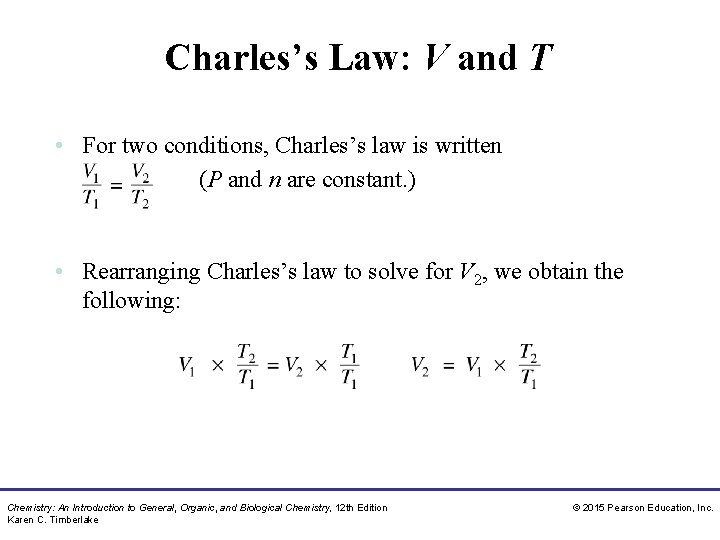
Charles’s Law: V and T • For two conditions, Charles’s law is written (P and n are constant. ) • Rearranging Charles’s law to solve for V 2, we obtain the following: Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

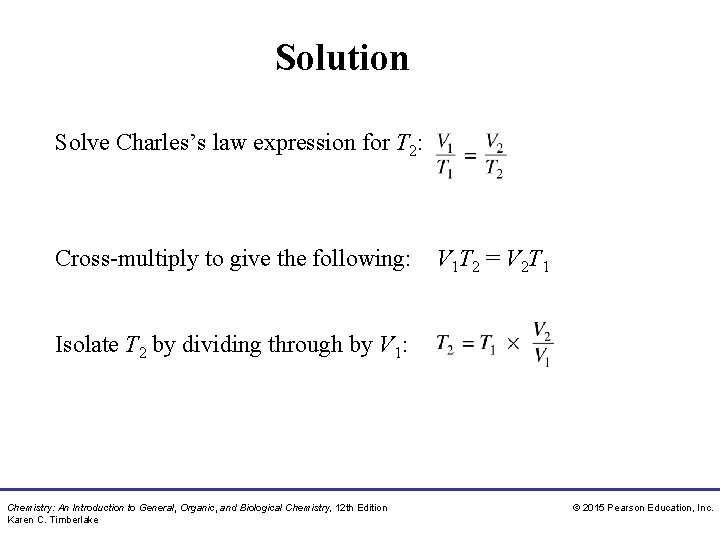
Study Check Solve Charles’s law expression for T 2. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

Solution Solve Charles’s law expression for T 2: Cross-multiply to give the following: V 1 T 2 = V 2 T 1 Isolate T 2 by dividing through by V 1: Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

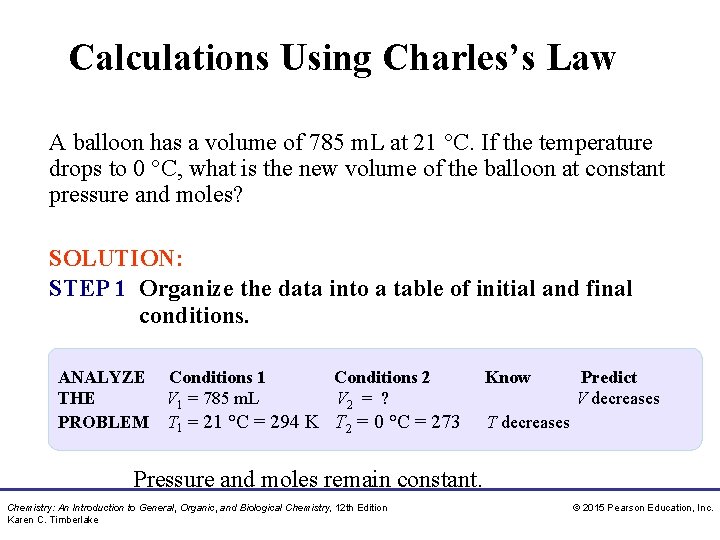
Calculations Using Charles’s Law A balloon has a volume of 785 m. L at 21 °C. If the temperature drops to 0 °C, what is the new volume of the balloon at constant pressure and moles? SOLUTION: STEP 1 Organize the data into a table of initial and final conditions. ANALYZE Conditions 1 Conditions 2 THE V 1 = 785 m. L V 2 = ? PROBLEM T 1 = 21 °C = 294 K T 2 = 0 °C = 273 Know Predict V decreases T decreases Pressure and moles remain constant. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

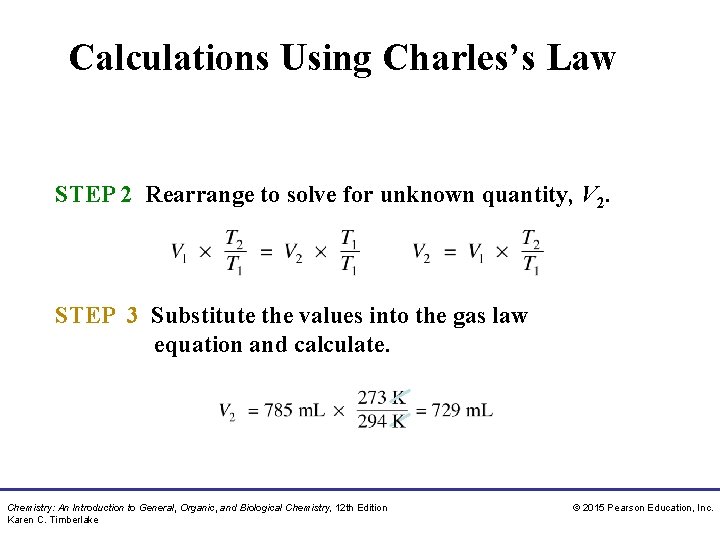
Calculations Using Charles’s Law STEP 2 Rearrange to solve for unknown quantity, V 2. STEP 3 Substitute the values into the gas law equation and calculate. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

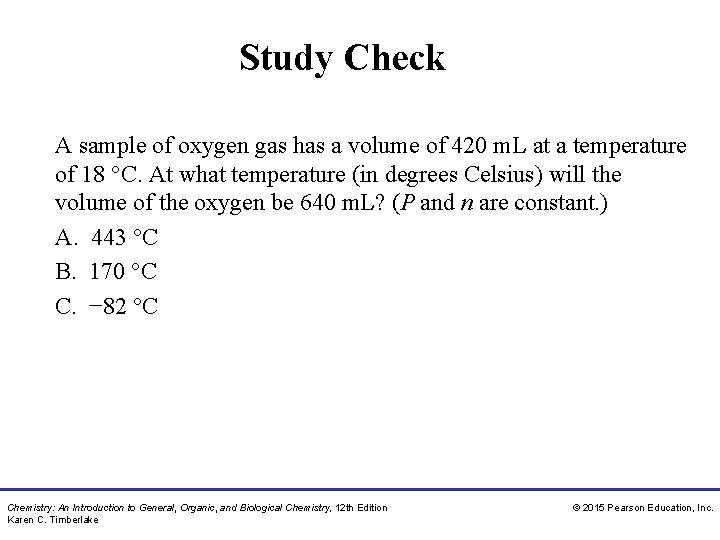
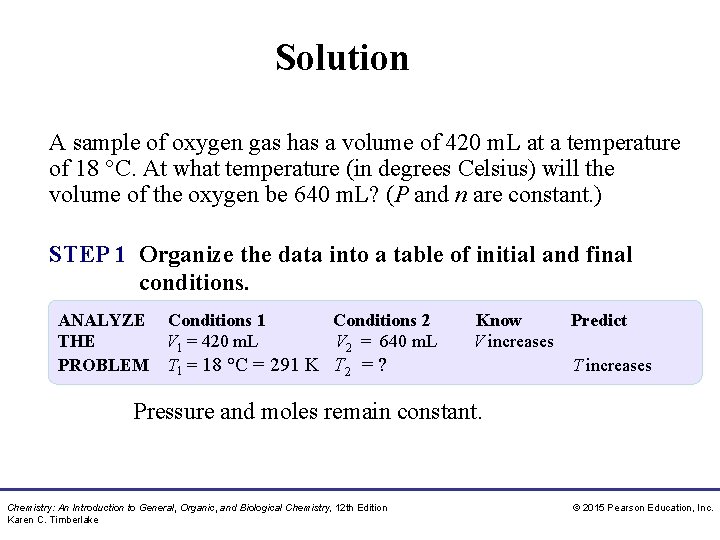
Study Check A sample of oxygen gas has a volume of 420 m. L at a temperature of 18 °C. At what temperature (in degrees Celsius) will the volume of the oxygen be 640 m. L? (P and n are constant. ) A. 443 °C B. 170 °C C. − 82 °C Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

Solution A sample of oxygen gas has a volume of 420 m. L at a temperature of 18 °C. At what temperature (in degrees Celsius) will the volume of the oxygen be 640 m. L? (P and n are constant. ) STEP 1 Organize the data into a table of initial and final conditions. ANALYZE Conditions 1 Conditions 2 THE V 1 = 420 m. L V 2 = 640 m. L PROBLEM T 1 = 18 °C = 291 K T 2 = ? Know Predict V increases T increases Pressure and moles remain constant. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

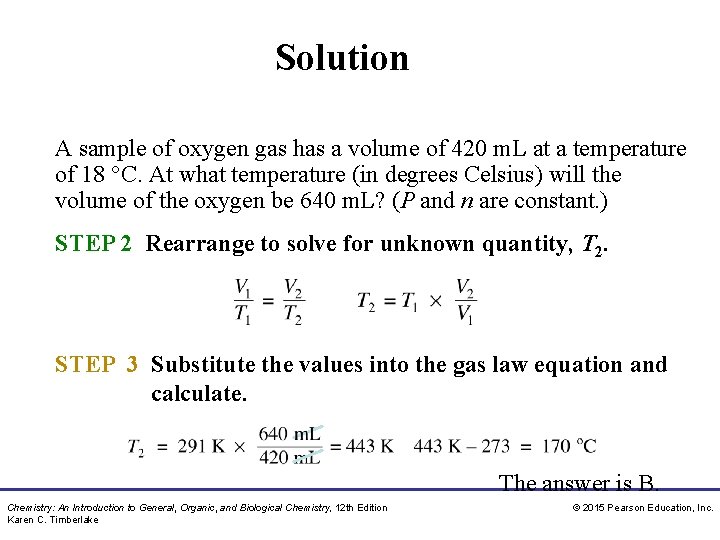
Solution A sample of oxygen gas has a volume of 420 m. L at a temperature of 18 °C. At what temperature (in degrees Celsius) will the volume of the oxygen be 640 m. L? (P and n are constant. ) STEP 2 Rearrange to solve for unknown quantity, T 2. STEP 3 Substitute the values into the gas law equation and calculate. The answer is B. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

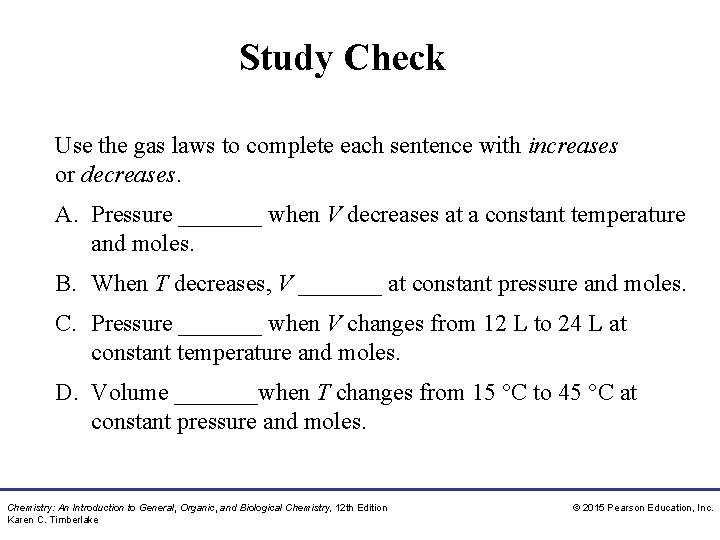
Study Check Use the gas laws to complete each sentence with increases or decreases. A. Pressure _______ when V decreases at a constant temperature and moles. B. When T decreases, V _______ at constant pressure and moles. C. Pressure _______ when V changes from 12 L to 24 L at constant temperature and moles. D. Volume _______when T changes from 15 °C to 45 °C at constant pressure and moles. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

Solution Use the gas laws to complete each sentence with increases or decreases. A. Pressure increases when V decreases at constant temperature and moles. B. When T decreases, V decreases at constant pressure and moles. C. Pressure decreases when V changes from 12 L to 24 L at constant temperature and moles. D. Volume increases when T changes from 15 °C to 45 °C at constant pressure and moles. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

CHAPTER 8: GASES 8. 4 A balloon has a volume of 2. 3 L at a pressure of 755 mm. Hg and 28 °C. What will be the new volume, in liters, of the balloon if the temperature is changed to 36 °C, assuming the pressure and moles do not change? A. B. C. D. 2. 3 L 2. 5 L 2. 1 L 2. 4 L Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

CHAPTER 8: GASES 8. 4 A balloon has a volume of 2. 3 L at a pressure of 755 mm. Hg and 28 °C. What will be the new volume, in liters, of the balloon if the temperature is changed to 36 °C, assuming the pressure and moles do not change? A. B. C. D. 2. 3 L 2. 5 L 2. 1 L 2. 4 L Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

