Chapter 10 Eternal Life Cell Immortalization and Tumorigenesis

- Slides: 53

Chapter 10 Eternal Life: Cell Immortalization and Tumorigenesis -10. 1 ~ 10. 9 - May 1, 8 & 15, 2007

10. 1 Normal cell populations register the number of cell generations separating them from their ancestors in the early embryo - Normal cells have a limited proliferative potential. - Cancer cells need to gain the ability to proliferate indefinitely – immortal. - The immortality is a critical component of the neoplastic growth program.

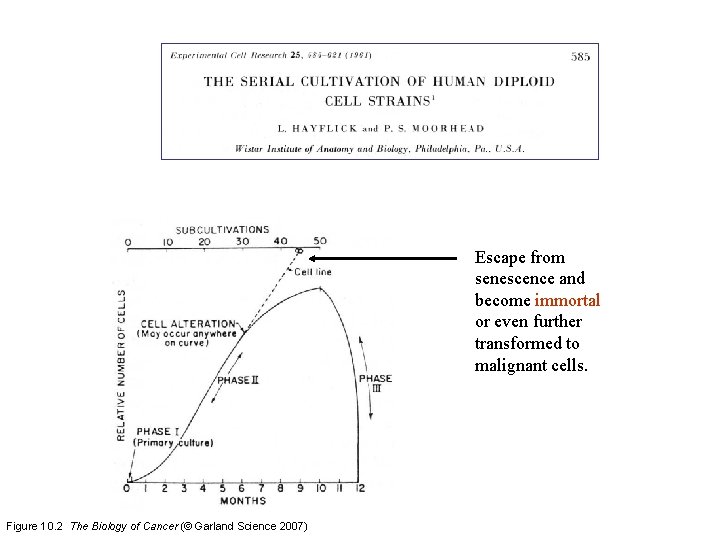
Escape from senescence and become immortal or even further transformed to malignant cells. Figure 10. 2 The Biology of Cancer (© Garland Science 2007)

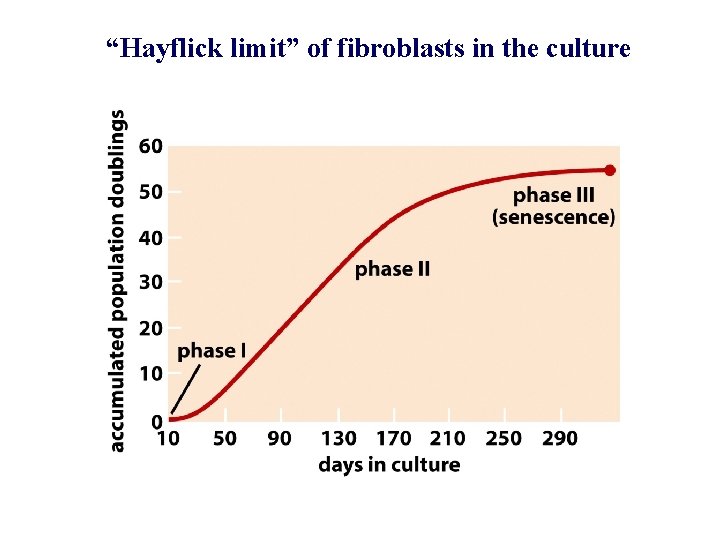
“Hayflick limit” of fibroblasts in the culture

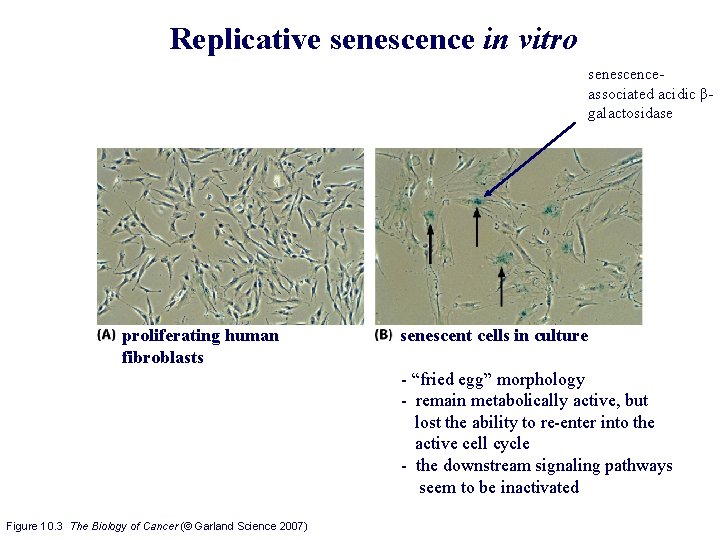
Replicative senescence in vitro senescenceassociated acidic βgalactosidase proliferating human fibroblasts senescent cells in culture - “fried egg” morphology - remain metabolically active, but lost the ability to re-enter into the active cell cycle - the downstream signaling pathways seem to be inactivated Figure 10. 3 The Biology of Cancer (© Garland Science 2007)

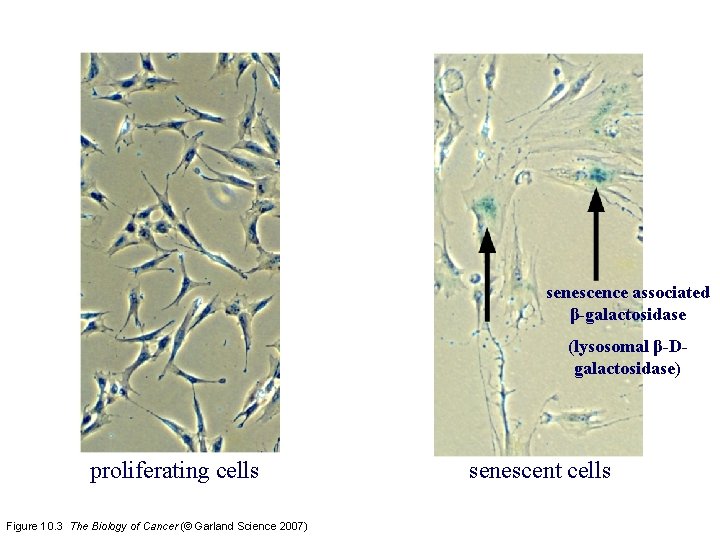
senescence associated β-galactosidase (lysosomal β-Dgalactosidase) proliferating cells Figure 10. 3 The Biology of Cancer (© Garland Science 2007) senescent cells

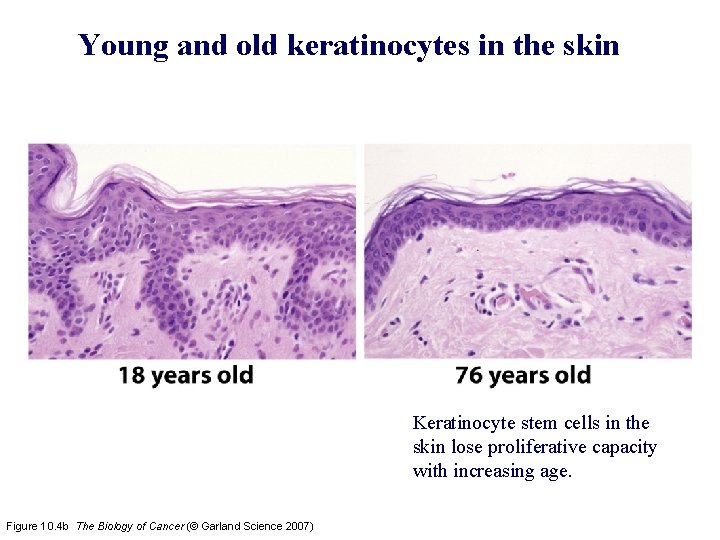
Young and old keratinocytes in the skin Keratinocyte stem cells in the skin lose proliferative capacity with increasing age. Figure 10. 4 b The Biology of Cancer (© Garland Science 2007)

Cancer cells and embryonic stem cells share some replicative properties - Embryonic stem (ES) cells show unlimited replicative potential in culture and are thus immortal. - The replicative behavior of cancer cells resembles that of ES cells. - Many types of cancer cells seem able to proliferate forever when provided with proper in vitro culture conditions. - He. La cells (Henrietta Lacks, 1951): - the 1 st human cell line and 1 st human cancer cell line established in culture - derived from the tissue of cervical adenocarcinoma

10. 2 & 3 Cells need to become immortal in order to form cancers Two regulatory mechanisms to govern the replicative capacity of cells: 1. senescence - Cumulative physiologic stress over extended periods of time halts further proliferation. These cells enter into a state of senescence. cumulation of oxidative damage contributes to senescence, e. g. , reactive oxygen species (ROS), DNA damage 2. crisis - Cells have used up the allowed “quota” of replicative doublings. These cells enter into a state of crisis, which leads to apoptosis.

