Chapter 10 Chemical bonding Molecular shapes valence bond

Chapter 10 Chemical bonding: Molecular shapes, valence bond theory, and molecular orbital theory
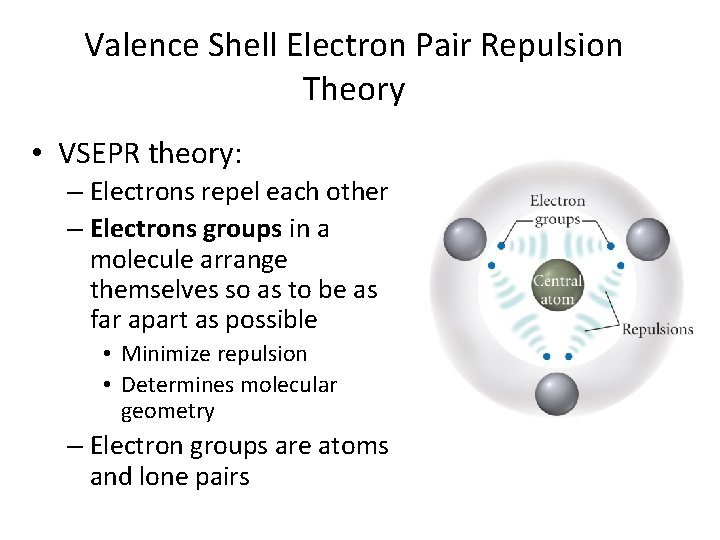
Valence Shell Electron Pair Repulsion Theory • VSEPR theory: – Electrons repel each other – Electrons groups in a molecule arrange themselves so as to be as far apart as possible • Minimize repulsion • Determines molecular geometry – Electron groups are atoms and lone pairs
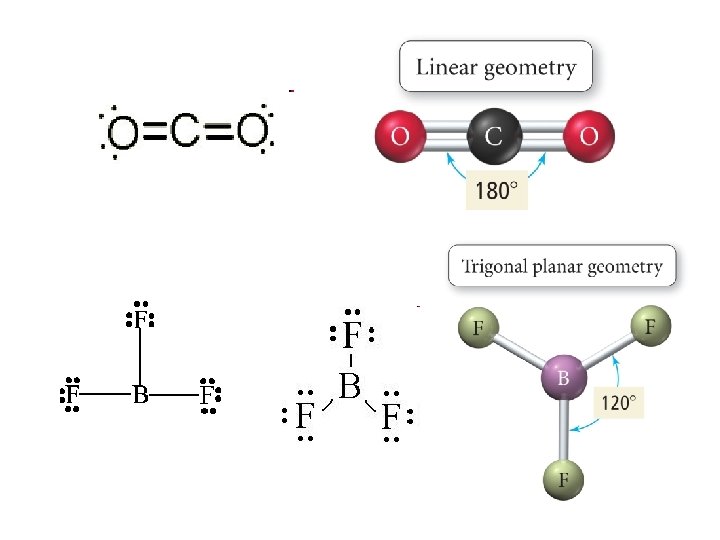
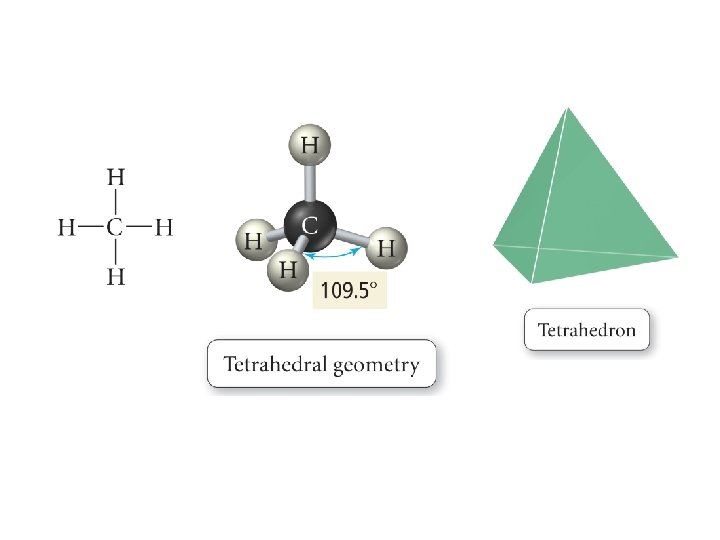
