Chapter 10 Chemical Equations l A chemical reaction





















- Slides: 26

Chapter 10 Chemical Equations

l A chemical reaction is a reaction in which at least one new substance is formed. l Example – Hydrogen and oxygen react to form water.

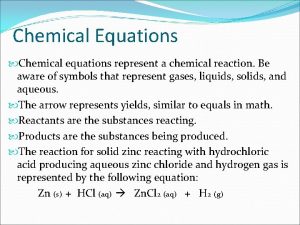
l A chemical equation is a shorthand method of writing a chemical reaction using chemical symbols and formulas. l Example – H 2 + O 2 H 2 O

Symbols Used in Equations (g) – gas l (cr) – crystalline solid l (l) – liquid l (aq) – aqueous solution l

Law of Conservation of Mass l In an ordinary chemical reaction, mass can neither be created nor destroyed. l Mass of products must equal the mass of the reactants.

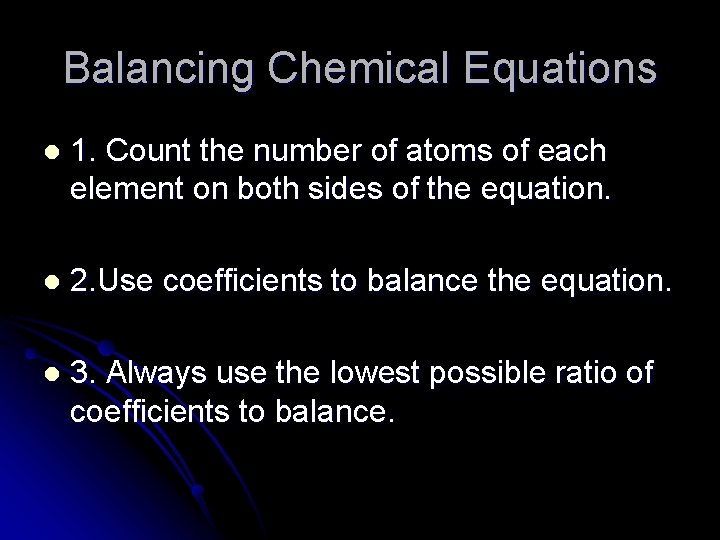
Balancing Chemical Equations l 1. Count the number of atoms of each element on both sides of the equation. l 2. Use coefficients to balance the equation. l 3. Always use the lowest possible ratio of coefficients to balance.

Examples

Practice Website l www. chem. vt. edu/RVGS/ACT/notes/script s/bal_eq 1. html

Balancing NO-NO’s l Coefficients can only be added BEFORE a chemical symbol or formula. l NEVER change a subscript to balance an equation.

Signs a Chemical Reaction Has Occurred: Color change l Liberation of a gas l Energy change l Formation of a precipitate l Change in odor l


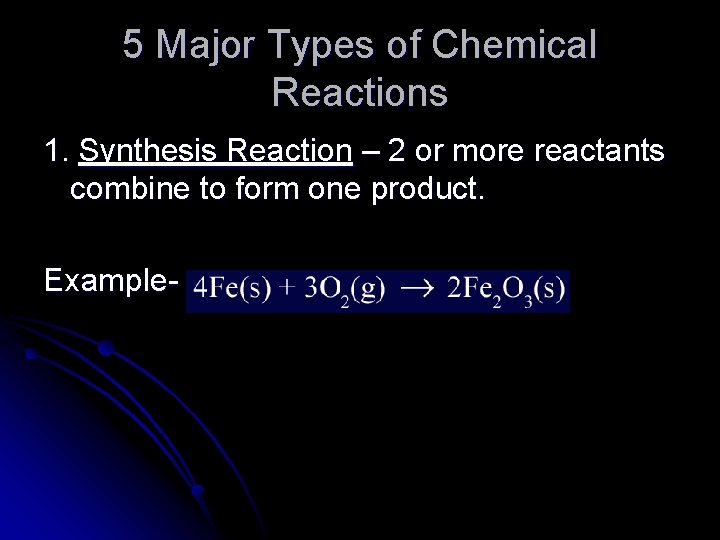
5 Major Types of Chemical Reactions 1. Synthesis Reaction – 2 or more reactants combine to form one product. Example-

Rust is the product of a synthesis reaction.