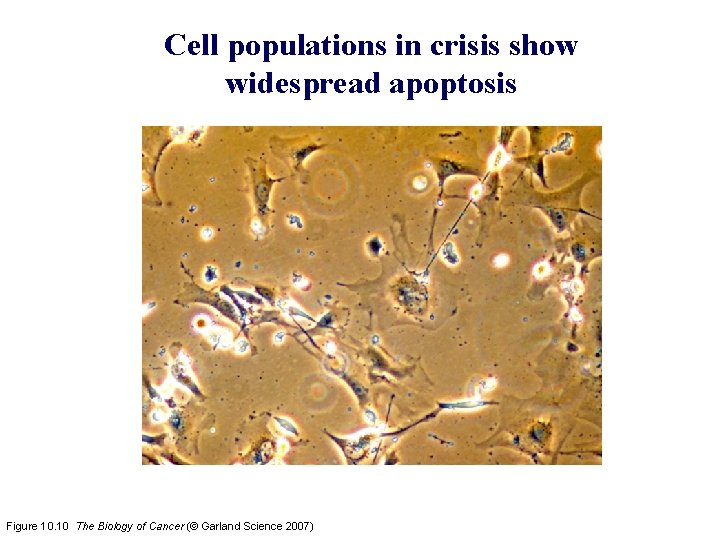
Cell populations in crisis show widespread apoptosis Figure 10. 10 The Biology of Cancer (© Garland Science 2007)

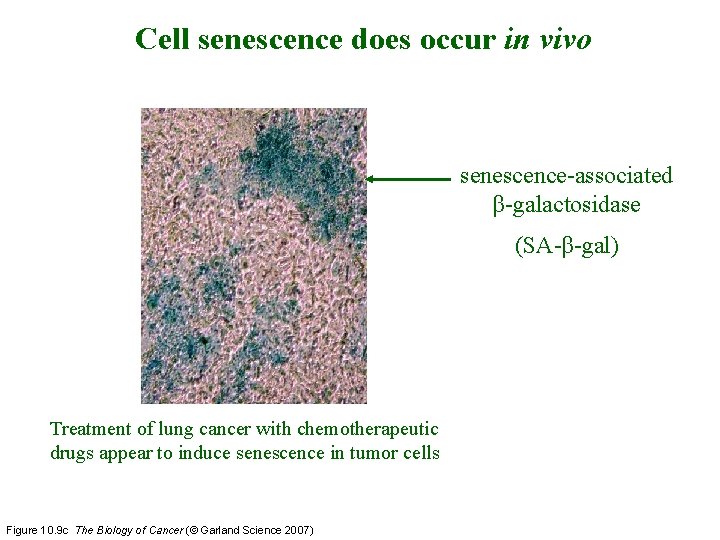
Cell senescence does occur in vivo senescence-associated β-galactosidase (SA-β-gal) Treatment of lung cancer with chemotherapeutic drugs appear to induce senescence in tumor cells Figure 10. 9 c The Biology of Cancer (© Garland Science 2007)

10. 4 The proliferation of cultured cells is limited by the telomeres of their chromosomes Barbara Mc. Clintoch discovered (1941) specialized structures at the ends of chromosomes, the telomeres, that protected chromosomes from end-to-end fusions. (She also demonstrated movable genetic elements in the corn genome, later called transposons. ) - Nobel prize in Physiology & Medicine in 1983

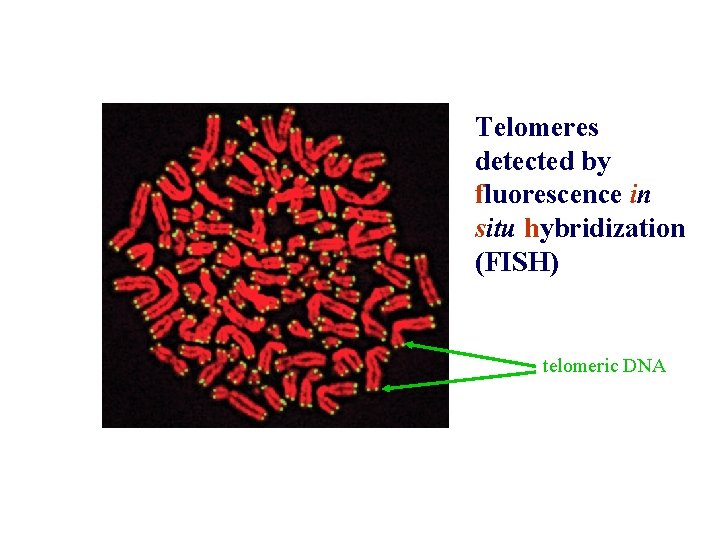
Telomeres detected by fluorescence in situ hybridization (FISH) telomeric DNA

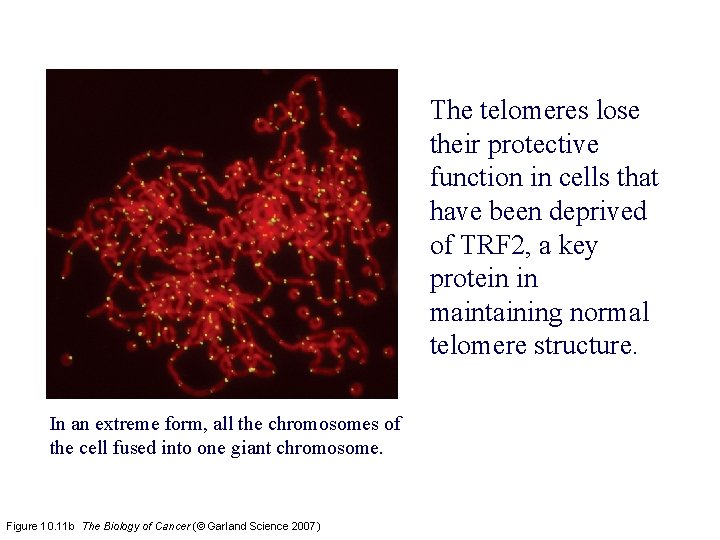
The telomeres lose their protective function in cells that have been deprived of TRF 2, a key protein in maintaining normal telomere structure. In an extreme form, all the chromosomes of the cell fused into one giant chromosome. Figure 10. 11 b The Biology of Cancer (© Garland Science 2007)

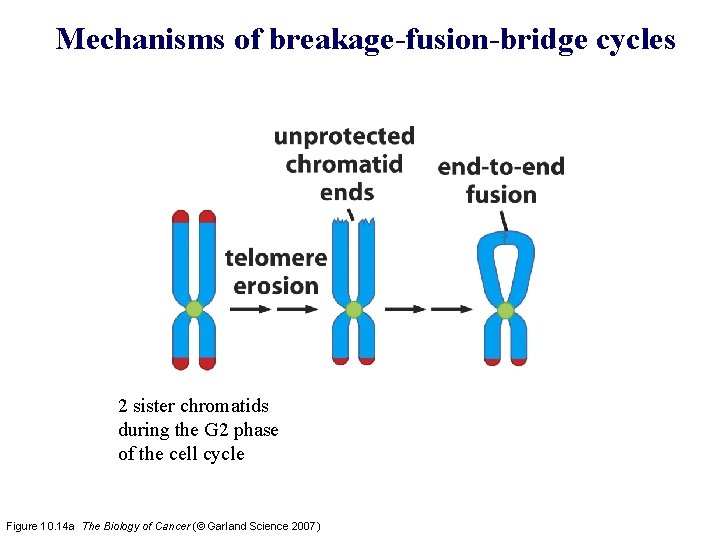
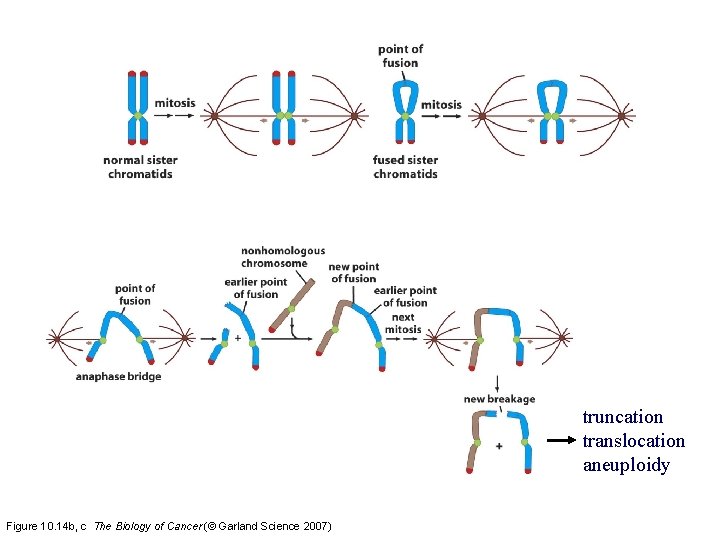
Mechanisms of breakage-fusion-bridge cycles 2 sister chromatids during the G 2 phase of the cell cycle Figure 10. 14 a The Biology of Cancer (© Garland Science 2007)

truncation translocation aneuploidy Figure 10. 14 b, c The Biology of Cancer (© Garland Science 2007)