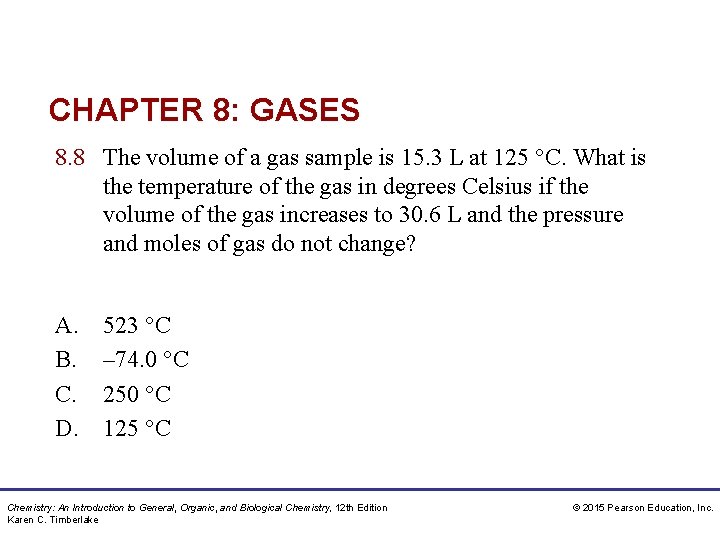
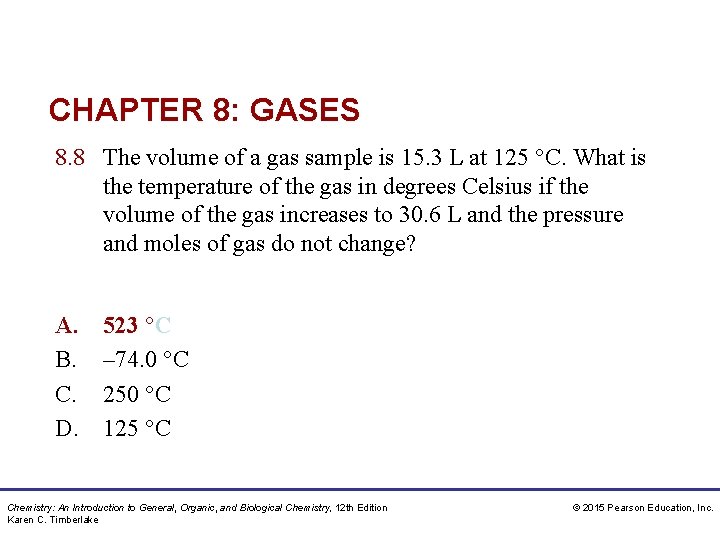
CHAPTER 8: GASES 8. 8 The volume of a gas sample is 15. 3 L at 125 °C. What is the temperature of the gas in degrees Celsius if the volume of the gas increases to 30. 6 L and the pressure and moles of gas do not change? A. B. C. D. 523 °C – 74. 0 °C 250 °C 125 °C Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

CHAPTER 8: GASES 8. 8 The volume of a gas sample is 15. 3 L at 125 °C. What is the temperature of the gas in degrees Celsius if the volume of the gas increases to 30. 6 L and the pressure and moles of gas do not change? A. B. C. D. 523 °C – 74. 0 °C 250 °C 125 °C Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

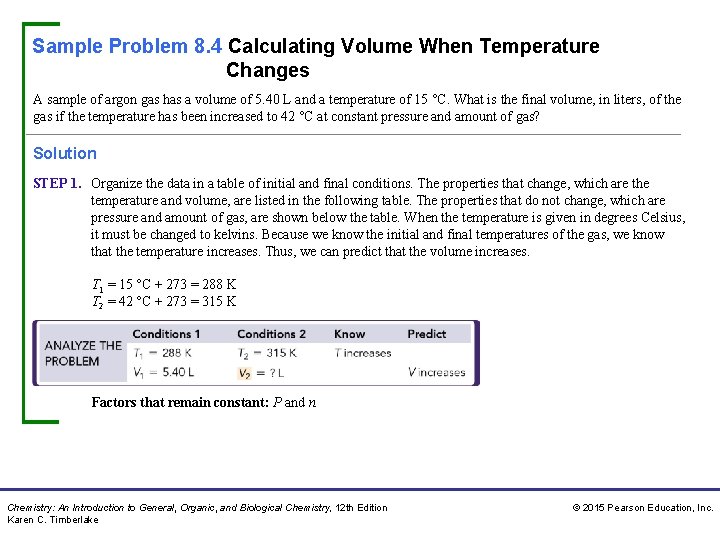
Sample Problem 8. 4 Calculating Volume When Temperature Changes A sample of argon gas has a volume of 5. 40 L and a temperature of 15 °C. What is the final volume, in liters, of the gas if the temperature has been increased to 42 °C at constant pressure and amount of gas? Solution STEP 1. Organize the data in a table of initial and final conditions. The properties that change, which are the temperature and volume, are listed in the following table. The properties that do not change, which are pressure and amount of gas, are shown below the table. When the temperature is given in degrees Celsius, it must be changed to kelvins. Because we know the initial and final temperatures of the gas, we know that the temperature increases. Thus, we can predict that the volume increases. T 1 = 15 °C + 273 = 288 K T 2 = 42 °C + 273 = 315 K Factors that remain constant: P and n Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

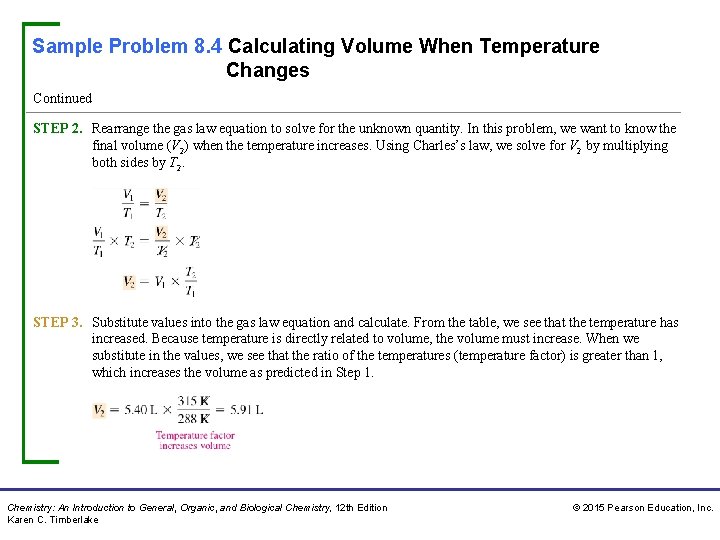
Sample Problem 8. 4 Calculating Volume When Temperature Changes Continued STEP 2. Rearrange the gas law equation to solve for the unknown quantity. In this problem, we want to know the final volume (V 2) when the temperature increases. Using Charles’s law, we solve for V 2 by multiplying both sides by T 2. STEP 3. Substitute values into the gas law equation and calculate. From the table, we see that the temperature has increased. Because temperature is directly related to volume, the volume must increase. When we substitute in the values, we see that the ratio of the temperatures (temperature factor) is greater than 1, which increases the volume as predicted in Step 1. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

Sample Problem 8. 4 Calculating Volume When Temperature Changes Continued Study Check 8. 4 A mountain climber with a body temperature of 37 °C inhales 486 m. L of air at a temperature of – 8 °C. What volume, in milliliters, will the air occupy in the lungs, if the pressure and amount of gas do not change? Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

8. 4 Temperature and Pressure: Gay-Lussac’s Law Gay-Lussac’s law: When the Kelvin temperature of a gas doubles at constant volume and amount of gas, the pressure also doubles. Learning Goal Use the temperature–pressure relationship (Gay -Lussac’s law) to determine the final temperature or pressure when the volume and amount of gas are constant. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

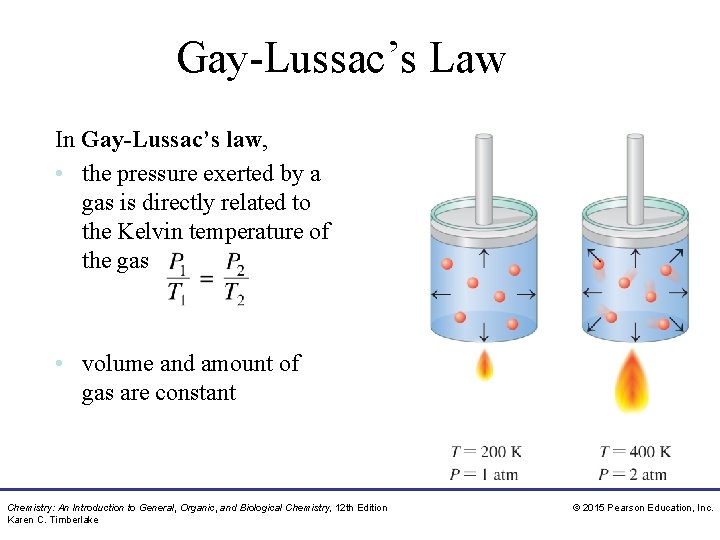
Gay-Lussac’s Law In Gay-Lussac’s law, • the pressure exerted by a gas is directly related to the Kelvin temperature of the gas • volume and amount of gas are constant Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

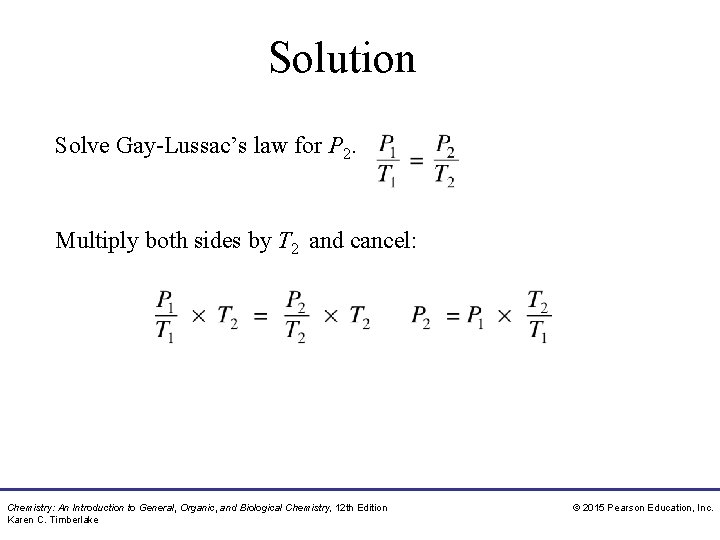
Study Check Solve Gay-Lussac’s law for P 2. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

Solution Solve Gay-Lussac’s law for P 2. Multiply both sides by T 2 and cancel: Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

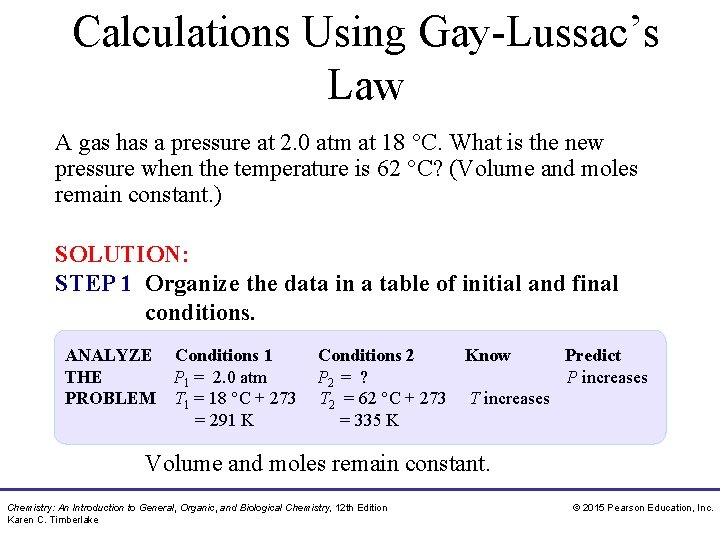
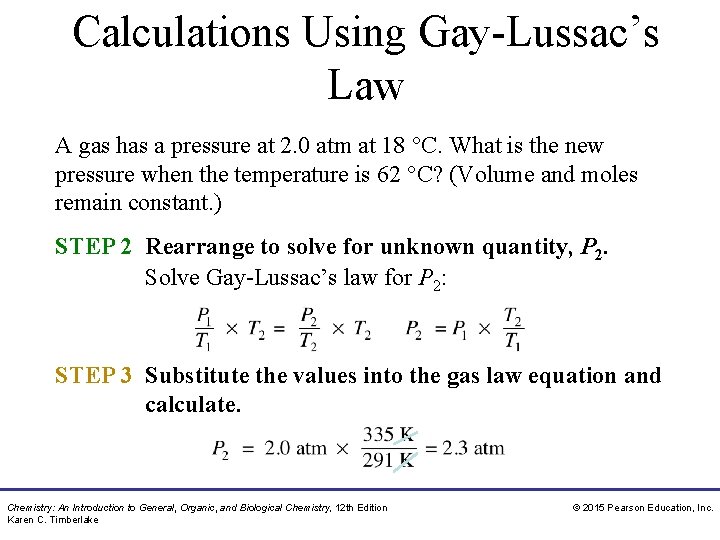
Calculations Using Gay-Lussac’s Law A gas has a pressure at 2. 0 atm at 18 °C. What is the new pressure when the temperature is 62 °C? (Volume and moles remain constant. ) SOLUTION: STEP 1 Organize the data in a table of initial and final conditions. ANALYZE Conditions 1 THE P 1 = 2. 0 atm PROBLEM T 1 = 18 °C + 273 = 291 K Conditions 2 Know Predict P 2 = ? P increases T 2 = 62 °C + 273 T increases = 335 K Volume and moles remain constant. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

Calculations Using Gay-Lussac’s Law A gas has a pressure at 2. 0 atm at 18 °C. What is the new pressure when the temperature is 62 °C? (Volume and moles remain constant. ) STEP 2 Rearrange to solve for unknown quantity, P 2. Solve Gay-Lussac’s law for P 2: STEP 3 Substitute the values into the gas law equation and calculate. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

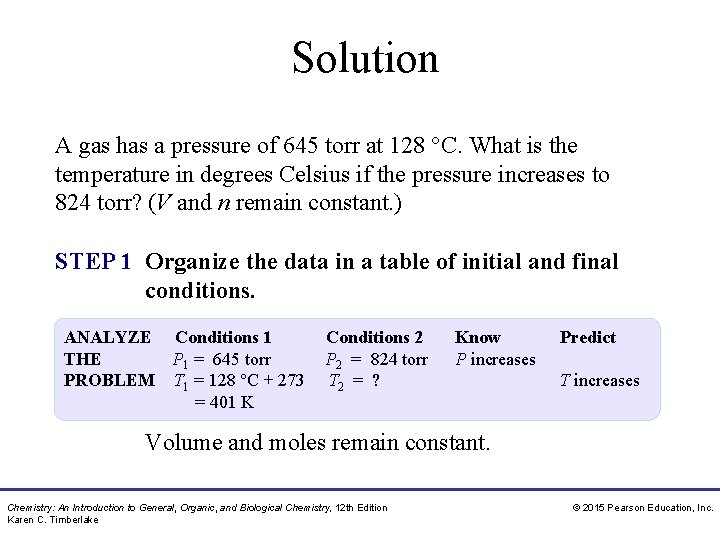
Study Check A gas has a pressure of 645 torr at 128 °C. What is the temperature in degrees Celsius if the pressure increases to 824 torr? (V and n remain constant. ) Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