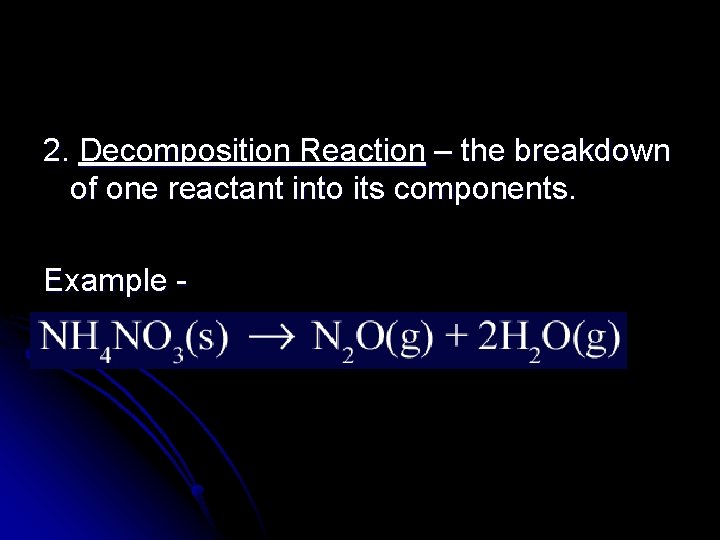
2. Decomposition Reaction – the breakdown of one reactant into its components. Example -

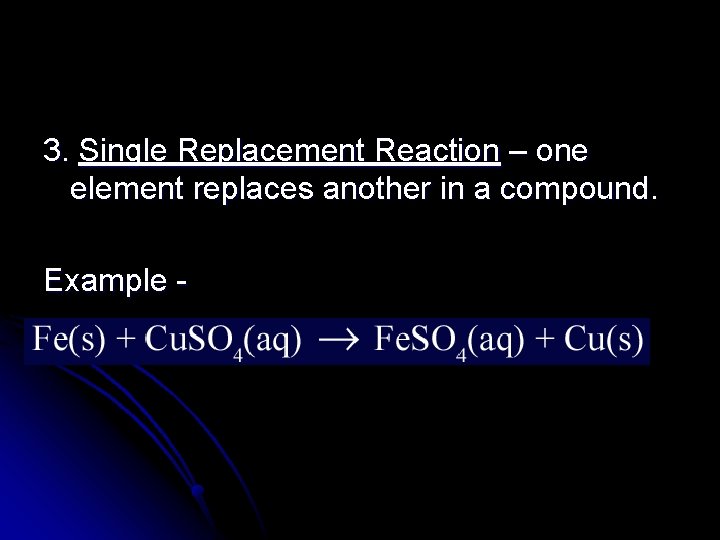
3. Single Replacement Reaction – one element replaces another in a compound. Example -

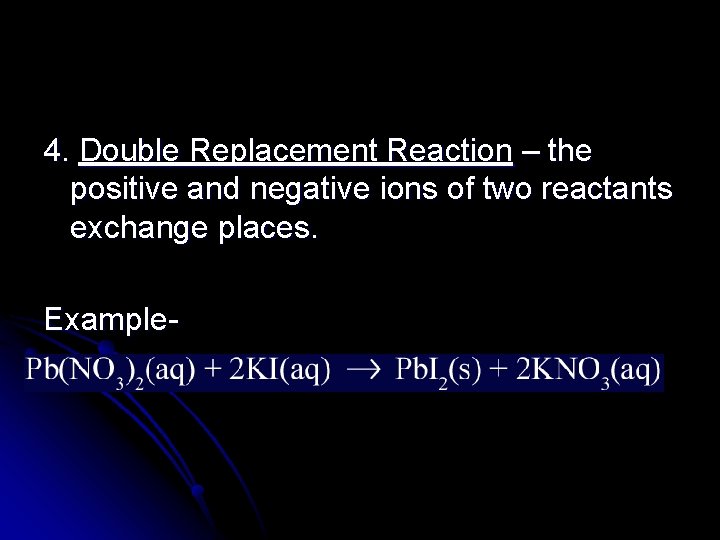
4. Double Replacement Reaction – the positive and negative ions of two reactants exchange places. Example-