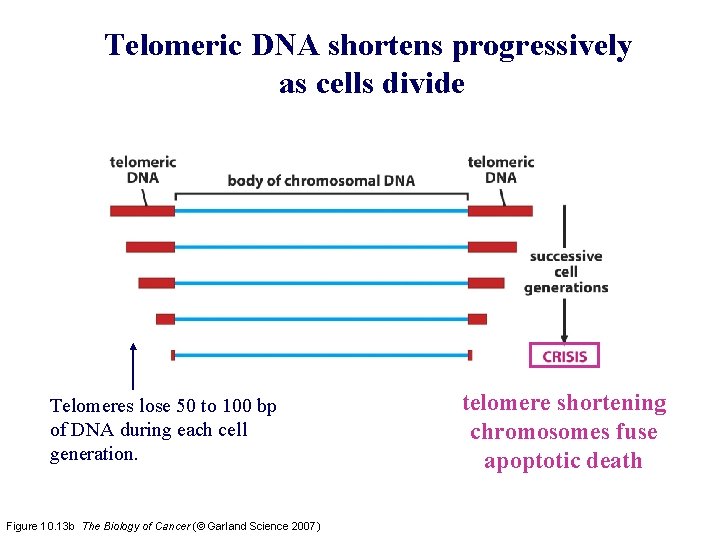
Telomeric DNA shortens progressively as cells divide Telomeres lose 50 to 100 bp of DNA during each cell generation. Figure 10. 13 b The Biology of Cancer (© Garland Science 2007) telomere shortening chromosomes fuse apoptotic death

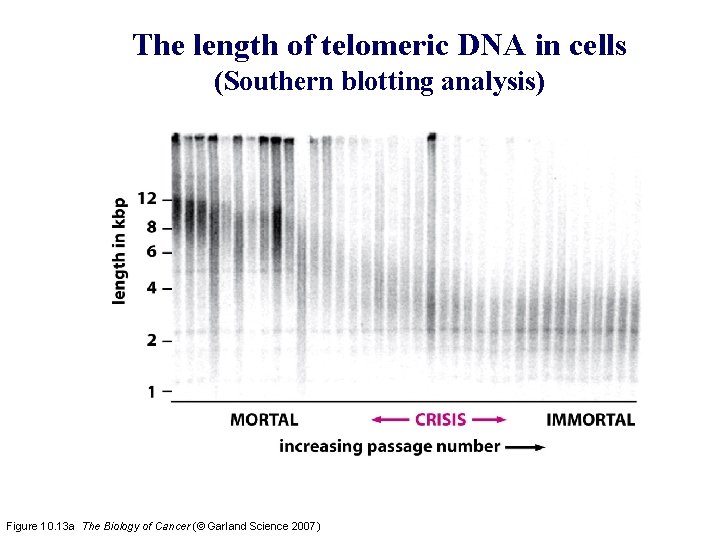
The length of telomeric DNA in cells (Southern blotting analysis) Figure 10. 13 a The Biology of Cancer (© Garland Science 2007)

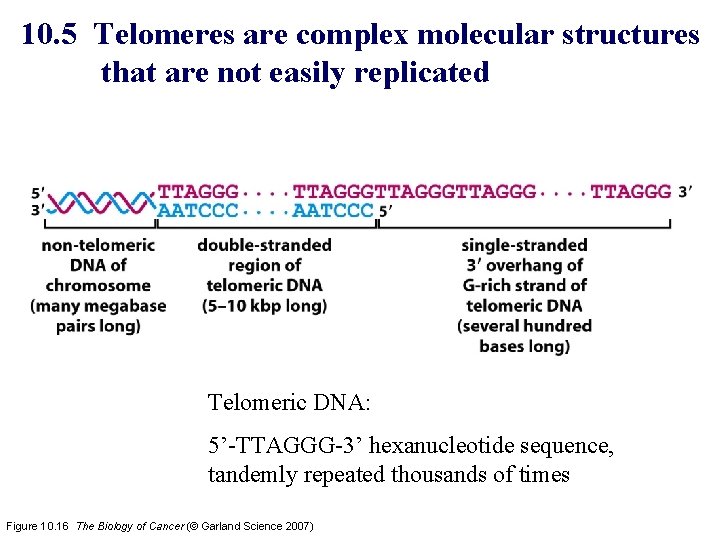
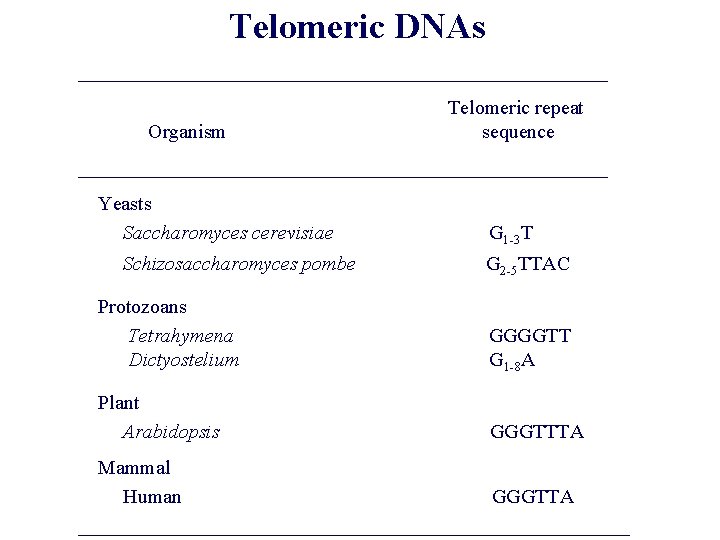
10. 5 Telomeres are complex molecular structures that are not easily replicated Telomeric DNA: 5’-TTAGGG-3’ hexanucleotide sequence, tandemly repeated thousands of times Figure 10. 16 The Biology of Cancer (© Garland Science 2007)

Telomeric DNAs ___________________________ Organism Telomeric repeat sequence ___________________________ Yeasts Saccharomyces cerevisiae Schizosaccharomyces pombe G 1 -3 T G 2 -5 TTAC Protozoans Tetrahymena Dictyostelium GGGGTT G 1 -8 A Plant Arabidopsis GGGTTTA Mammal Human GGGTTA _______________________

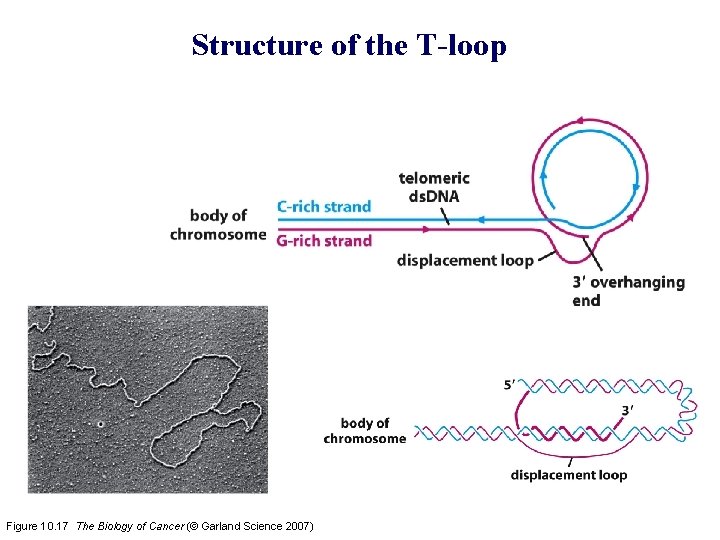
Structure of the T-loop Figure 10. 17 The Biology of Cancer (© Garland Science 2007)