Solution A gas has a pressure of 645 torr at 128 °C. What is the temperature in degrees Celsius if the pressure increases to 824 torr? (V and n remain constant. ) STEP 1 Organize the data in a table of initial and final conditions. ANALYZE Conditions 1 THE P 1 = 645 torr PROBLEM T 1 = 128 °C + 273 = 401 K Conditions 2 P 2 = 824 torr T 2 = ? Know P increases Predict T increases Volume and moles remain constant. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

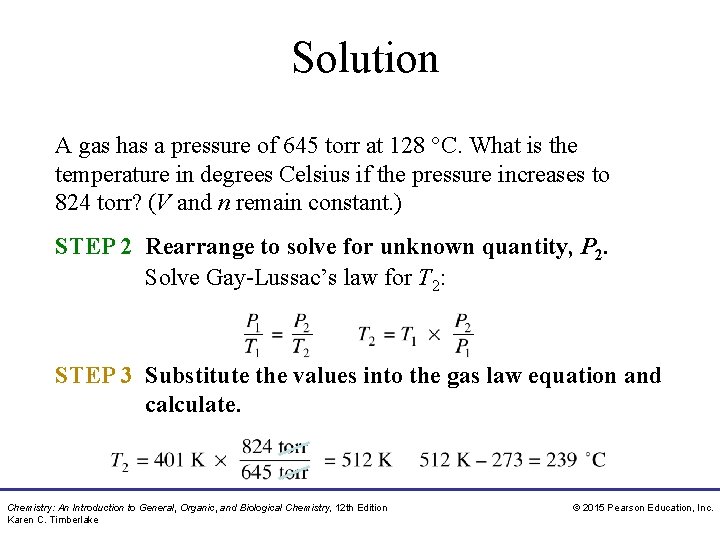
Solution A gas has a pressure of 645 torr at 128 °C. What is the temperature in degrees Celsius if the pressure increases to 824 torr? (V and n remain constant. ) STEP 2 Rearrange to solve for unknown quantity, P 2. Solve Gay-Lussac’s law for T 2: STEP 3 Substitute the values into the gas law equation and calculate. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

Study Check Explain why water boils at a lower temperature in the mountains than at sea level. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

Solution Explain why water boils at a lower temperature in the mountains than at sea level. Atmospheric pressure in the mountains is less than at sea level. The vapor pressure of the water reaches the atmospheric pressure at a lower temperature. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

CHAPTER 8: GASES 8. 9 A fire extinguisher has a pressure of 10. 0 atm at 25. 0 °C. What would be the pressure in atmospheres if the fire extinguisher is used at a temperature of 75. 0 °C and the moles and volume are held constant? A. B. C. D. 11. 0 atm 11. 7 atm 19. 0 atm 21. 0 atm Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

CHAPTER 8: GASES 8. 9 A fire extinguisher has a pressure of 10. 0 atm at 25. 0 °C. What would be the pressure in atmospheres if the fire extinguisher is used at a temperature of 75. 0 °C and the moles and volume are held constant? A. B. C. D. 11. 0 atm 11. 7 atm 19. 0 atm 21. 0 atm Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

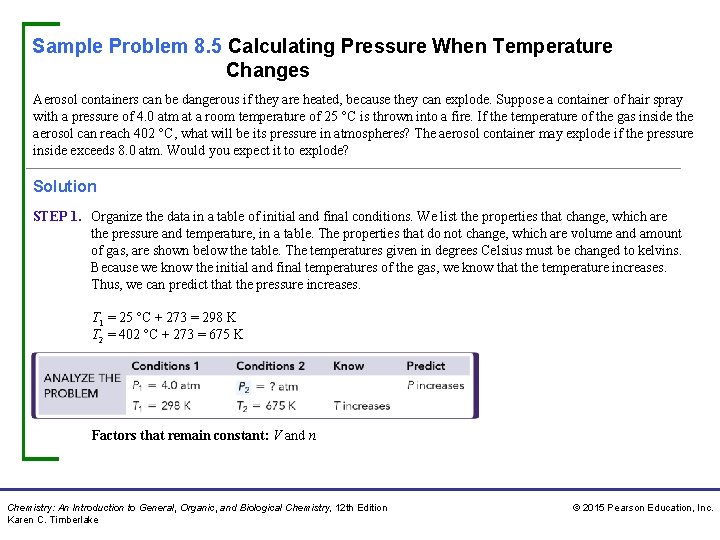
Sample Problem 8. 5 Calculating Pressure When Temperature Changes Aerosol containers can be dangerous if they are heated, because they can explode. Suppose a container of hair spray with a pressure of 4. 0 atm at a room temperature of 25 °C is thrown into a fire. If the temperature of the gas inside the aerosol can reach 402 °C, what will be its pressure in atmospheres? The aerosol container may explode if the pressure inside exceeds 8. 0 atm. Would you expect it to explode? Solution STEP 1. Organize the data in a table of initial and final conditions. We list the properties that change, which are the pressure and temperature, in a table. The properties that do not change, which are volume and amount of gas, are shown below the table. The temperatures given in degrees Celsius must be changed to kelvins. Because we know the initial and final temperatures of the gas, we know that the temperature increases. Thus, we can predict that the pressure increases. T 1 = 25 °C + 273 = 298 K T 2 = 402 °C + 273 = 675 K Factors that remain constant: V and n Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

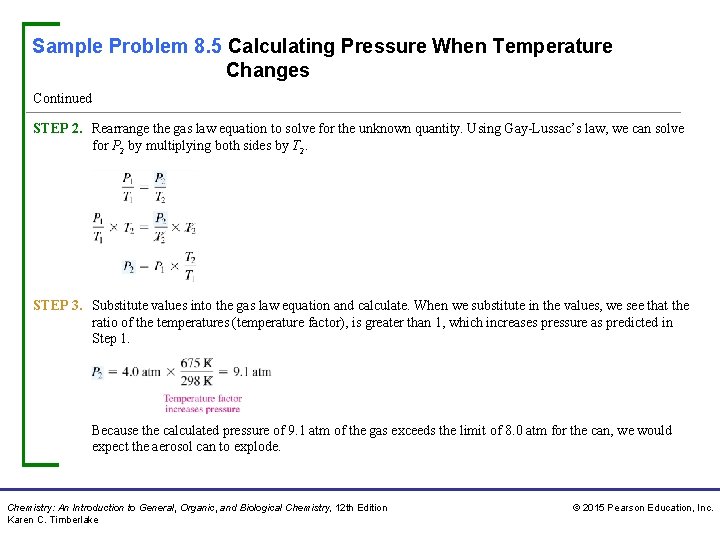
Sample Problem 8. 5 Calculating Pressure When Temperature Changes Continued STEP 2. Rearrange the gas law equation to solve for the unknown quantity. Using Gay-Lussac’s law, we can solve for P 2 by multiplying both sides by T 2. STEP 3. Substitute values into the gas law equation and calculate. When we substitute in the values, we see that the ratio of the temperatures (temperature factor), is greater than 1, which increases pressure as predicted in Step 1. Because the calculated pressure of 9. 1 atm of the gas exceeds the limit of 8. 0 atm for the can, we would expect the aerosol can to explode. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

Sample Problem 8. 5 Calculating Pressure When Temperature Changes Continued Study Check 8. 5 In a storage area where the temperature has reached 55 °C, the pressure of oxygen gas in a 15. 0 -L steel cylinder is 965 torr. To what temperature, in degrees Celsius, would the gas have to be cooled to reduce the pressure to 850. torr when the volume and amount of the gas do not change? Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

8. 5 The Combined Gas Law Under water, the pressure on a diver is greater than the atmospheric pressure. The combined gas law comes from the pressure–volume–temperature relationships for gases that we have studied. Learning Goal Use the combined gas law to calculate the final pressure, volume, or temperature of a gas when changes in two of these properties are given and the amount of gas is constant. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

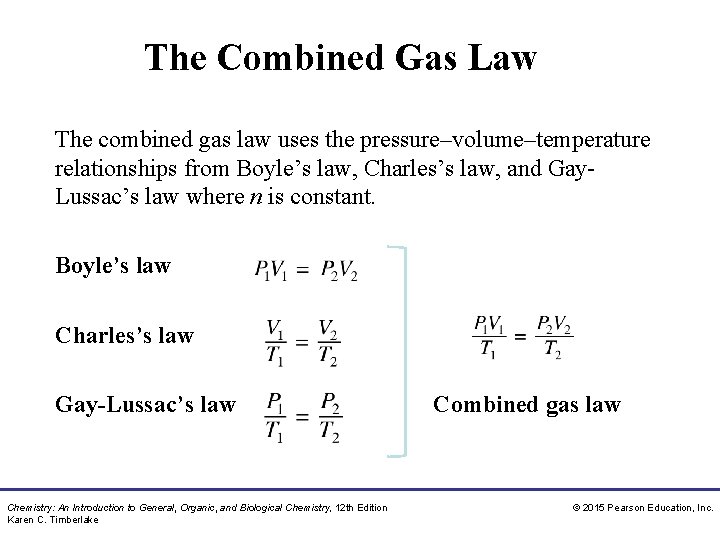
The Combined Gas Law The combined gas law uses the pressure–volume–temperature relationships from Boyle’s law, Charles’s law, and Gay. Lussac’s law where n is constant. Boyle’s law Charles’s law Gay-Lussac’s law Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake Combined gas law © 2015 Pearson Education, Inc.

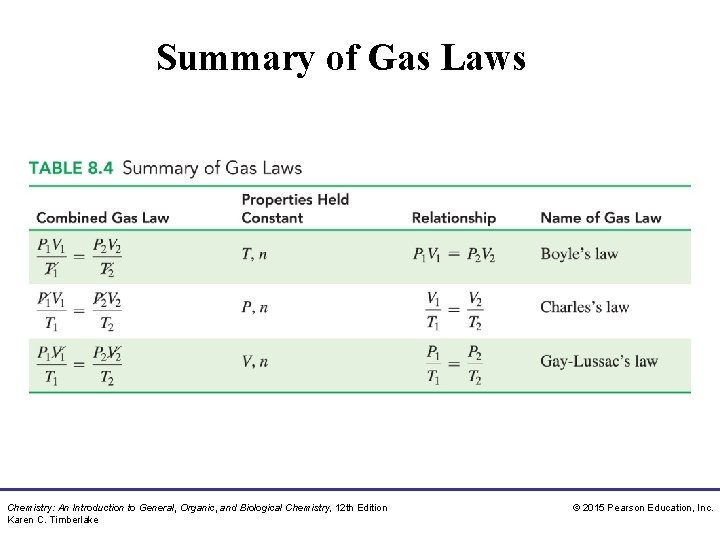
Summary of Gas Laws Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

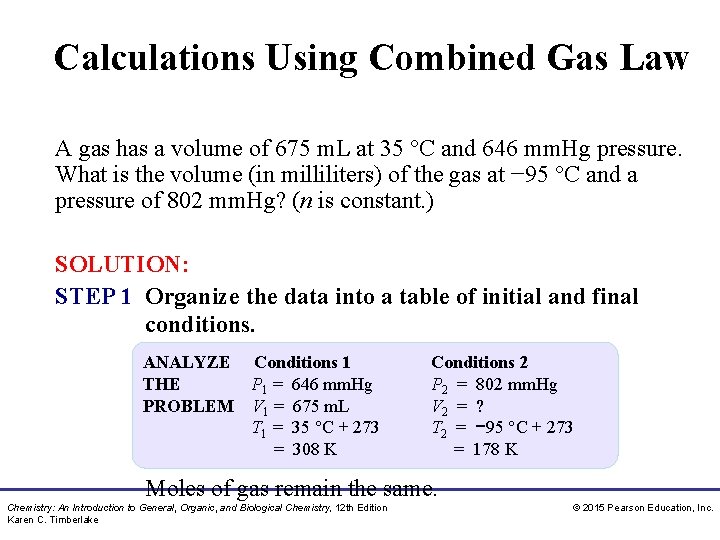
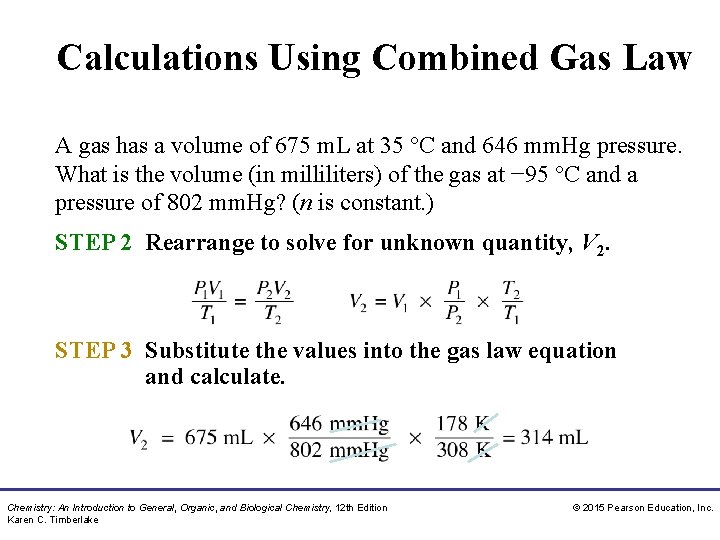
Calculations Using Combined Gas Law A gas has a volume of 675 m. L at 35 °C and 646 mm. Hg pressure. What is the volume (in milliliters) of the gas at − 95 °C and a pressure of 802 mm. Hg? (n is constant. ) SOLUTION: STEP 1 Organize the data into a table of initial and final conditions. ANALYZE Conditions 1 THE P 1 = 646 mm. Hg PROBLEM V 1 = 675 m. L T 1 = 35 °C + 273 = 308 K Conditions 2 P 2 = 802 mm. Hg V 2 = ? T 2 = − 95 °C + 273 = 178 K Moles of gas remain the same. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