Defining Molecular Shape • Electron pair geometry: the geometrical arrangement of electron groups around a central atom – Atoms and lone pairs count as electron groups • Molecular Geometry: the geometrical arrangement of atoms around a central atom – Ignore lone pair electrons
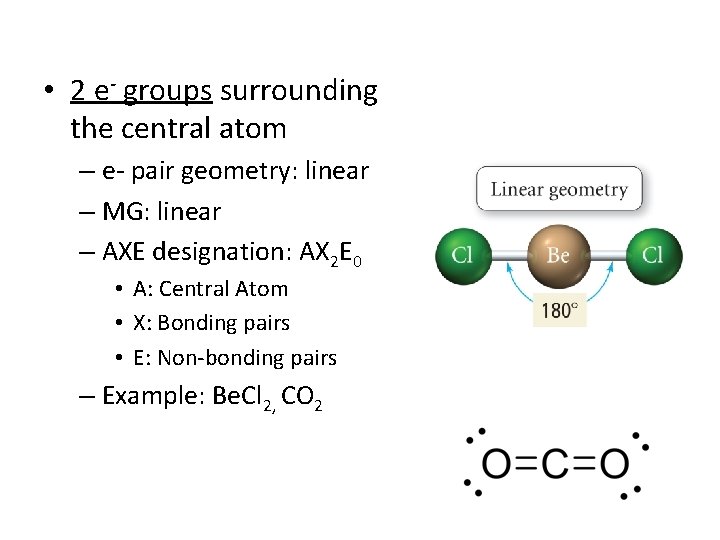
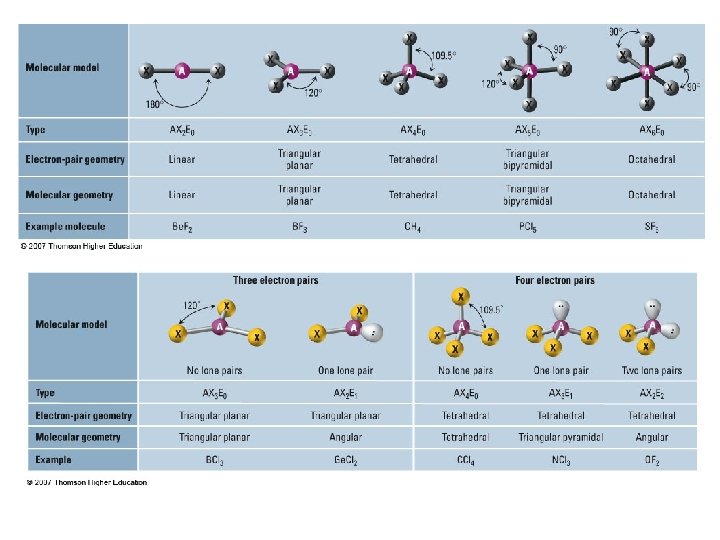
• 2 e- groups surrounding the central atom – e- pair geometry: linear – MG: linear – AXE designation: AX 2 E 0 • A: Central Atom • X: Bonding pairs • E: Non-bonding pairs – Example: Be. Cl 2, CO 2