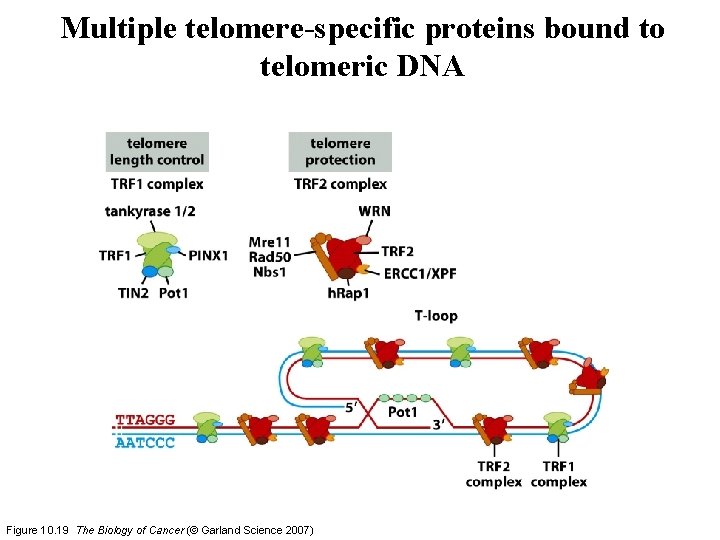
Multiple telomere-specific proteins bound to telomeric DNA Figure 10. 19 The Biology of Cancer (© Garland Science 2007)

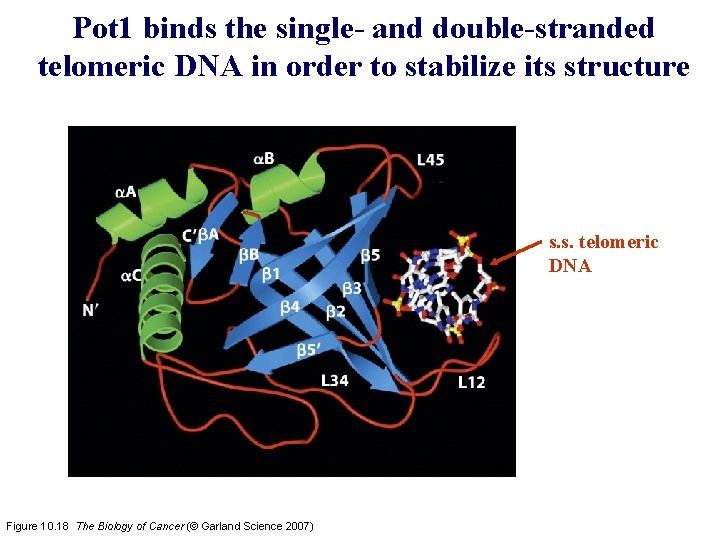
Pot 1 binds the single- and double-stranded telomeric DNA in order to stabilize its structure s. s. telomeric DNA Figure 10. 18 The Biology of Cancer (© Garland Science 2007)

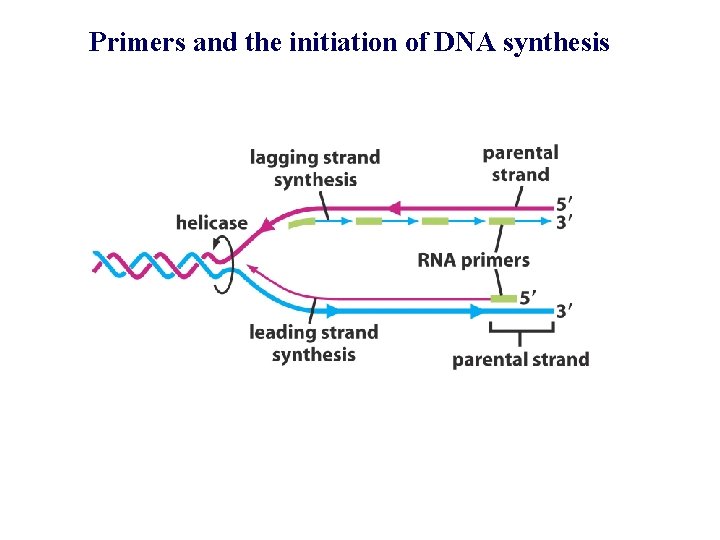
Primers and the initiation of DNA synthesis

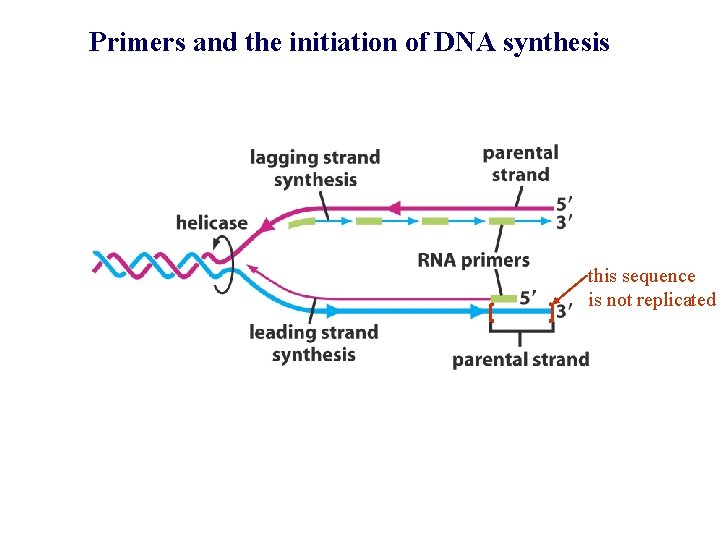
Primers and the initiation of DNA synthesis this sequence is not replicated

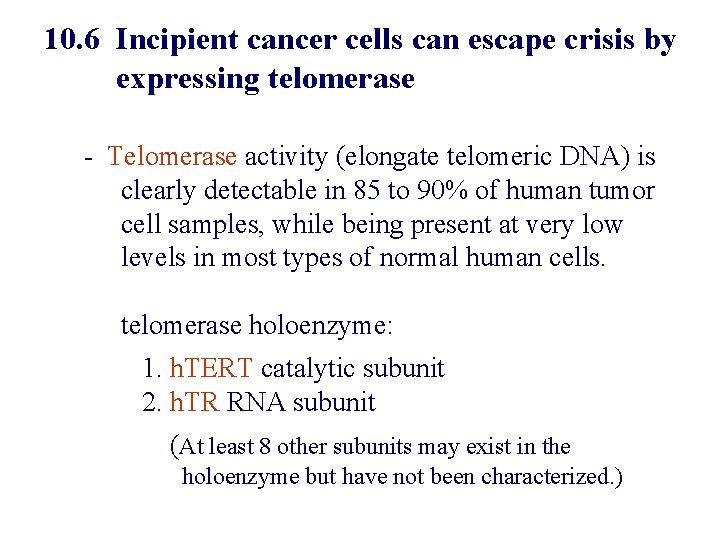
10. 6 Incipient cancer cells can escape crisis by expressing telomerase - Telomerase activity (elongate telomeric DNA) is clearly detectable in 85 to 90% of human tumor cell samples, while being present at very low levels in most types of normal human cells. telomerase holoenzyme: 1. h. TERT catalytic subunit 2. h. TR RNA subunit (At least 8 other subunits may exist in the holoenzyme but have not been characterized. )

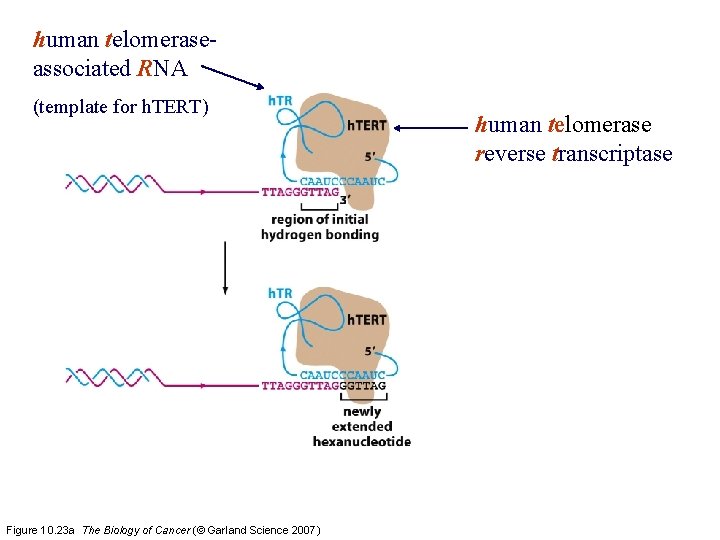
human telomeraseassociated RNA (template for h. TERT) Figure 10. 23 a The Biology of Cancer (© Garland Science 2007) human telomerase reverse transcriptase