Calculations Using Combined Gas Law A gas has a volume of 675 m. L at 35 °C and 646 mm. Hg pressure. What is the volume (in milliliters) of the gas at − 95 °C and a pressure of 802 mm. Hg? (n is constant. ) STEP 2 Rearrange to solve for unknown quantity, V 2. STEP 3 Substitute the values into the gas law equation and calculate. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

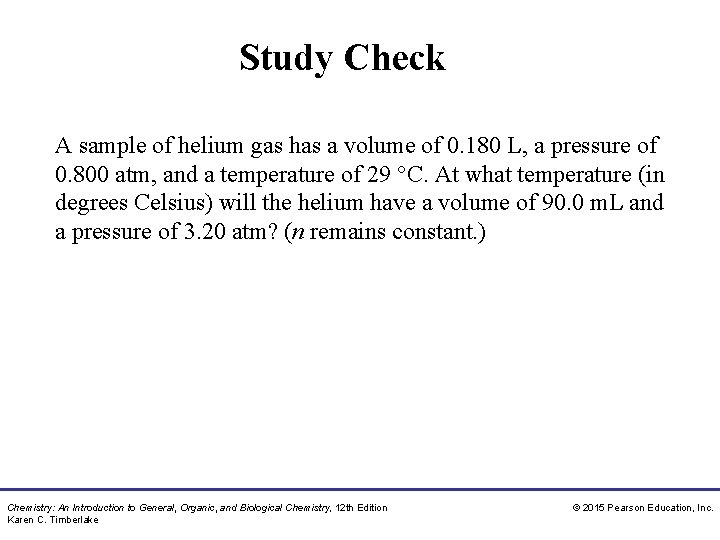
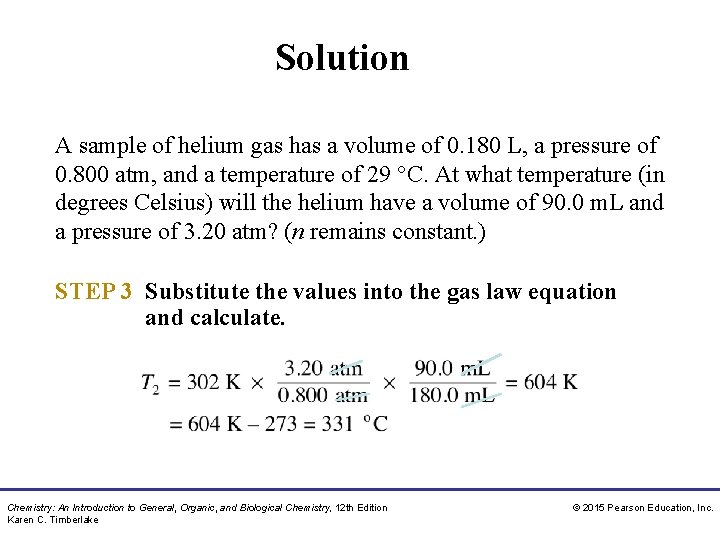
Study Check A sample of helium gas has a volume of 0. 180 L, a pressure of 0. 800 atm, and a temperature of 29 °C. At what temperature (in degrees Celsius) will the helium have a volume of 90. 0 m. L and a pressure of 3. 20 atm? (n remains constant. ) Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

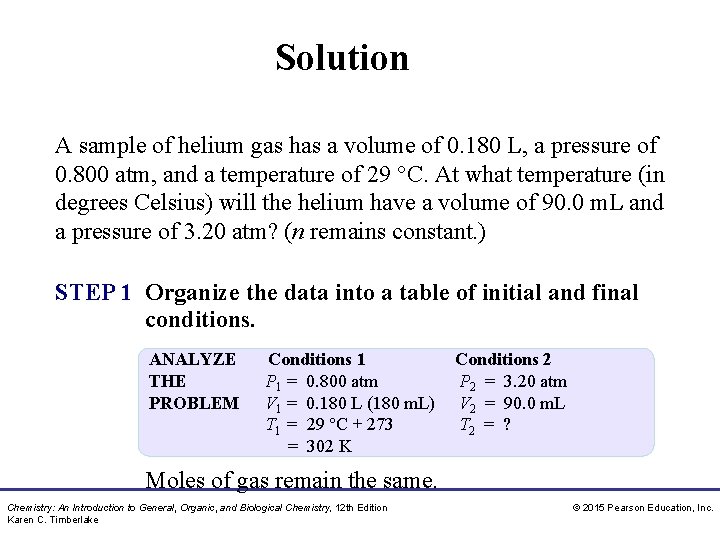
Solution A sample of helium gas has a volume of 0. 180 L, a pressure of 0. 800 atm, and a temperature of 29 °C. At what temperature (in degrees Celsius) will the helium have a volume of 90. 0 m. L and a pressure of 3. 20 atm? (n remains constant. ) STEP 1 Organize the data into a table of initial and final conditions. ANALYZE THE PROBLEM Conditions 1 P 1 = 0. 800 atm V 1 = 0. 180 L (180 m. L) T 1 = 29 °C + 273 = 302 K Conditions 2 P 2 = 3. 20 atm V 2 = 90. 0 m. L T 2 = ? Moles of gas remain the same. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

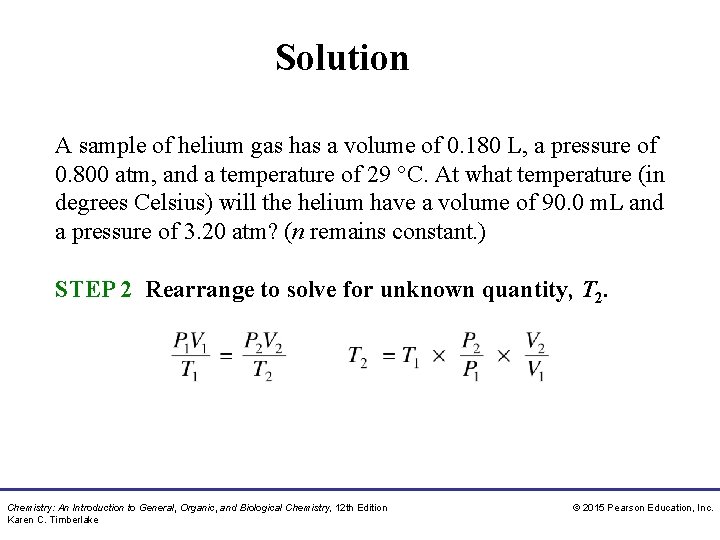
Solution A sample of helium gas has a volume of 0. 180 L, a pressure of 0. 800 atm, and a temperature of 29 °C. At what temperature (in degrees Celsius) will the helium have a volume of 90. 0 m. L and a pressure of 3. 20 atm? (n remains constant. ) STEP 2 Rearrange to solve for unknown quantity, T 2. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

Solution A sample of helium gas has a volume of 0. 180 L, a pressure of 0. 800 atm, and a temperature of 29 °C. At what temperature (in degrees Celsius) will the helium have a volume of 90. 0 m. L and a pressure of 3. 20 atm? (n remains constant. ) STEP 3 Substitute the values into the gas law equation and calculate. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

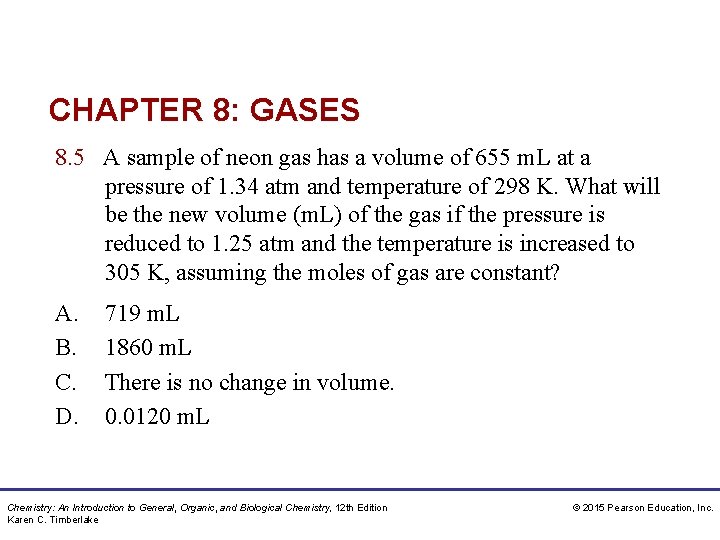
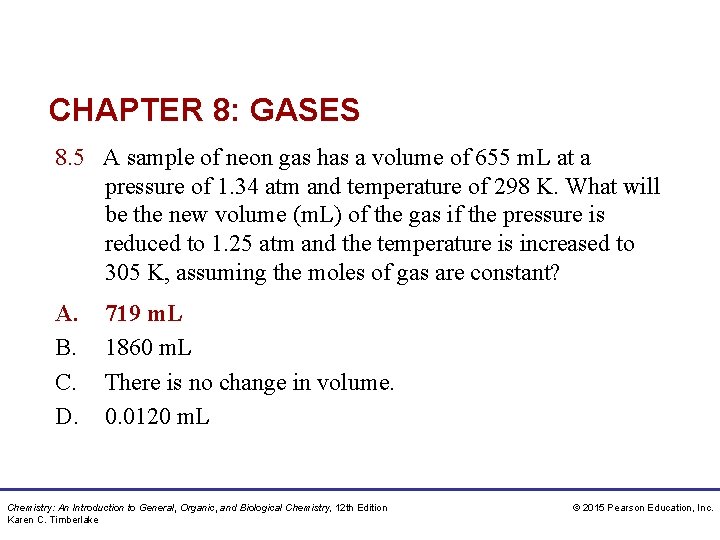
CHAPTER 8: GASES 8. 5 A sample of neon gas has a volume of 655 m. L at a pressure of 1. 34 atm and temperature of 298 K. What will be the new volume (m. L) of the gas if the pressure is reduced to 1. 25 atm and the temperature is increased to 305 K, assuming the moles of gas are constant? A. B. C. D. 719 m. L 1860 m. L There is no change in volume. 0. 0120 m. L Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

CHAPTER 8: GASES 8. 5 A sample of neon gas has a volume of 655 m. L at a pressure of 1. 34 atm and temperature of 298 K. What will be the new volume (m. L) of the gas if the pressure is reduced to 1. 25 atm and the temperature is increased to 305 K, assuming the moles of gas are constant? A. B. C. D. 719 m. L 1860 m. L There is no change in volume. 0. 0120 m. L Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

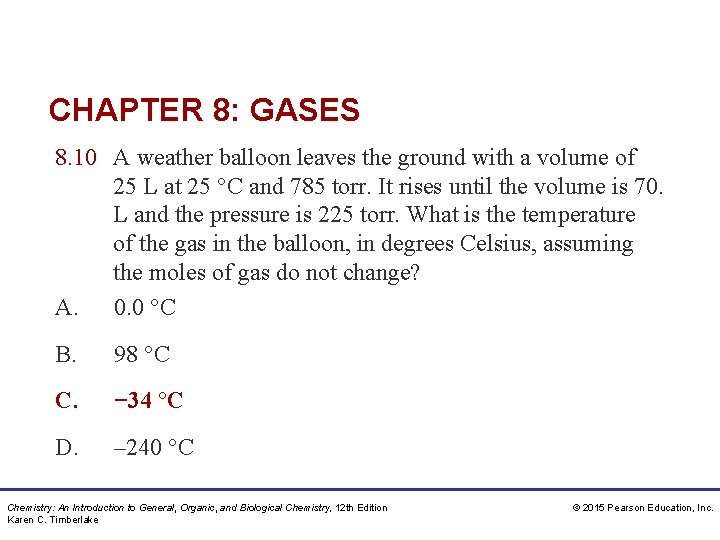
CHAPTER 8: GASES 8. 10 A weather balloon leaves the ground with a volume of 25 L at 25 °C and 785 torr. It rises until the volume is 70. L and the pressure is 225 torr. What is the temperature of the gas in the balloon, in degrees Celsius, assuming the moles of gas do not change? A. 0. 0 °C B. 98 °C C. − 34 °C D. – 240 °C Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

CHAPTER 8: GASES 8. 10 A weather balloon leaves the ground with a volume of 25 L at 25 °C and 785 torr. It rises until the volume is 70. L and the pressure is 225 torr. What is the temperature of the gas in the balloon, in degrees Celsius, assuming the moles of gas do not change? A. 0. 0 °C B. 98 °C C. − 34 °C D. – 240 °C Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

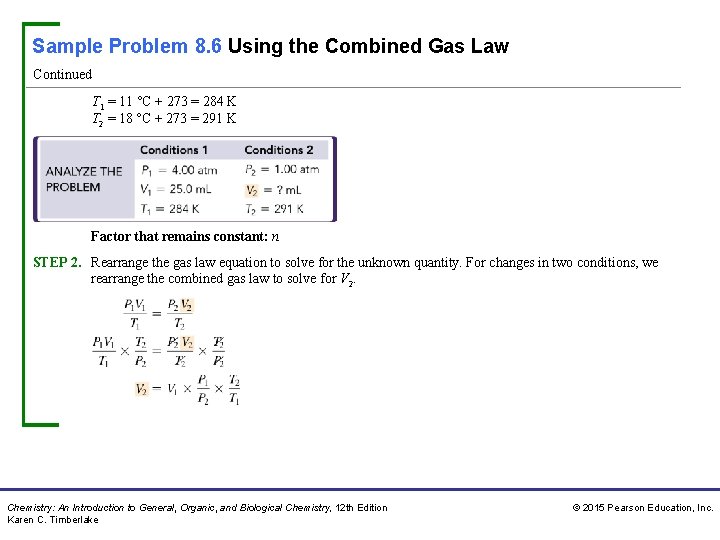
Sample Problem 8. 6 Using the Combined Gas Law A 25. 0 -m. L bubble is released from a diver’s air tank at a pressure of 4. 00 atm and a temperature of 11 °C. What is the volume, in milliliters, of the bubble when it reaches the ocean surface, where the pressure is 1. 00 atm and the temperature is 18 °C? (Assume the amount of gas in the bubble does not change. ) Solution STEP 1. Organize the data in a table of initial and final conditions. We list the properties that change, which are the pressure, volume, and temperature, in a table. The property that remains constant, which is the amount of gas, is shown below the table. The temperatures in degrees Celsius must be changed to kelvins. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