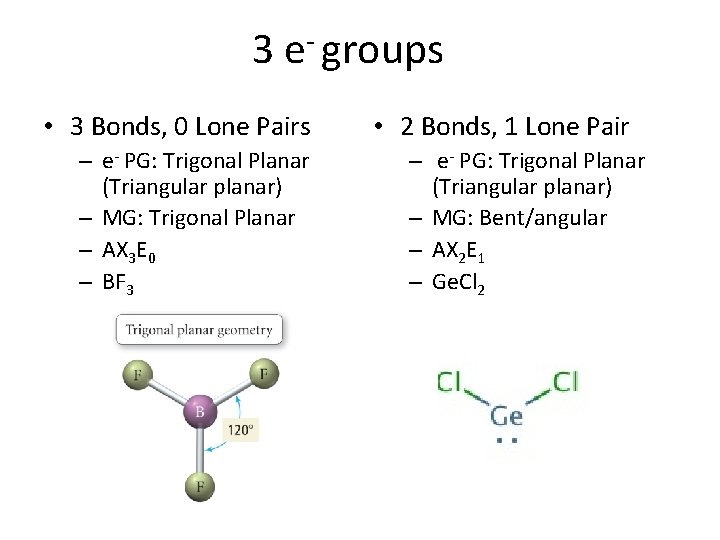
3 e- groups • 3 Bonds, 0 Lone Pairs – e- PG: Trigonal Planar (Triangular planar) – MG: Trigonal Planar – AX 3 E 0 – BF 3 • 2 Bonds, 1 Lone Pair – e- PG: Trigonal Planar (Triangular planar) – MG: Bent/angular – AX 2 E 1 – Ge. Cl 2
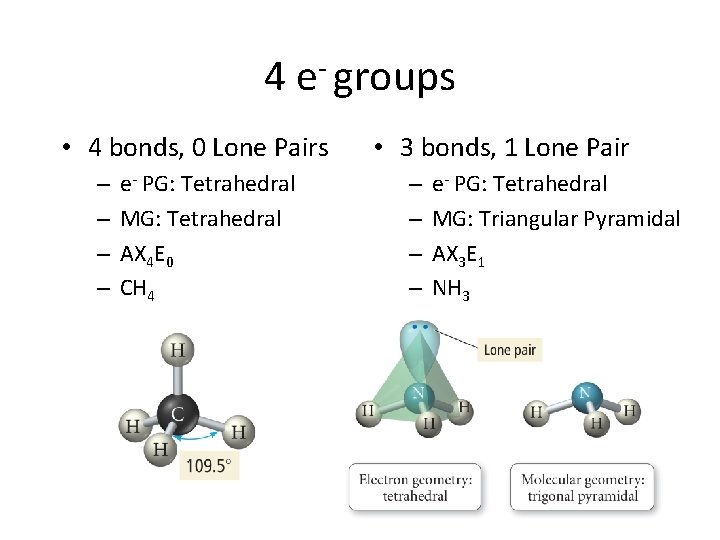
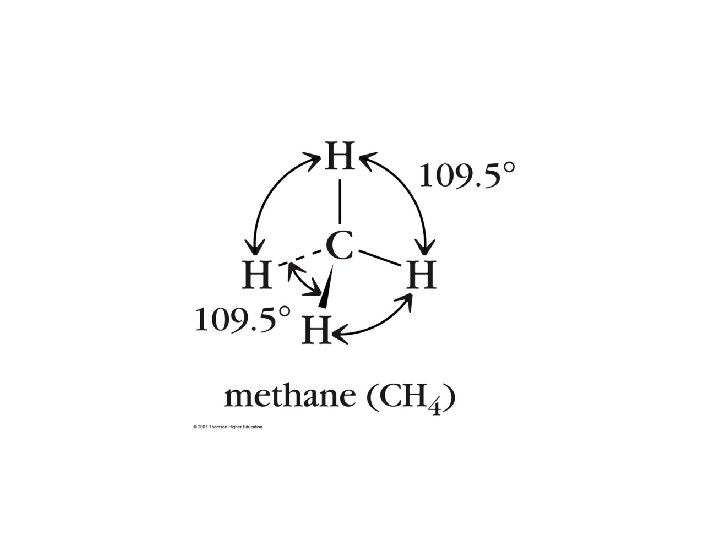
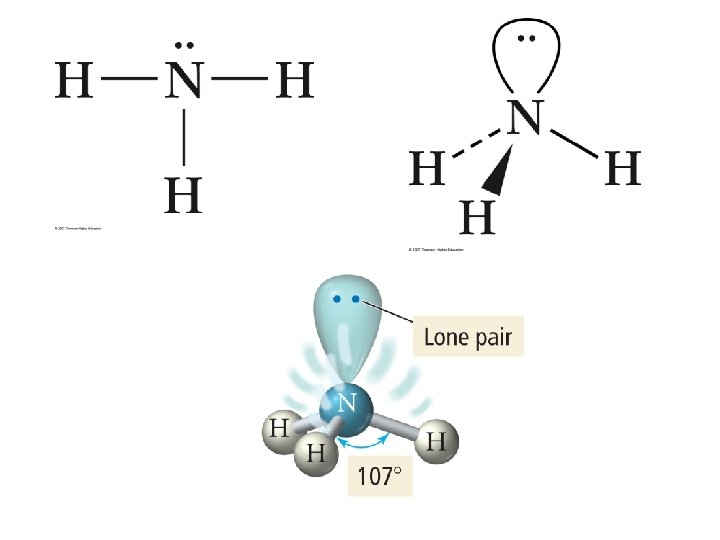
4 e- groups • 4 bonds, 0 Lone Pairs – – e- PG: Tetrahedral MG: Tetrahedral AX 4 E 0 CH 4 • 3 bonds, 1 Lone Pair – – e- PG: Tetrahedral MG: Triangular Pyramidal AX 3 E 1 NH 3