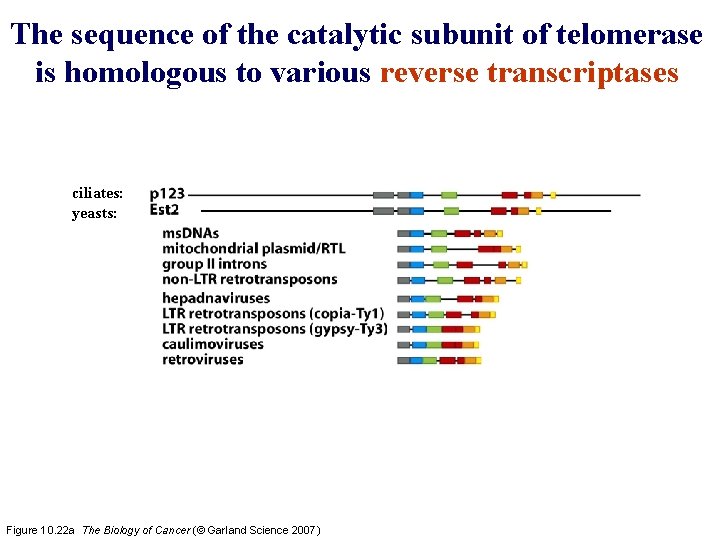
The sequence of the catalytic subunit of telomerase is homologous to various reverse transcriptases ciliates: yeasts: Figure 10. 22 a The Biology of Cancer (© Garland Science 2007)

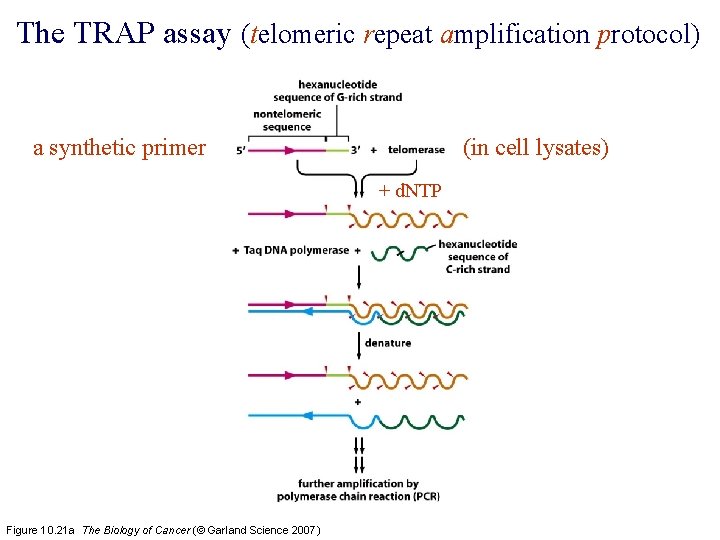
The TRAP assay (telomeric repeat amplification protocol) a synthetic primer (in cell lysates) + d. NTP Figure 10. 21 a The Biology of Cancer (© Garland Science 2007)

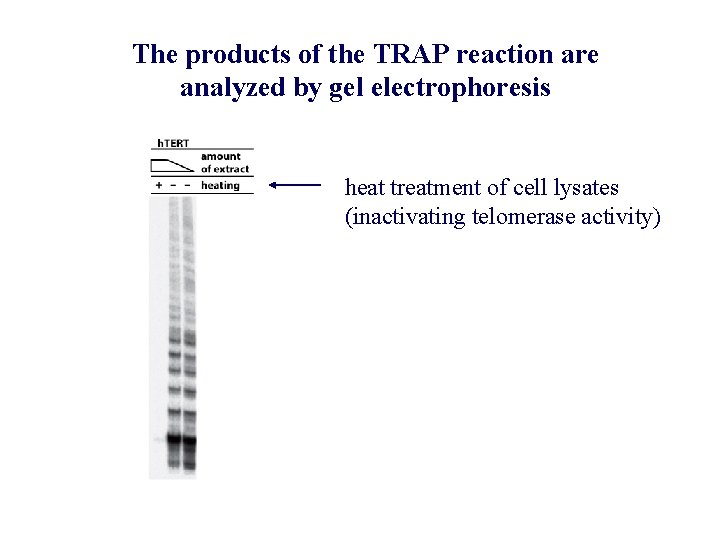
The products of the TRAP reaction are analyzed by gel electrophoresis heat treatment of cell lysates (inactivating telomerase activity)

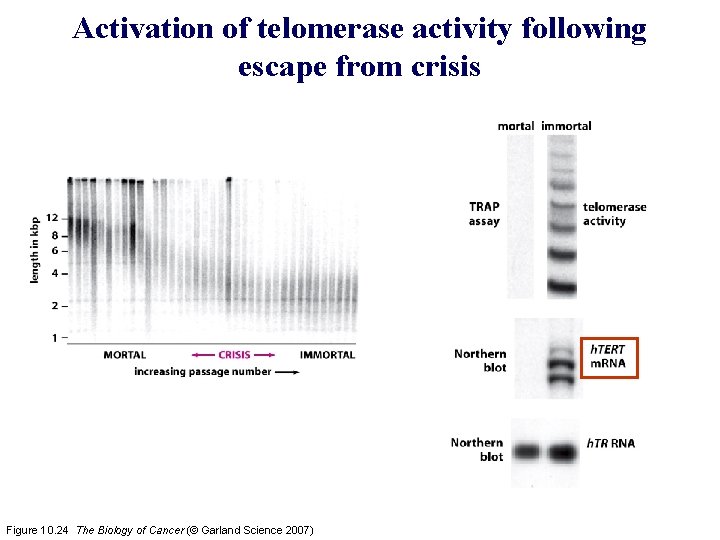
Activation of telomerase activity following escape from crisis Figure 10. 24 The Biology of Cancer (© Garland Science 2007)

Sidebar 10. 5 Oncoproteins and tumor suppressor proteins play critical roles in governing h. TERT expression - The mechanisms that lead to the de-repression of h. TERT transcription during tumor progression in humans are complex and still quite obscure. - Multiple transcription factors appear to collaborate to activate the h. TERT promoter. - For example, the Myc protein (Section 8. 9) and Menin (the product of the MEN 1 tumor suppressor gene), deregulate the cell clock.

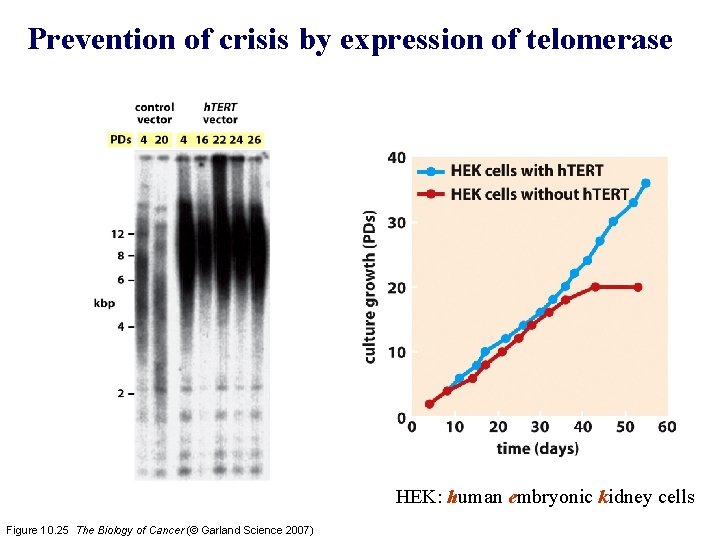
Prevention of crisis by expression of telomerase HEK: human embryonic kidney cells Figure 10. 25 The Biology of Cancer (© Garland Science 2007)

Sidebar 10. 6 The role of telomeres in replicative senescence - In cultured human fibroblasts, senescence can be postponed by expressing h. TERT prior to the expected time for entering replicative senescence. - However, senescence is also observed in cells that still possess quite long telomeres. Why?