Many times a precipitate is the result of a double replacement reaction.

5. Combustion – the burning of an organic compound in oxygen to form CO 2 and H 2 O and energy. Example -

Welding is an example of a combustion reaction.

Practice – Classifying Reactions 1. 2. 3. 4. Cl 2 + 2 KBr KCl + Br 2 2 Ag 2 O 4 Ag + O 2 2 Na + Cl 2 2 Na. Cl Pb. Cl 2 + Li 2 SO 4 Pb. SO 4 + 2 Li. Cl

Activote Quiz Ca. O + H 2 O Ca(OH)2 This equation is an example of which type of chemical reaction: a. Decomposition b. Synthesis c. Combustion d. Single Replacement

LAB – Chemical Reactions


Writing Chemical Equations 7 Diatomic elements Chlorine Fluorine Iodine Bromine Oxygen Nitrogen Hydrogen

Common Acids Sulfuric acid l Nitric acid l Carbonic acid l Hydrochloric acid l Acetic acid l Phosphoric acid l

 Are kc and kp equal
Are kc and kp equal Difference between nuclear reaction and chemical reaction
Difference between nuclear reaction and chemical reaction Translate word equations to chemical equations
Translate word equations to chemical equations Chemical equations and reactions chapter 8 review
Chemical equations and reactions chapter 8 review Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Chemical equations and reactions chapter 8
Chemical equations and reactions chapter 8 Synthesis reaction
Synthesis reaction Reaction order
Reaction order Ictahedron
Ictahedron Leukoerythroblastic reaction vs leukemoid reaction
Leukoerythroblastic reaction vs leukemoid reaction Empirical formula and molecular formula pogil
Empirical formula and molecular formula pogil Love formula in chemistry
Love formula in chemistry Rectangular equations to polar equations
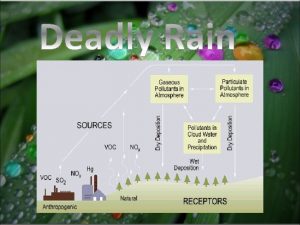
Rectangular equations to polar equations Conclusion of acid rain
Conclusion of acid rain Word equation examples
Word equation examples Tyoes of chemical reactions
Tyoes of chemical reactions Breathalyzer redox reaction
Breathalyzer redox reaction In a chemical reaction, stoichiometry refers to
In a chemical reaction, stoichiometry refers to Stability measurement instruments
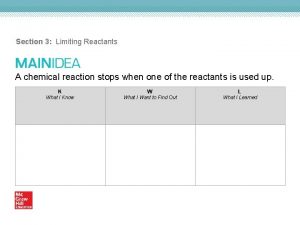
Stability measurement instruments What is used up in and stops a chemical reaction
What is used up in and stops a chemical reaction Which chemical reaction switches 2 elements
Which chemical reaction switches 2 elements Percent yield efficiency
Percent yield efficiency Reduction of pyrrole
Reduction of pyrrole Rancidity chemical reaction
Rancidity chemical reaction Factors affecting rate of chemical reaction
Factors affecting rate of chemical reaction Phosphorus oxygen reaction
Phosphorus oxygen reaction Incomplete combustion of methane
Incomplete combustion of methane