Sample Problem 8. 6 Using the Combined Gas Law Continued T 1 = 11 °C + 273 = 284 K T 2 = 18 °C + 273 = 291 K Factor that remains constant: n STEP 2. Rearrange the gas law equation to solve for the unknown quantity. For changes in two conditions, we rearrange the combined gas law to solve for V 2. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

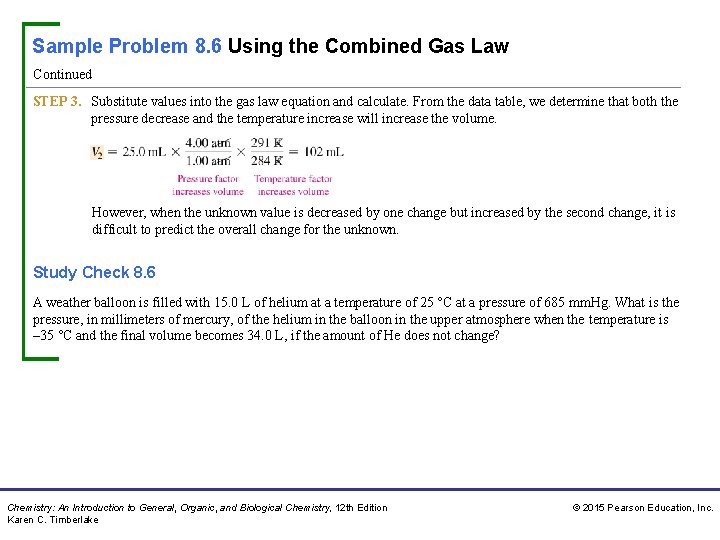
Sample Problem 8. 6 Using the Combined Gas Law Continued STEP 3. Substitute values into the gas law equation and calculate. From the data table, we determine that both the pressure decrease and the temperature increase will increase the volume. However, when the unknown value is decreased by one change but increased by the second change, it is difficult to predict the overall change for the unknown. Study Check 8. 6 A weather balloon is filled with 15. 0 L of helium at a temperature of 25 °C at a pressure of 685 mm. Hg. What is the pressure, in millimeters of mercury, of the helium in the balloon in the upper atmosphere when the temperature is – 35 °C and the final volume becomes 34. 0 L, if the amount of He does not change? Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

8. 6 Volume and Moles: Avogadro’s Law The molar volume of a gas at STP is about the same as the volume of three basketballs. The volume of 1 mole of gas is 22. 4 liters. Learning Goal Use Avogadro’s law to calculate the amount or volume of a gas when the pressure and temperature are constant. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

Avogadro’s Law: Volume and Moles In Avogadro’s law, • the volume of a gas is directly related to the number of moles (n) of gas • T and P are constant Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

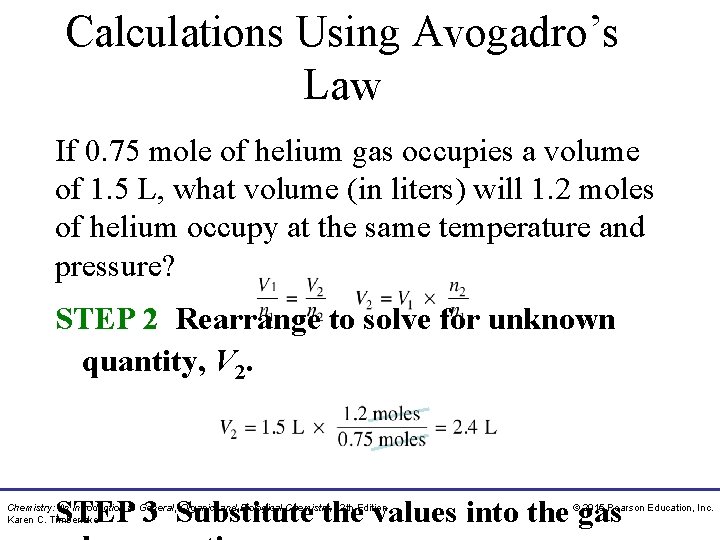
Calculations Using Avogadro’s Law If 0. 75 mole of helium gas occupies a volume of 1. 5 L, what volume (in liters) will 1. 2 moles of helium occupy at the same temperature and pressure? A. 0. 94 L B. 1. 8 L C. 2. 4 L Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

Calculations Using Avogadro’s Law If 0. 75 mole of helium gas occupies a volume of 1. 5 L, what volume (in liters) will 1. 2 moles of helium occupy at the same temperature and pressure? SOLUTION: ANALYZE Conditions 1 THE V 1 = 1. 5 L PROBLEM n 1 = 0. 75 mole Conditions 2 V 2 = ? n 2 = 1. 2 moles Know Predict V increases STEP 1 Organize the data into a table of n increases initial and final conditions. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

Calculations Using Avogadro’s Law If 0. 75 mole of helium gas occupies a volume of 1. 5 L, what volume (in liters) will 1. 2 moles of helium occupy at the same temperature and pressure? STEP 2 Rearrange to solve for unknown quantity, V 2. STEP 3 Substitute the values into the gas Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

Sample Problem 8. 7 Calculating Volume for a Change in Moles A weather balloon with a volume of 44 L is filled with 2. 0 moles of helium. What is the final volume, in liters, if 3. 0 moles of helium gas are added, to give a total of 5. 0 moles of helium gas, if the pressure and temperature do not change? Solution STEP 1. Organize the data in a table of initial and final conditions. We list those properties that change, which are volume and amount (moles) of gas, in a table. The properties that do not change, which are pressure and temperature, are shown below the table. Because there is an increase in the number of moles of gas, we can predict that the volume increases. Factors that remain constant: P and T Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

Sample Problem 8. 7 Calculating Volume for a Change in Moles Continued STEP 2. Rearrange the gas law equation to solve for the unknown quantity. Using Avogadro’s law, we can solve for V 2 by multiplying both sides by n 2. STEP 3. Substitute values into the gas law equation and calculate. When we substitute in the values, we see that the ratio of the moles (mole factor) is greater than 1, which increases the volume as predicted in Step 1. Study Check 8. 7 A sample containing 8. 00 g of oxygen gas has a volume of 5. 00 L. What is the final volume, in liters, after 4. 00 g of oxygen gas is added to the 8. 00 g of oxygen in the balloon, if the temperature and pressure do not change? Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

Standard Temperature and Pressure The volumes of gases can be compared at STP, Standard Temperature and Pressure, when they have • the same temperature standard temperature (T ) 0 °C or 273 K • the same pressure standard pressure (P ) 1 atm (760 mm. Hg) Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

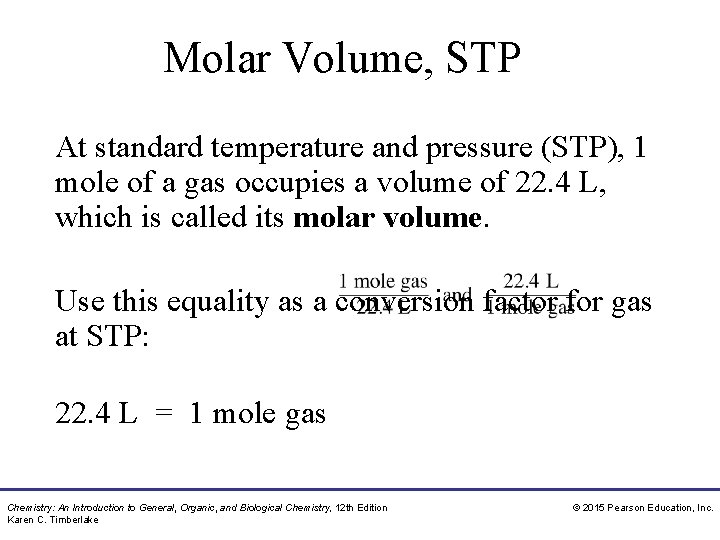
Molar Volume, STP At standard temperature and pressure (STP), 1 mole of a gas occupies a volume of 22. 4 L, which is called its molar volume. Use this equality as a conversion factor for gas at STP: 22. 4 L = 1 mole gas Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

Molar Volume Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

Guide to Using Molar Volume Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

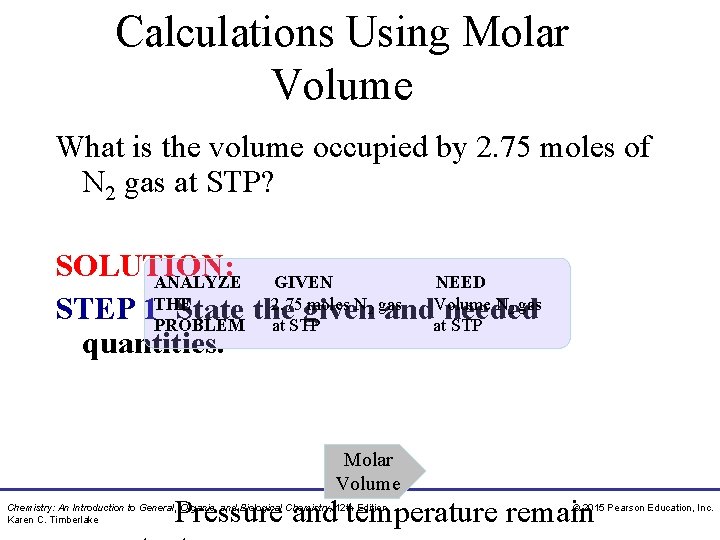
Calculations Using Molar Volume What is the volume occupied by 2. 75 moles of N 2 gas at STP? SOLUTION: ANALYZE GIVEN NEED 2. 75 moles N gas Volume N gas STEP 1 THE State the given and needed PROBLEM at STP quantities. 2 2 Molar Volume Pressure and temperature remain Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

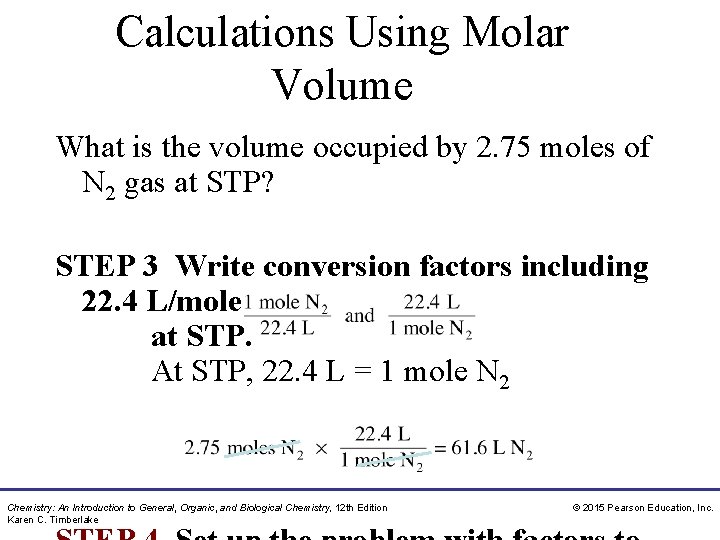
Calculations Using Molar Volume What is the volume occupied by 2. 75 moles of N 2 gas at STP? STEP 3 Write conversion factors including 22. 4 L/mole at STP. At STP, 22. 4 L = 1 mole N 2 Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

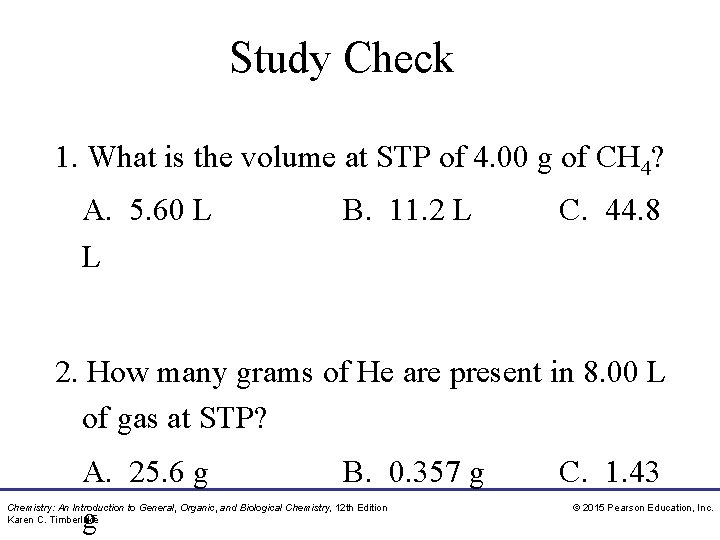
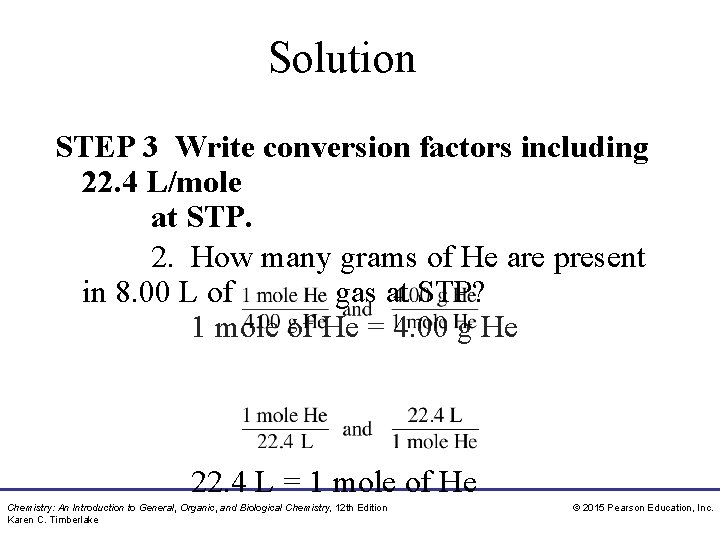
Study Check 1. What is the volume at STP of 4. 00 g of CH 4? A. 5. 60 L L B. 11. 2 L C. 44. 8 2. How many grams of He are present in 8. 00 L of gas at STP? A. 25. 6 g g B. 0. 357 g Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake C. 1. 43 © 2015 Pearson Education, Inc.