Possible explanations: - When cells encounter cell-physiologic stress or the stress of tissue culture, telomeric DNA loses many of the single-stranded overhangs at the ends. The resulting degraded telomeric ends may release a DNA damage signal, thereby provoking a p 53 -mediated halt in cell proliferation that is manifested as the senescent growth state

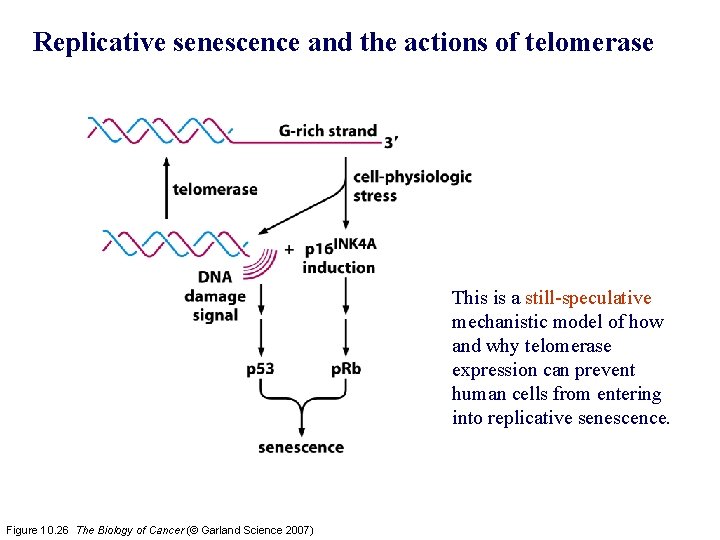
Replicative senescence and the actions of telomerase This is a still-speculative mechanistic model of how and why telomerase expression can prevent human cells from entering into replicative senescence. Figure 10. 26 The Biology of Cancer (© Garland Science 2007)

10. 7 Telomerase plays a key role in the proliferation of human cancer cells - Expression of antisense RNA in the telomerase (+) He. La cells causes them stop growing 23 to 26 days. - Expression of the dn (dominant negative) h. TERT subunit in telomerase (+) human tumor cell lines also causes them to lose all detectable telomerase activity and, with some delay, to enter crisis.

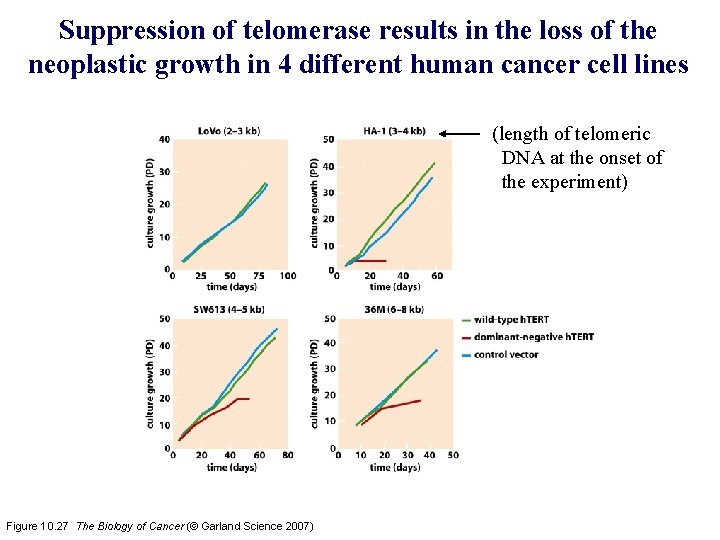
Suppression of telomerase results in the loss of the neoplastic growth in 4 different human cancer cell lines (length of telomeric DNA at the onset of the experiment) Figure 10. 27 The Biology of Cancer (© Garland Science 2007)

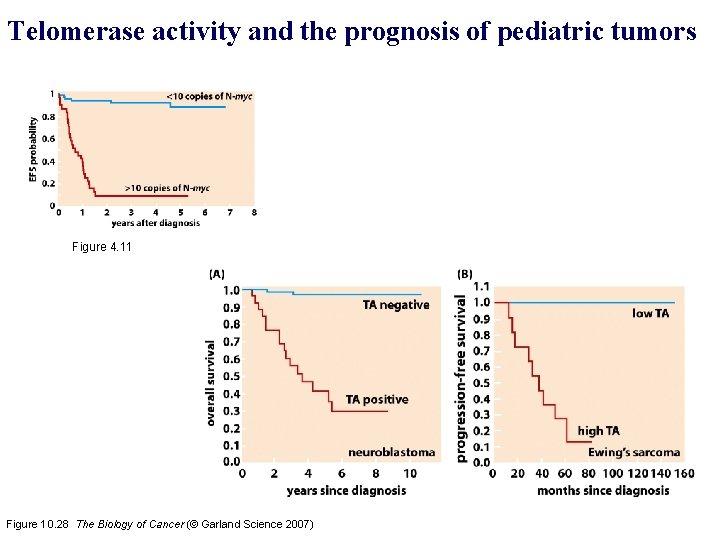
Telomerase activity and the prognosis of pediatric tumors Figure 4. 11 Figure 10. 28 The Biology of Cancer (© Garland Science 2007)

10. 8 Some immortalized cells can maintain telomeres without telomerase - 85 to 90% of human tumors have been found to be telomerase-positive. - The remaining 10 to 15% lack detectable telomerase activity, yet these cells need to maintain their telomeres above some minimum length in order to proliferate indefinitely. - These cells obtain the ability to maintain their telomeric DNA using a mechanism that does not depend on the actions of telomerase.

- In the yeast Saccharomyces cervisiae, following inactivation of genes encoding subunits of the telomerase holoenzyme, the vast majority of the cells enter a state of crisis and die. - Rare variants emerged from these populations of dying cells that used the alternative lengthening of telomerase (ALT) mechanism to construct and maintain their telomeres. - This ALT mechanism is also used by the minority of human tumor cells that lack significant telomerase activity, e. g. , 50% osteosarcomas and soft-tissue sarcomas and many glioblastomas.

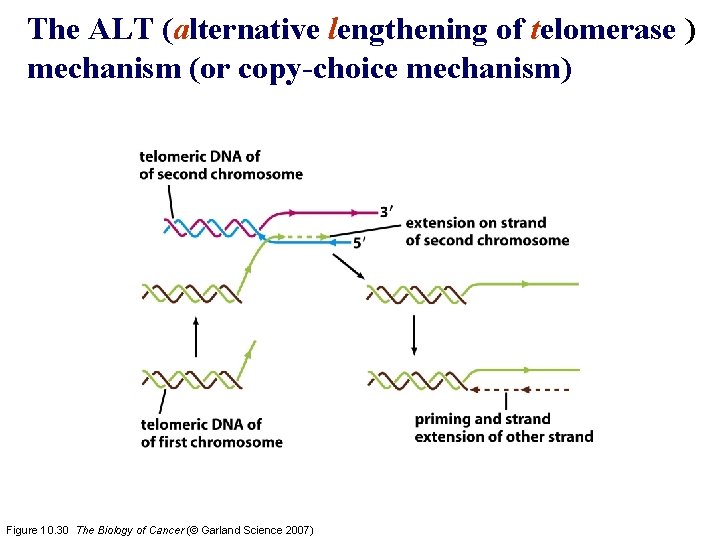
The ALT (alternative lengthening of telomerase ) mechanism (or copy-choice mechanism) Figure 10. 30 The Biology of Cancer (© Garland Science 2007)

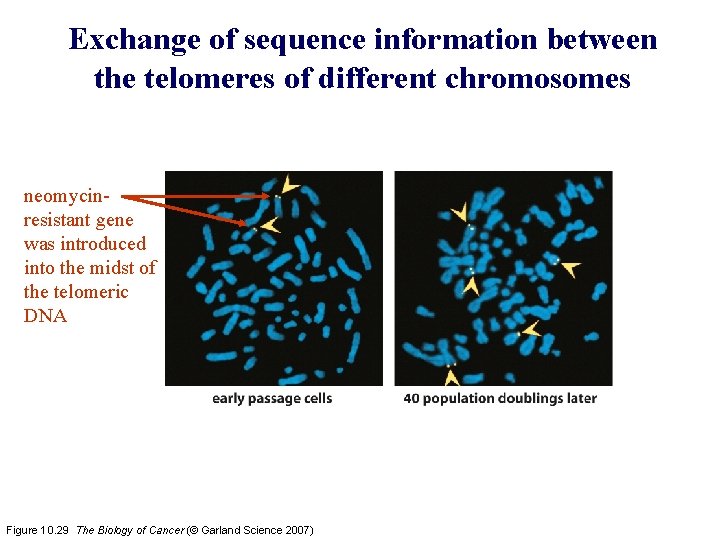
Exchange of sequence information between the telomeres of different chromosomes neomycinresistant gene was introduced into the midst of the telomeric DNA Figure 10. 29 The Biology of Cancer (© Garland Science 2007)