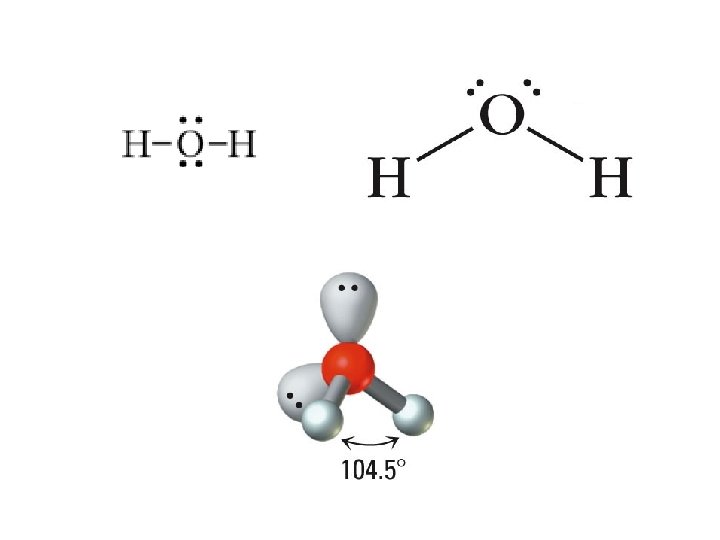
• 2 bonds, 2 Lone Pairs – e- PG: Tetrahedral – MG: Bent/Angular – AX 2 E 2 – H 2 O
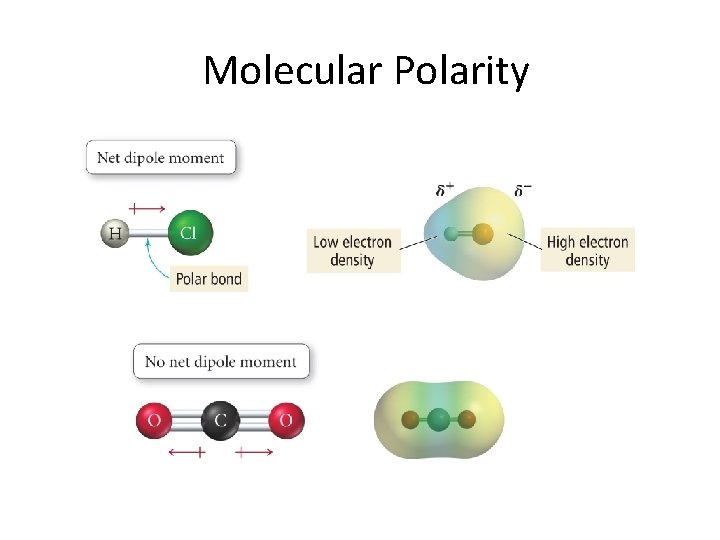
Molecular Polarity
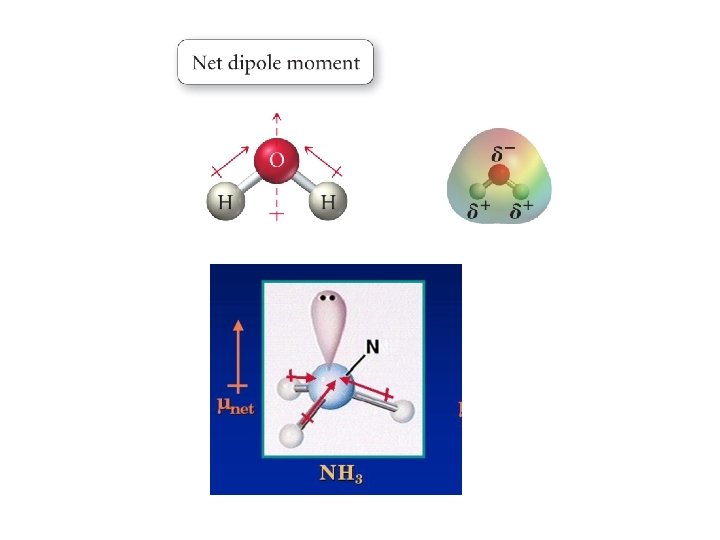
Determining molecular polarity • Draw Lewis dot structure taking into account 3 -D molecular geometry • Indicate the dipole moment for each bond using EN values for each atom • Sum up the dipole moments as vectors and determine if there is a net dipole moment
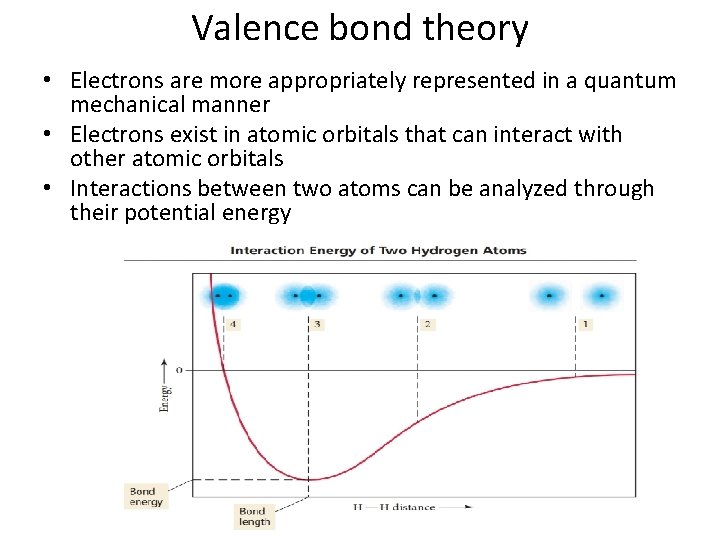