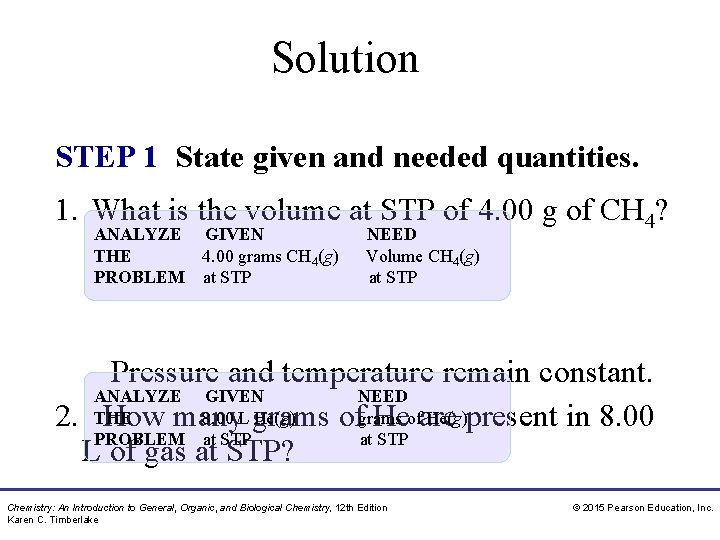
Solution STEP 1 State given and needed quantities. 1. What is the volume at STP of 4. 00 g of CH 4? ANALYZE GIVEN THE 4. 00 grams CH 4(g) PROBLEM at STP NEED Volume CH 4(g) at STP Pressure and temperature remain constant. ANALYZE GIVEN NEED 8. 00 L grams He(g)present in 8. 00 2. THE How many ofgrams Heofare PROBLEM at STP L of gas at STP? Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

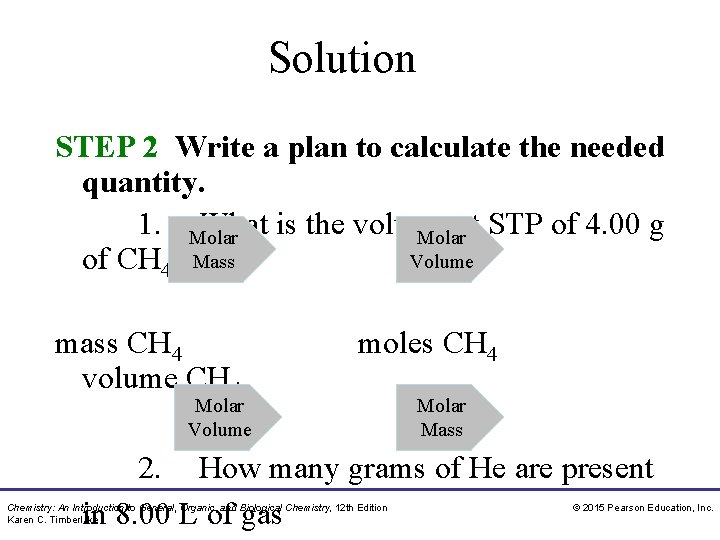
Solution STEP 2 Write a plan to calculate the needed quantity. 1. Molar What is the volume at STP of 4. 00 g Molar Volume of CH 4? Mass mass CH 4 volume CH 4 moles CH 4 Molar Volume Molar Mass 2. How many grams of He are present in 8. 00 L of gas Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

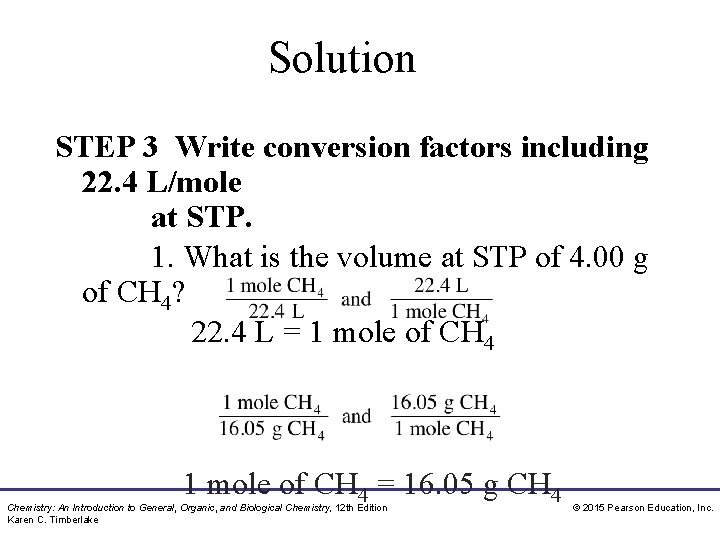
Solution STEP 3 Write conversion factors including 22. 4 L/mole at STP. 1. What is the volume at STP of 4. 00 g of CH 4? 22. 4 L = 1 mole of CH 4 = 16. 05 g CH 4 Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

Solution STEP 3 Write conversion factors including 22. 4 L/mole at STP. 2. How many grams of He are present in 8. 00 L of gas at STP? 1 mole of He = 4. 00 g He 22. 4 L = 1 mole of He Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

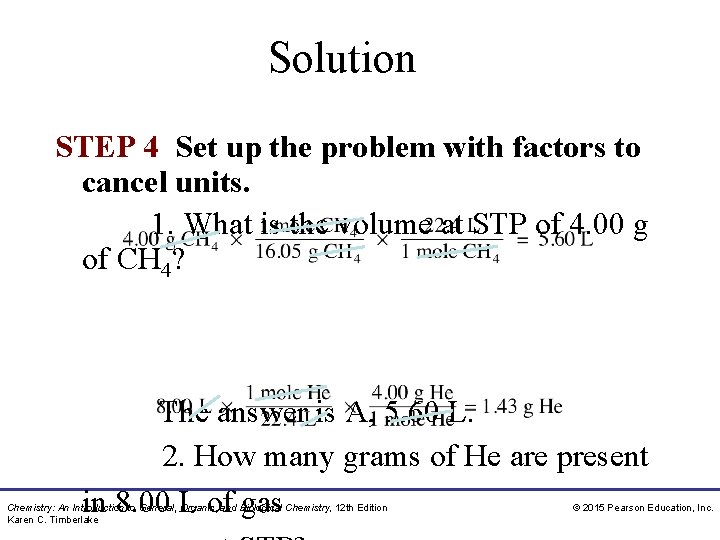
Solution STEP 4 Set up the problem with factors to cancel units. 1. What is the volume at STP of 4. 00 g of CH 4? The answer is A, 5. 60 L. 2. How many grams of He are present in 8. 00 L of gas Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

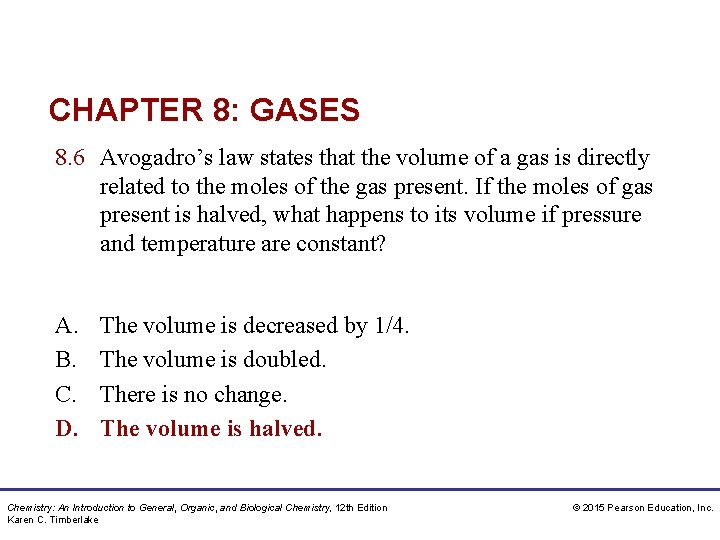
CHAPTER 8: GASES 8. 6 Avogadro’s law states that the volume of a gas is directly related to the moles of the gas present. If the moles of gas present is halved, what happens to its volume if pressure and temperature are constant? A. B. C. D. The volume is decreased by 1/4. The volume is doubled. There is no change. The volume is halved. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

CHAPTER 8: GASES 8. 6 Avogadro’s law states that the volume of a gas is directly related to the moles of the gas present. If the moles of gas present is halved, what happens to its volume if pressure and temperature are constant? A. B. C. D. The volume is decreased by 1/4. The volume is doubled. There is no change. The volume is halved. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

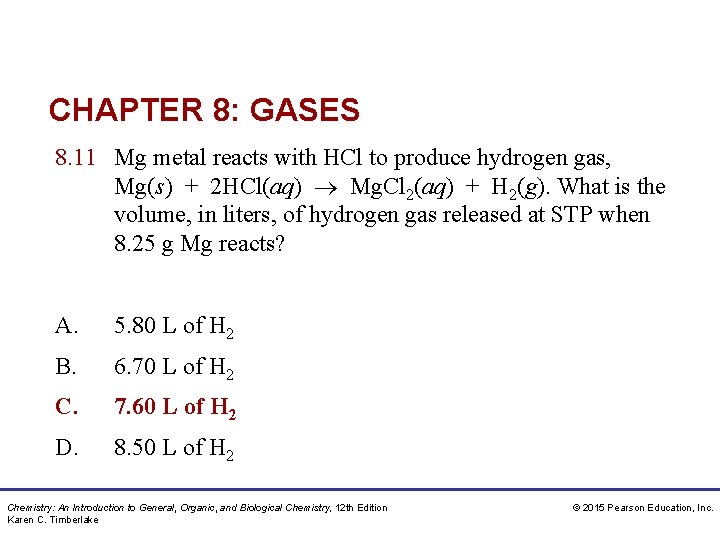
CHAPTER 8: GASES 8. 11 Mg metal reacts with HCl to produce hydrogen gas, Mg(s) + 2 HCl(aq) Mg. Cl 2(aq) + H 2(g). What is the volume, in liters, of hydrogen gas released at STP when 8. 25 g Mg reacts? A. 5. 80 L of H 2 B. 6. 70 L of H 2 C. 7. 60 L of H 2 D. 8. 50 L of H 2 Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

CHAPTER 8: GASES 8. 11 Mg metal reacts with HCl to produce hydrogen gas, Mg(s) + 2 HCl(aq) Mg. Cl 2(aq) + H 2(g). What is the volume, in liters, of hydrogen gas released at STP when 8. 25 g Mg reacts? A. 5. 80 L of H 2 B. 6. 70 L of H 2 C. 7. 60 L of H 2 D. 8. 50 L of H 2 Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

CHAPTER 8: GASES 8. 12 A bottle of argon gas has a volume of 6. 35 L at STP. How many moles of argon gas are in the bottle? A. B. C. D. 0. 106 mole 0. 283 mole 3. 53 moles 12. 7 moles Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

CHAPTER 8: GASES 8. 12 A bottle of argon gas has a volume of 6. 35 L at STP. How many moles of argon gas are in the bottle? A. B. C. D. 0. 106 mole 0. 283 mole 3. 53 moles 12. 7 moles Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

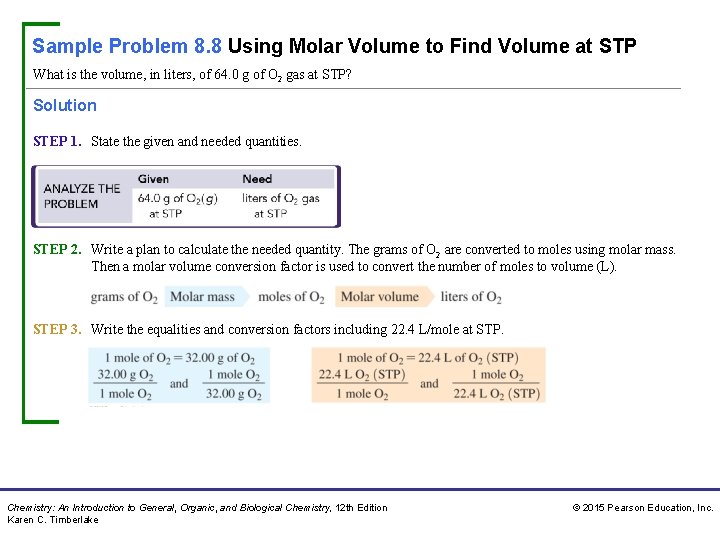
Sample Problem 8. 8 Using Molar Volume to Find Volume at STP What is the volume, in liters, of 64. 0 g of O 2 gas at STP? Solution STEP 1. State the given and needed quantities. STEP 2. Write a plan to calculate the needed quantity. The grams of O 2 are converted to moles using molar mass. Then a molar volume conversion factor is used to convert the number of moles to volume (L). STEP 3. Write the equalities and conversion factors including 22. 4 L/mole at STP. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

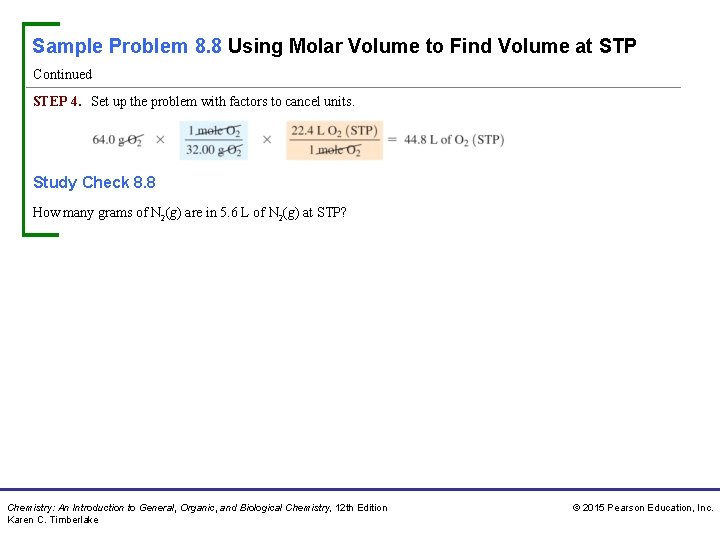
Sample Problem 8. 8 Using Molar Volume to Find Volume at STP Continued STEP 4. Set up the problem with factors to cancel units. Study Check 8. 8 How many grams of N 2(g) are in 5. 6 L of N 2(g) at STP? Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