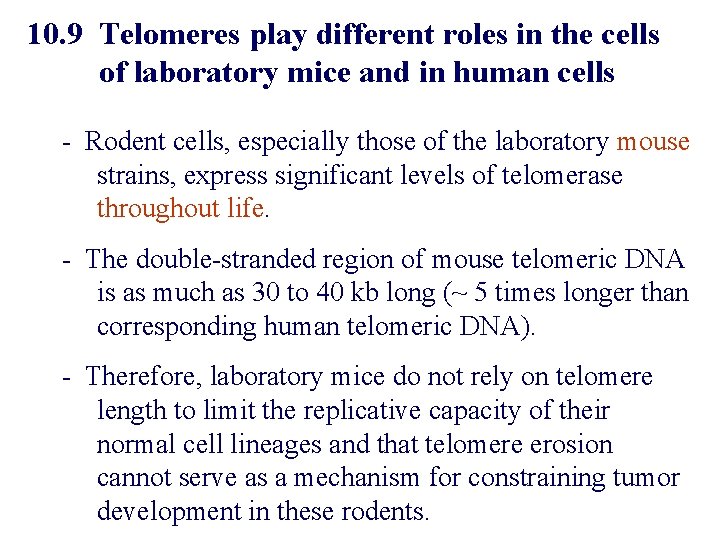
10. 9 Telomeres play different roles in the cells of laboratory mice and in human cells - Rodent cells, especially those of the laboratory mouse strains, express significant levels of telomerase throughout life. - The double-stranded region of mouse telomeric DNA is as much as 30 to 40 kb long (~ 5 times longer than corresponding human telomeric DNA). - Therefore, laboratory mice do not rely on telomere length to limit the replicative capacity of their normal cell lineages and that telomere erosion cannot serve as a mechanism for constraining tumor development in these rodents.

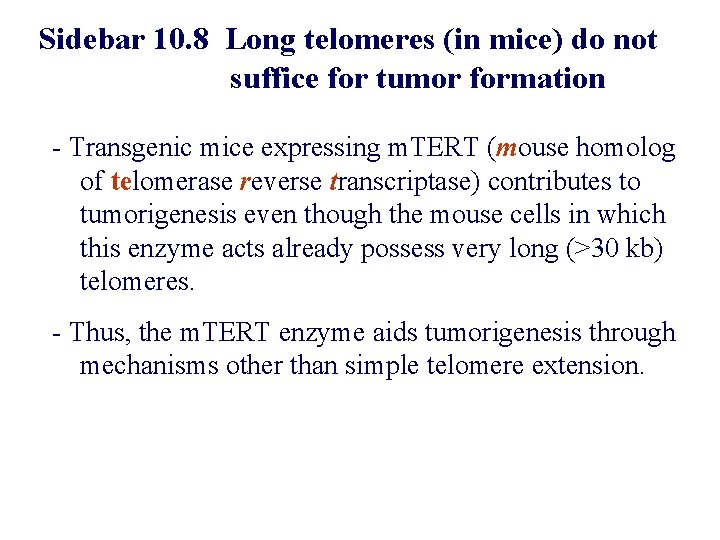
Sidebar 10. 8 Long telomeres (in mice) do not suffice for tumor formation - Transgenic mice expressing m. TERT (mouse homolog of telomerase reverse transcriptase) contributes to tumorigenesis even though the mouse cells in which this enzyme acts already possess very long (>30 kb) telomeres. - Thus, the m. TERT enzyme aids tumorigenesis through mechanisms other than simple telomere extension.

- Mouse cells can be immortalized relatively easily following extended propagation in culture. - Human cells require, instead, the introduction of both the SV 40 large T oncogene (to avoid senescence) and the h. TERT gene (to avoid crisis).

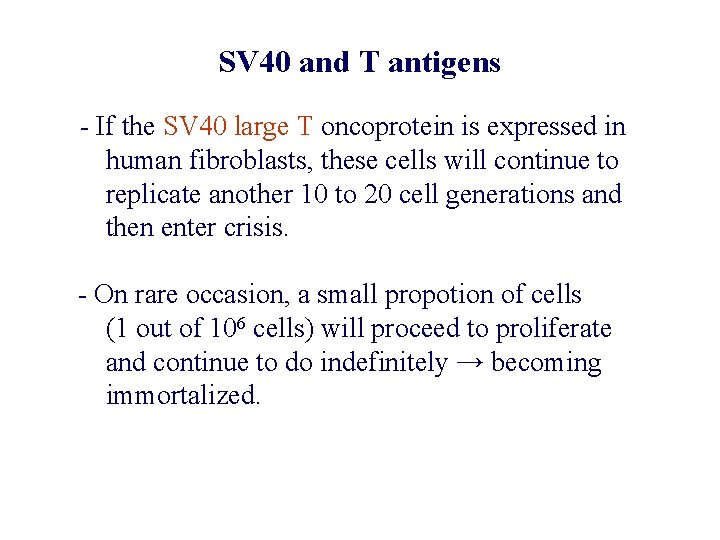
SV 40 and T antigens - If the SV 40 large T oncoprotein is expressed in human fibroblasts, these cells will continue to replicate another 10 to 20 cell generations and then enter crisis. - On rare occasion, a small propotion of cells (1 out of 106 cells) will proceed to proliferate and continue to do indefinitely → becoming immortalized.

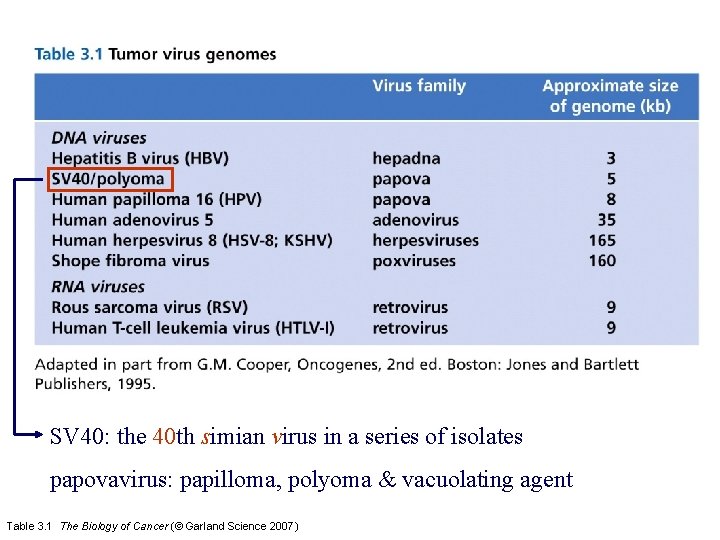
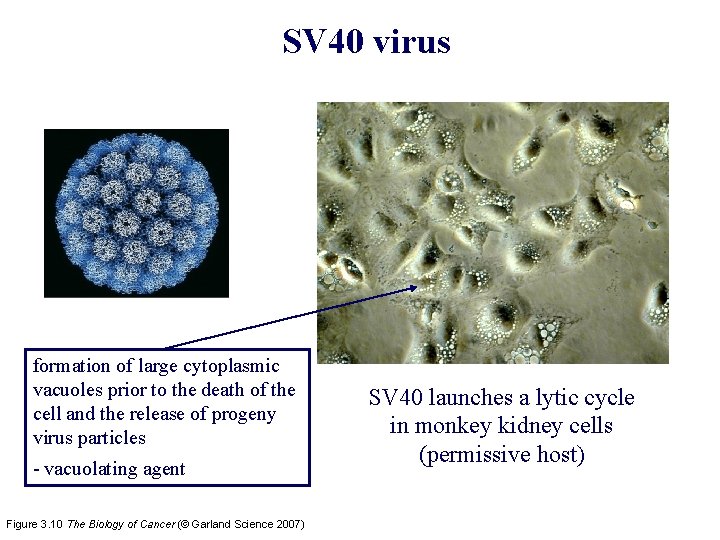
SV 40: the 40 th simian virus in a series of isolates papovavirus: papilloma, polyoma & vacuolating agent Table 3. 1 The Biology of Cancer (© Garland Science 2007)

SV 40 virus formation of large cytoplasmic vacuoles prior to the death of the cell and the release of progeny virus particles - vacuolating agent Figure 3. 10 The Biology of Cancer (© Garland Science 2007) SV 40 launches a lytic cycle in monkey kidney cells (permissive host)

