Chapter 10 Chemical Reactions Section 10 1 Reactions











- Slides: 18

Chapter 10 Chemical Reactions

Section 10. 1 Reactions and Equations

Section 10. 1 Objectives n Recognize evidence of chemical change n Represent chemical reactions with equations

Key Terms Chemical reaction n Reactant n Product n Chemical equations n coefficient n

Chemical Reactions n n n Atoms of one or more substances are rearranged to form different substances Another name= chemical change Important part of everyday life

Evidence of a Reaction n How to tell if a chemical reaction has taken place – Temperature change – Color change – Odor – Gas bubbles

Representing Chemical Reactions n Reactants: the starting substances n Products: resulting or ending substances n Arrow: “react to produce” or “yield” n Reactant 1 + reactant 2 product 1 + product 2

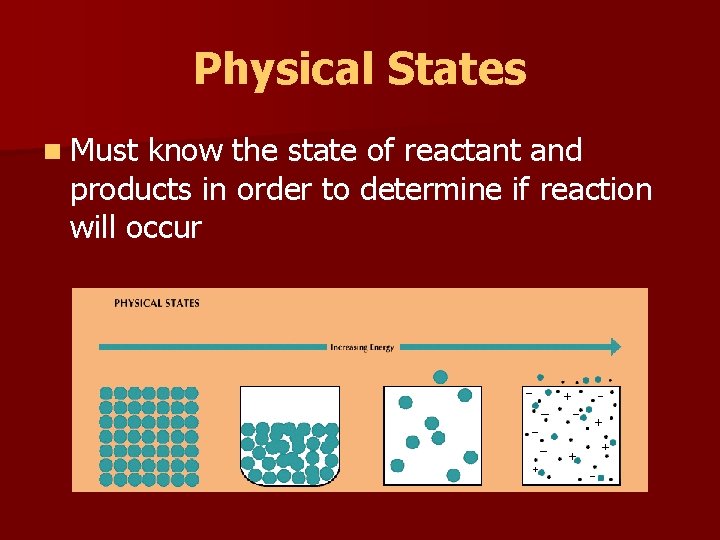
Symbols used in Equations n Table 10. 1 on page 278 n Why do these symbols mean? § (g) § § (s) § (aq) §+ § (l)

Physical States n Must know the state of reactant and products in order to determine if reaction will occur

Types of Equations n Three types of equations – Word – Skeleton – Chemical

Word Equations n Used to indicate the reactants and products of chemical reactions n Uses the actual names of the substances involved n Setup: Reactant 1 + Reactant 2 Product 1 Ex: Iron(s) + Chlorine(g) Iron(III) Chloride(s)

Skeleton Equations n Uses chemical formulas to represent the substances unlike the word equation n Chemical formulas take the place of the words n Iron(s) + Chlorine(g) Iron(III) Chloride(s) would become: Fe(s) + Cl 2(g) Fe Cl 3(s)

Practice Problems n Page 279

Chemical Equations n Uses chemical formulas like the skeleton n Shows matter is conserved during a reaction (Law of Conservation of Mass) n Uses identities and relative amounts of the substances involved in a chemical reaction n Everything is balanced in a chemical equation

Balancing Chemical Equations – Find correct coefficients for chemical formulas § Coefficient is usually a whole number – 1 is understood- not written

Steps to Balance a Chemical Equation n n n 1: Write the skeleton equation 2: Count the atoms of elements in the reactants 3: Count the atoms of elements in the products 4: Change the coefficients to make both sides of the equation the equal 5: Write the coefficient(s) in the lowest ratio possible 6: Check your work – Ex: 2 H 2(g) + O 2(g) 2 H 2 O(g)

Practice Problems n Page 282

Homework n 7 -13 on page 283