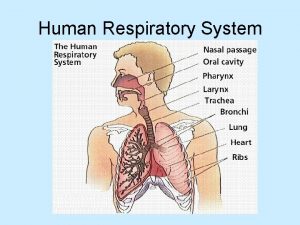
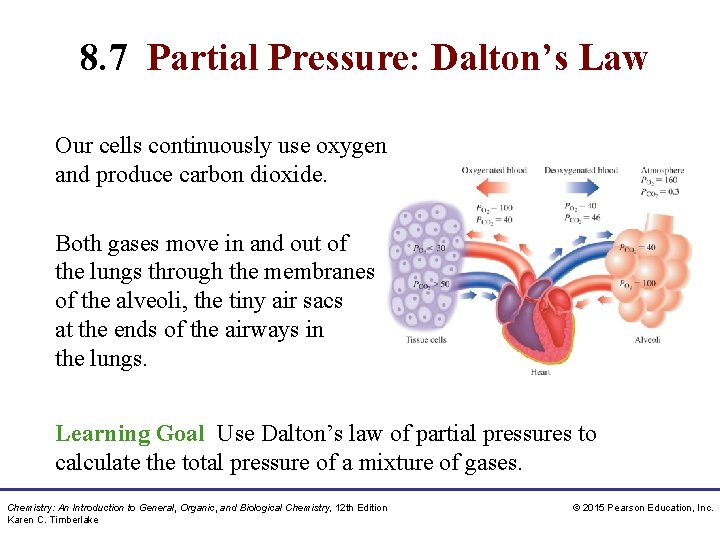
8. 7 Partial Pressure: Dalton’s Law Our cells continuously use oxygen and produce carbon dioxide. Both gases move in and out of the lungs through the membranes of the alveoli, the tiny air sacs at the ends of the airways in the lungs. Learning Goal Use Dalton’s law of partial pressures to calculate the total pressure of a mixture of gases. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

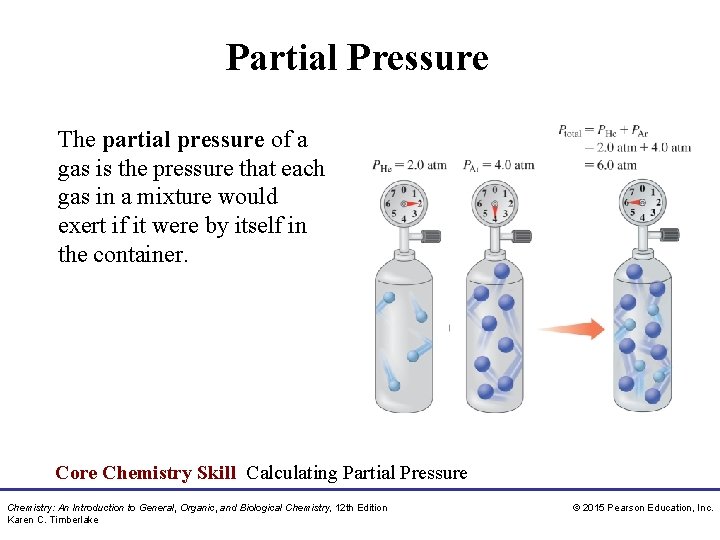
Partial Pressure The partial pressure of a gas is the pressure that each gas in a mixture would exert if it were by itself in the container. Core Chemistry Skill Calculating Partial Pressure Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

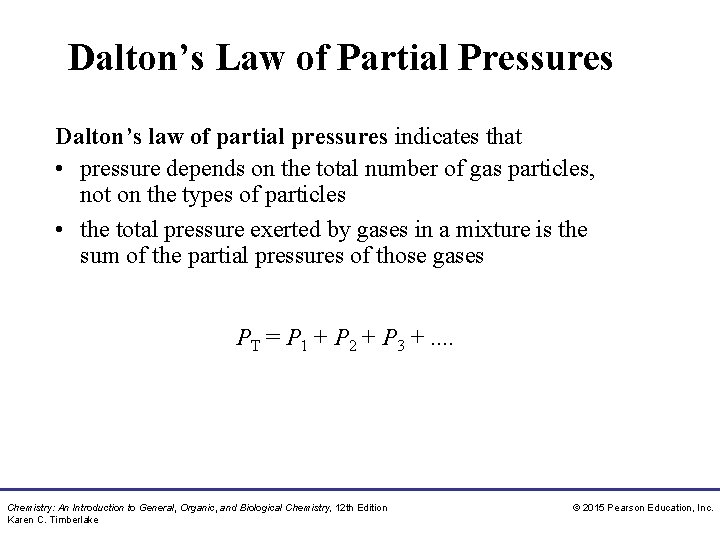
Dalton’s Law of Partial Pressures Dalton’s law of partial pressures indicates that • pressure depends on the total number of gas particles, not on the types of particles • the total pressure exerted by gases in a mixture is the sum of the partial pressures of those gases PT = P 1 + P 2 + P 3 +. . Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

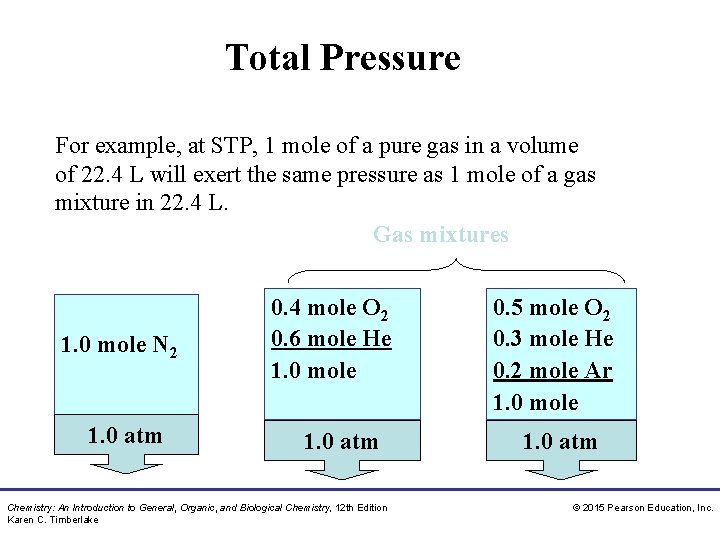
Total Pressure For example, at STP, 1 mole of a pure gas in a volume of 22. 4 L will exert the same pressure as 1 mole of a gas mixture in 22. 4 L. Gas mixtures 1. 0 mole N 2 1. 0 atm 0. 4 mole O 2 0. 6 mole He 1. 0 mole 1. 0 atm Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake 0. 5 mole O 2 0. 3 mole He 0. 2 mole Ar 1. 0 mole 1. 0 atm © 2015 Pearson Education, Inc.

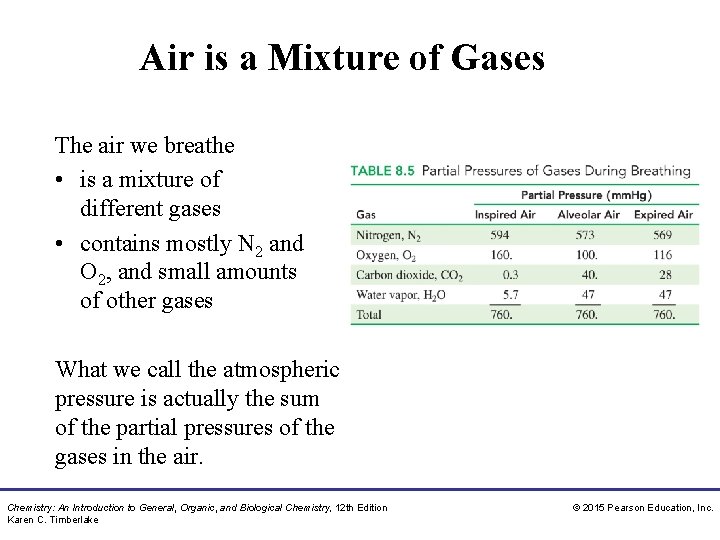
Air is a Mixture of Gases The air we breathe • is a mixture of different gases • contains mostly N 2 and O 2, and small amounts of other gases What we call the atmospheric pressure is actually the sum of the partial pressures of the gases in the air. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

Guide to Solving for Partial Pressure Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

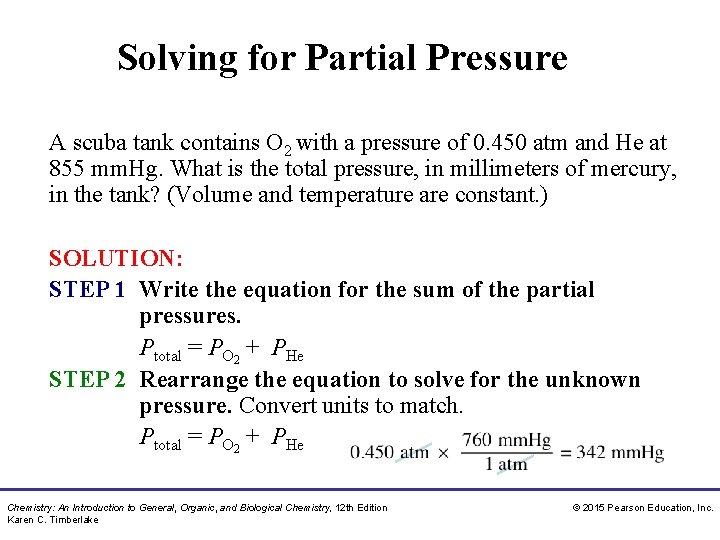
Solving for Partial Pressure A scuba tank contains O 2 with a pressure of 0. 450 atm and He at 855 mm. Hg. What is the total pressure, in millimeters of mercury, in the tank? (Volume and temperature are constant. ) SOLUTION: STEP 1 Write the equation for the sum of the partial pressures. Ptotal = PO 2 + PHe STEP 2 Rearrange the equation to solve for the unknown pressure. Convert units to match. Ptotal = PO 2 + PHe Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

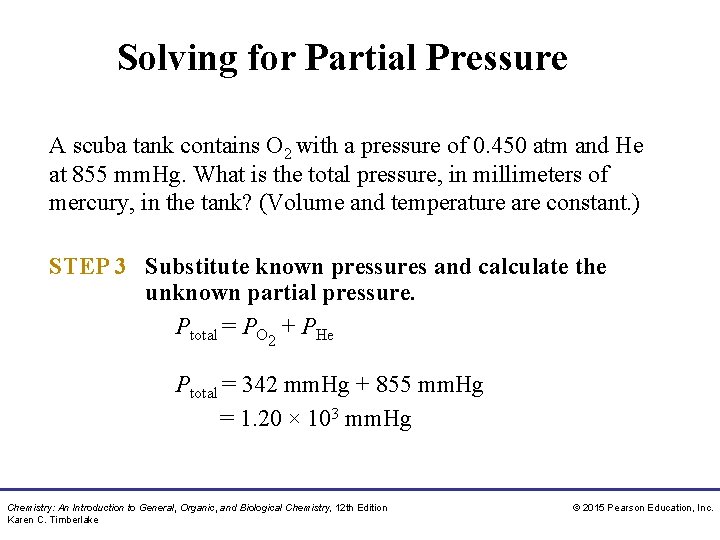
Solving for Partial Pressure A scuba tank contains O 2 with a pressure of 0. 450 atm and He at 855 mm. Hg. What is the total pressure, in millimeters of mercury, in the tank? (Volume and temperature are constant. ) STEP 3 Substitute known pressures and calculate the unknown partial pressure. Ptotal = PO 2 + PHe Ptotal = 342 mm. Hg + 855 mm. Hg = 1. 20 × 103 mm. Hg Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

Study Check For a deep dive, a scuba diver uses a mixture of helium and oxygen with a pressure of 8. 00 atm. If the oxygen has a partial pressure of 1280 mm. Hg, what is the partial pressure of the helium? (Volume and temperature are constant. ) A. 520 mm. Hg B. 2040 mm. Hg C. 4800 mm. Hg Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

Solution For a deep dive, a scuba diver uses a mixture of helium and oxygen with a pressure of 8. 00 atm. If the oxygen has a partial pressure of 1280 mm. Hg, what is the partial pressure of the helium? (Volume and temperature are constant. ) STEP 1 Write the equation for the sum of the partial pressures. Ptotal = PO 2 + PHe STEP 2 Rearrange the equation to solve for the unknown pressure. Convert units to match. PHe = Ptotal − PO 2 Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

Solution For a deep dive, a scuba diver uses a mixture of helium and oxygen with a pressure of 8. 00 atm. If the oxygen has a partial pressure of 1280 mm. Hg, what is the partial pressure of the helium? (Volume and temperature are constant. ) STEP 3 Substitute known pressures and calculate the unknown partial pressure. PHe = 6080 mm. Hg – 1280 mm. Hg = 4800 mm. Hg The answer is C, 4800 mm Hg. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

Sample Problem 8. 9 Partial Pressure of a Gas in a Mixture A heliox breathing mixture of oxygen and helium is prepared for a scuba diver who is going to descend 200 ft below the ocean surface. At that depth, the diver breathes a gas mixture that has a total pressure of 7. 00 atm. If the partial pressure of the oxygen in the tank at that depth is 1140 mm. Hg, what is the partial pressure, in atmospheres, of the helium in the breathing mixture? Solution STEP 1. Write the equation for the sum of the partial pressures. STEP 2. Rearrange the equation to solve for the unknown pressure. To solve for the partial pressure of helium (PHe), we rearrange the equation to give the following: Convert units to match. STEP 3. Substitute known pressures into the equation and calculate the unknown partial pressure. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

Sample Problem 8. 9 Partial Pressure of a Gas in a Mixture Continued Study Check 8. 9 A common anesthetic in dentistry known as Nitronox consists of a mixture of dinitrogen oxide, N 2 O, and oxygen gas, O 2, which is administered through an inhaler over the nose. If the anesthetic mixture has a total pressure of 740 mm. Hg, and the partial pressure of the oxygen is 370 mm. Hg, what is the partial pressure, in torr, of the dinitrogen oxide? Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