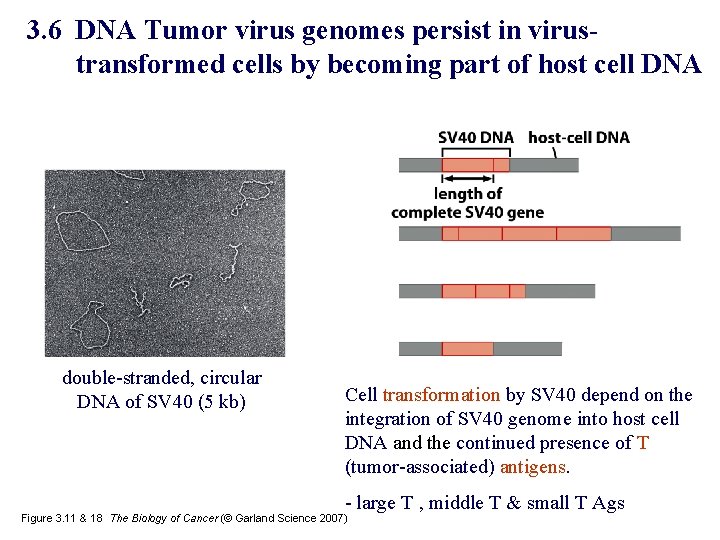
3. 6 DNA Tumor virus genomes persist in virustransformed cells by becoming part of host cell DNA double-stranded, circular DNA of SV 40 (5 kb) Cell transformation by SV 40 depend on the integration of SV 40 genome into host cell DNA and the continued presence of T (tumor-associated) antigens. - large T , middle T & small T Ags Figure 3. 11 & 18 The Biology of Cancer (© Garland Science 2007)

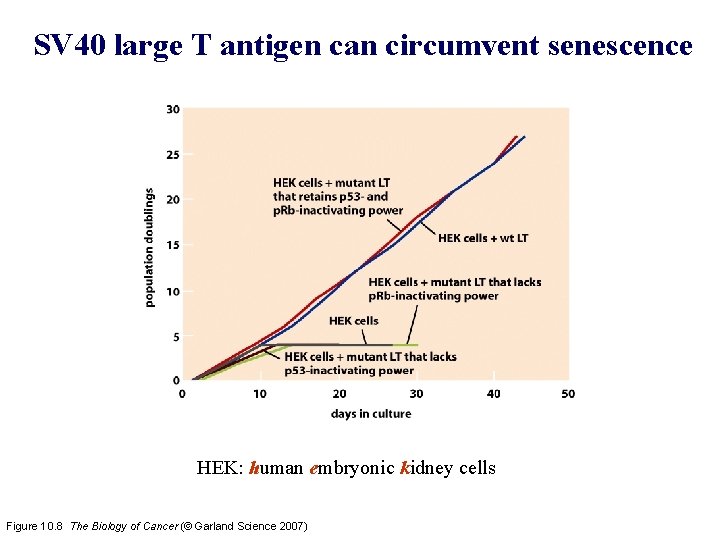
SV 40 large T antigen can circumvent senescence HEK: human embryonic kidney cells Figure 10. 8 The Biology of Cancer (© Garland Science 2007)

HEK 293 - HEK 293 cells were generated by transformation of normal human embryonic kidney (HEK) cells with adenovirus 5 DNA in the late 1977. - HEK 293 T cell line contains, in addition, the SV 40 large T antigen, that allows for episomal replication of transfected plasmids containing the SV 40 origin of replication. This allows for amplification of transfected plasmids and expression of the desired gene products.

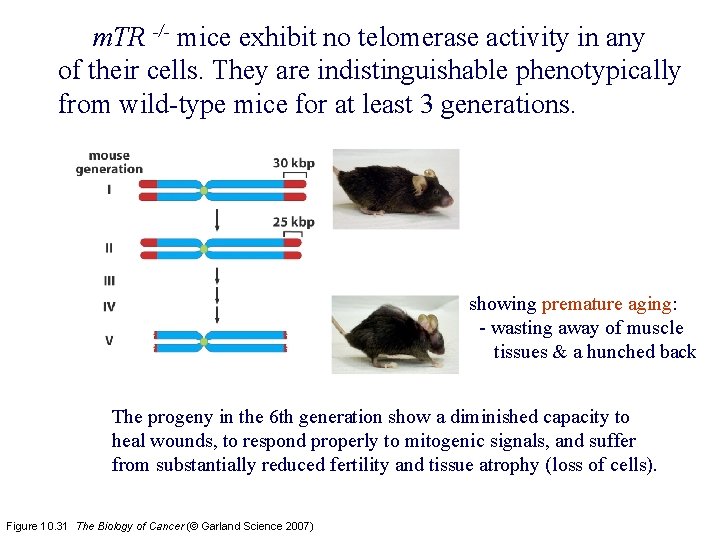
m. TR -/- mice exhibit no telomerase activity in any of their cells. They are indistinguishable phenotypically from wild-type mice for at least 3 generations. showing premature aging: - wasting away of muscle tissues & a hunched back The progeny in the 6 th generation show a diminished capacity to heal wounds, to respond properly to mitogenic signals, and suffer from substantially reduced fertility and tissue atrophy (loss of cells). Figure 10. 31 The Biology of Cancer (© Garland Science 2007)
 Multistep tumorigenesis
Multistep tumorigenesis Eternal life chapter 36
Eternal life chapter 36 Free to choose liberty and eternal life
Free to choose liberty and eternal life Eternal cycle of life
Eternal cycle of life Eternal life
Eternal life Eternal life
Eternal life The scientist mathias schleiden studied _______ in ______.
The scientist mathias schleiden studied _______ in ______. Our country sinks beneath the yoke
Our country sinks beneath the yoke Eternal covenant 20
Eternal covenant 20 Eternal creator
Eternal creator God's law is eternal
God's law is eternal Acquiring spiritual knowledge part 1
Acquiring spiritual knowledge part 1 Eternal ideas to behavioral actions
Eternal ideas to behavioral actions The eternal jew youtube
The eternal jew youtube Eternal weight of glory
Eternal weight of glory Eternal german
Eternal german Eternal german
Eternal german Athena zeus
Athena zeus Kitaro eternal spring
Kitaro eternal spring What is virtue ethics
What is virtue ethics God's eternal plan drawing
God's eternal plan drawing The eternal god is our refuge
The eternal god is our refuge Extended metaphor poem examples
Extended metaphor poem examples At the going down of the sun poem
At the going down of the sun poem How does thanatopsis reflect romanticism
How does thanatopsis reflect romanticism Eternal love wedding
Eternal love wedding The eternal jew poster
The eternal jew poster The eternal jew propaganda
The eternal jew propaganda Enkratic
Enkratic Campus martius
Campus martius Eternal inflation
Eternal inflation Developing an eternal perspective
Developing an eternal perspective Advantages of diaphragm cell
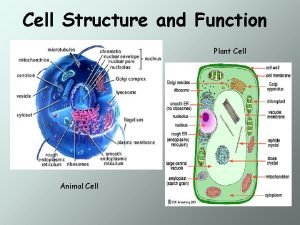
Advantages of diaphragm cell Prokaryotic and eukaryotic cells
Prokaryotic and eukaryotic cells Animal vs plant cell venn diagram
Animal vs plant cell venn diagram Plant cell and animal cell diagram
Plant cell and animal cell diagram Structure of a plant cell
Structure of a plant cell Primary and secondary cells
Primary and secondary cells Difference between bacteria and plant cell
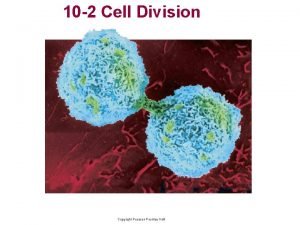
Difference between bacteria and plant cell Cell cycle and cell division
Cell cycle and cell division Life
Life Idealized animal cell and plant cell
Idealized animal cell and plant cell Walker cell and hadley cell
Walker cell and hadley cell Cell cycle and cell division
Cell cycle and cell division Venn diagram for animal and plant cells
Venn diagram for animal and plant cells Steps of cell cycle
Steps of cell cycle Chapter 21 electrochemistry
Chapter 21 electrochemistry What is the gooey liquid in plant and animal cells
What is the gooey liquid in plant and animal cells Volvox diagram
Volvox diagram Site:slidetodoc.com
Site:slidetodoc.com Ecell vs log cu2+
Ecell vs log cu2+ Dry cell vs wet cell
Dry cell vs wet cell What is the function of a cell
What is the function of a cell Cell wall vs cell membrane
Cell wall vs cell membrane