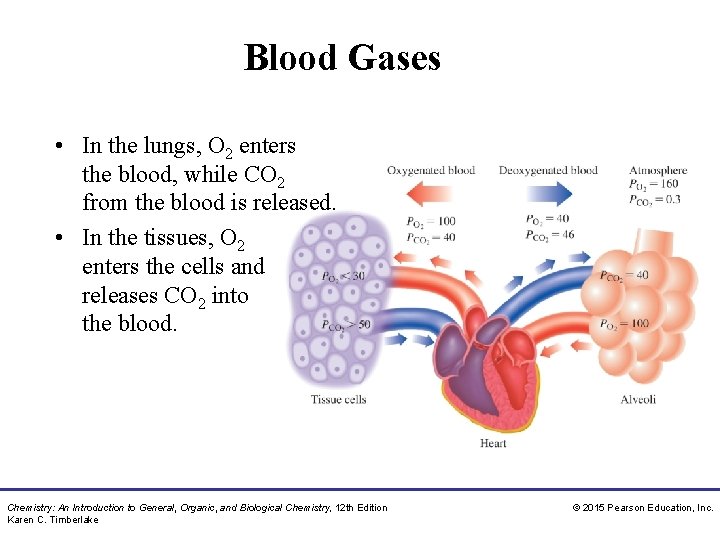
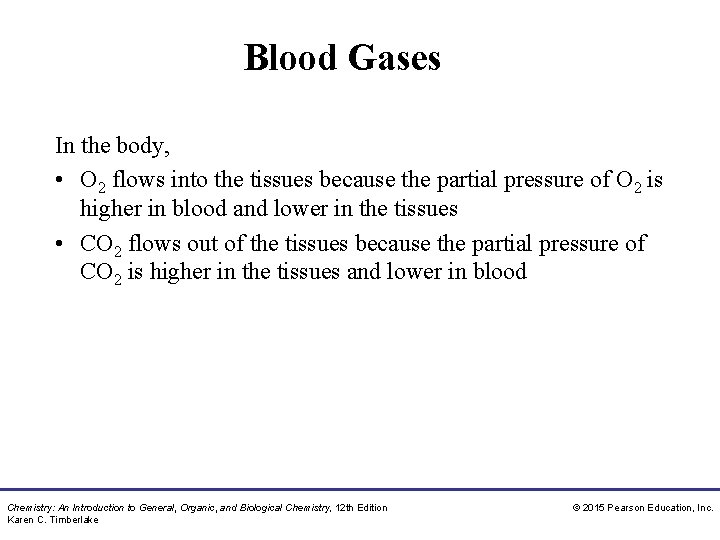
Blood Gases • In the lungs, O 2 enters the blood, while CO 2 from the blood is released. • In the tissues, O 2 enters the cells and releases CO 2 into the blood. Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

Blood Gases In the body, • O 2 flows into the tissues because the partial pressure of O 2 is higher in blood and lower in the tissues • CO 2 flows out of the tissues because the partial pressure of CO 2 is higher in the tissues and lower in blood Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

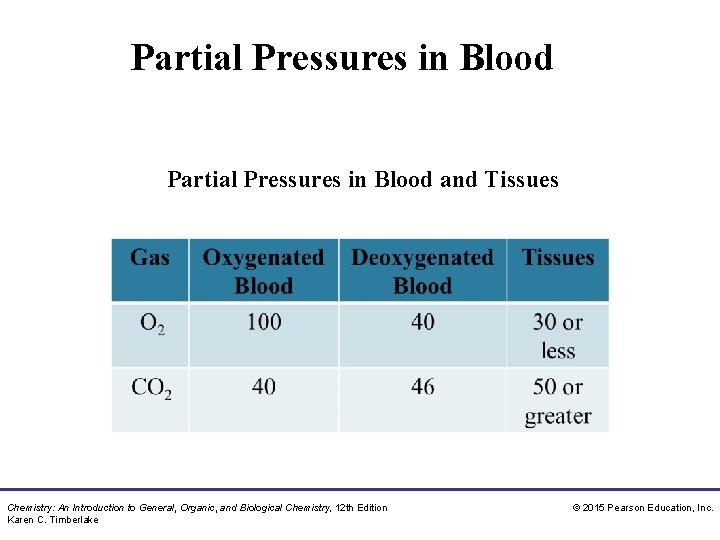
Partial Pressures in Blood and Tissues Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

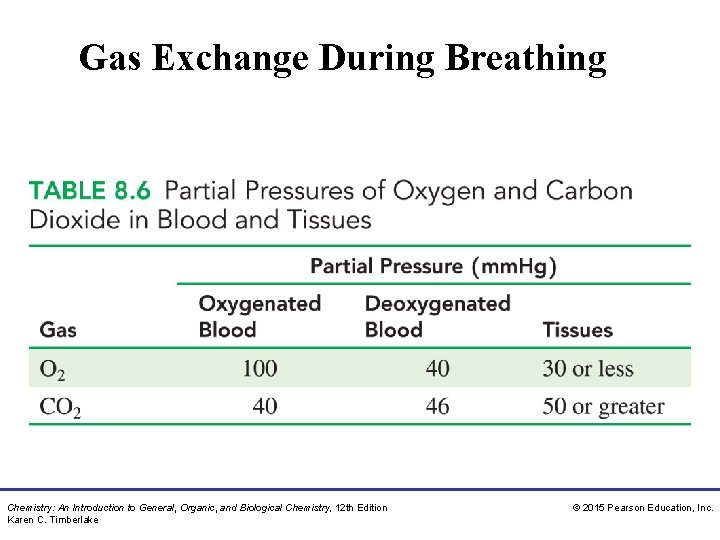
Gas Exchange During Breathing Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.

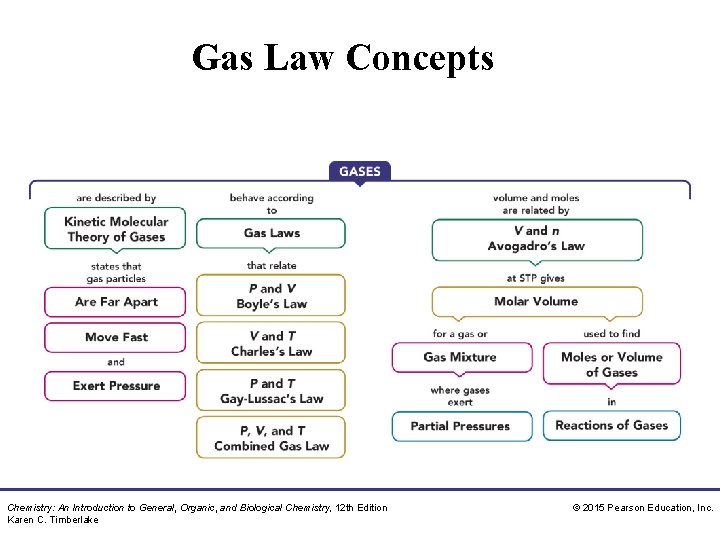
Gas Law Concepts Chemistry: An Introduction to General, Organic, and Biological Chemistry, 12 th Edition Karen C. Timberlake © 2015 Pearson Education, Inc.
 Boundaries meme
Boundaries meme Royal college of occupational therapists
Royal college of occupational therapists Client centered therapists try to appreciate
Client centered therapists try to appreciate Which of the following therapists would most likely
Which of the following therapists would most likely Diffusion of gases across respiratory membranes:
Diffusion of gases across respiratory membranes: Respiratory zone and conducting zone
Respiratory zone and conducting zone How to assess alert and oriented
How to assess alert and oriented How to assess alert and oriented
How to assess alert and oriented City & guilds evolve
City & guilds evolve City and guilds secure assess
City and guilds secure assess Who is mr. kirwin and how does he treat victor?
Who is mr. kirwin and how does he treat victor? Chapter 14 solids liquids and gases worksheet answers
Chapter 14 solids liquids and gases worksheet answers Chapter 14 solids liquids and gases
Chapter 14 solids liquids and gases Fremitus lungs
Fremitus lungs Assess current hr capacity
Assess current hr capacity What does tactile fremitus assess
What does tactile fremitus assess Orm abcds
Orm abcds Abbott nutrition focused physical assessment
Abbott nutrition focused physical assessment Retail organization
Retail organization Tactile fremitus
Tactile fremitus Aatsecureassess
Aatsecureassess Sqa personal finance
Sqa personal finance Projective testing
Projective testing Permuted block randomization
Permuted block randomization Cranial nerve smiling
Cranial nerve smiling Standart başarı testleri
Standart başarı testleri Assess plan do review template
Assess plan do review template Assess plan act
Assess plan act Hazard profile examples
Hazard profile examples Acfi assess incontinence for
Acfi assess incontinence for Site:slidetodoc.com
Site:slidetodoc.com Why assess
Why assess Assess your understanding
Assess your understanding Techniques to assess country risk
Techniques to assess country risk Ask assess advise
Ask assess advise Assess your understanding
Assess your understanding To assess achievement at the end of instruction is
To assess achievement at the end of instruction is Assess plan do review model
Assess plan do review model Humanistic psychologists may assess personality by
Humanistic psychologists may assess personality by Chapter 33 section 1 the circulatory system
Chapter 33 section 1 the circulatory system Cambysus
Cambysus Equation for number needed to treat
Equation for number needed to treat Invitation to treat
Invitation to treat How does paris react to juliet's death
How does paris react to juliet's death Which seedless plants have been used to treat bee stings?
Which seedless plants have been used to treat bee stings? Number needed to treat calculation
Number needed to treat calculation Offer
Offer How to treat laryngopharyngeal reflux
How to treat laryngopharyngeal reflux Calculate number needed to harm
Calculate number needed to harm Number need to treat
Number need to treat Interpreting relative risk
Interpreting relative risk Number needed to treat
Number needed to treat Guthing vs lynn case
Guthing vs lynn case Number needed to treat formula
Number needed to treat formula Invitation to treat meaning
Invitation to treat meaning Mffmm
Mffmm How does mercutio treat the nurse?
How does mercutio treat the nurse? Is byetta used to treat pcos
Is byetta used to treat pcos Treat everyone with sincerity
Treat everyone with sincerity Diener 1976 trick or treat
Diener 1976 trick or treat Mole day treat ideas
Mole day treat ideas How to treat bursitis in the hip
How to treat bursitis in the hip Treat others the way you would like to be
Treat others the way you would like to be Treat with confidence
Treat with confidence How do the nuns treat the pack at first?
How do the nuns treat the pack at first? Felicia gizycka
Felicia gizycka Treat someone with contempt
Treat someone with contempt Cystitis how to treat
Cystitis how to treat How does aunt alexandra treat calpurnia
How does aunt alexandra treat calpurnia Intention to treat analysis
Intention to treat analysis Colours of halloween song
Colours of halloween song Factors that helped spread christianity
Factors that helped spread christianity Eraser challenge urban dictionary
Eraser challenge urban dictionary Treat the earth well
Treat the earth well Treat the earth well
Treat the earth well What is the main idea of the ant and the grasshopper
What is the main idea of the ant and the grasshopper Chapter questions for to kill a mockingbird part 1
Chapter questions for to kill a mockingbird part 1 How did cyrus treat the people he conquered
How did cyrus treat the people he conquered Suspension trauma treatment
Suspension trauma treatment Why are seedless plants important
Why are seedless plants important Jay gould childhood
Jay gould childhood How did the scotch half breed treat the dogs
How did the scotch half breed treat the dogs How did henry ford spend his money
How did henry ford spend his money Avogadro's law relationship
Avogadro's law relationship Chapter 11 review gases section 1
Chapter 11 review gases section 1 How to solve ideal gas law
How to solve ideal gas law Chapter 14 the behavior of gases
Chapter 14 the behavior of gases Section 13.2 the combined gas law and avogadro's principle
Section 13.2 the combined gas law and avogadro's principle Chapter 7 cengage
Chapter 7 cengage Bronchide
Bronchide 22
22 Chapter 15 lesson 4
Chapter 15 lesson 4 Chapter 16 respiratory emergencies
Chapter 16 respiratory emergencies Chapter 7 the respiratory system labeling exercises
Chapter 7 the respiratory system labeling exercises Chapter 34 circulation in humans concept mapping answer key
Chapter 34 circulation in humans concept mapping answer key Respiratory system organs
Respiratory system organs Figure 13-1 respiratory system
Figure 13-1 respiratory system Chapter 16 respiratory emergencies
Chapter 16 respiratory emergencies Chapter 17 respiratory system workbook answers
Chapter 17 respiratory system workbook answers The child with a respiratory disorder chapter 25
The child with a respiratory disorder chapter 25 Boron
Boron Thermal expansion and contraction examples
Thermal expansion and contraction examples Solids liquids and gases section 2 properties of fluids
Solids liquids and gases section 2 properties of fluids Venn diagram about solid liquid and gas
Venn diagram about solid liquid and gas What are the properties of solids
What are the properties of solids Molecular theory of gases and liquids
Molecular theory of gases and liquids Solid liquid gas examples
Solid liquid gas examples 14.4 gases: mixtures and movements answers
14.4 gases: mixtures and movements answers Lesson outline lesson 1 solids liquids and gases answer key
Lesson outline lesson 1 solids liquids and gases answer key Red liquid element
Red liquid element Unit 24 cutting with oxyfuels and other gases
Unit 24 cutting with oxyfuels and other gases Solid liquid and gases
Solid liquid and gases How does sound travel through solids liquids and gases
How does sound travel through solids liquids and gases Matter and its composition
Matter and its composition Motion of particles in solids, liquids and gases
Motion of particles in solids, liquids and gases Spirogram diagram
Spirogram diagram Upper and lower respiratory system
Upper and lower respiratory system Circulatory system and respiratory system work together
Circulatory system and respiratory system work together Site:slidetodoc.com
Site:slidetodoc.com Stress and respiratory system
Stress and respiratory system Identify the cell
Identify the cell Alveolar ducts
Alveolar ducts Compensated respiratory acidosis
Compensated respiratory acidosis Human respiratory system diagram
Human respiratory system diagram Eficiencia de carnot
Eficiencia de carnot Periodic table metals nonmetals metalloids noble gases
Periodic table metals nonmetals metalloids noble gases Gases on the periodic table
Gases on the periodic table Greenhouse gases are good or bad
Greenhouse gases are good or bad Characteristics of gases
Characteristics of gases The atmosphere is a mixture of
The atmosphere is a mixture of Gas stoichiometry
Gas stoichiometry The actual exchange of gases occurs at the site of the *
The actual exchange of gases occurs at the site of the * Degree of freedom of gases
Degree of freedom of gases 2ª série
2ª série Lei de dalton
Lei de dalton Gases have low densities
Gases have low densities Mendeleev
Mendeleev Noble gas configuration for zinc
Noble gas configuration for zinc Fraccion molar gases
Fraccion molar gases El cero absoluto
El cero absoluto Constante dos gases ideais
Constante dos gases ideais Objetivo de la ley de charles
Objetivo de la ley de charles Globo aerostatico ley de los gases
Globo aerostatico ley de los gases Constante r de los gases
Constante r de los gases Ecuación general de los gases
Ecuación general de los gases Ley de avogadro
Ley de avogadro Internal energy of real gas
Internal energy of real gas Kinetic molecular theory of gases
Kinetic molecular theory of gases Características de los gases nobles
Características de los gases nobles Camadas de ar
Camadas de ar