Valence bond theory • Electrons are more appropriately represented in a quantum mechanical manner • Electrons exist in atomic orbitals that can interact with other atomic orbitals • Interactions between two atoms can be analyzed through their potential energy
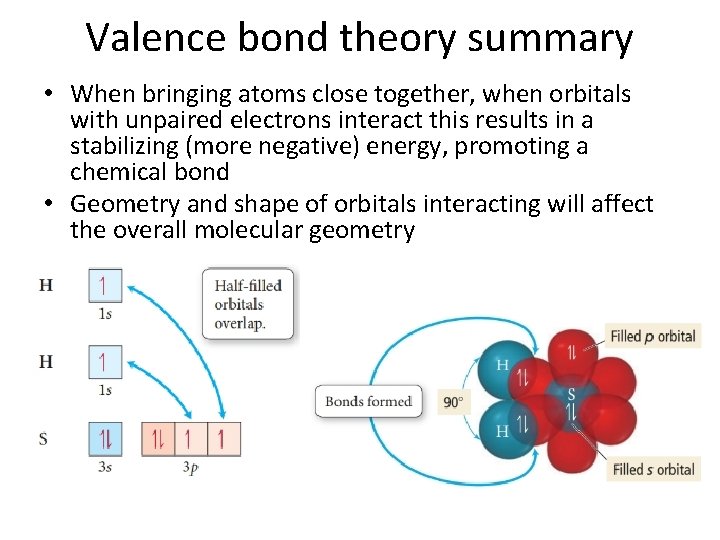
Valence bond theory summary • When bringing atoms close together, when orbitals with unpaired electrons interact this results in a stabilizing (more negative) energy, promoting a chemical bond • Geometry and shape of orbitals interacting will affect the overall molecular geometry
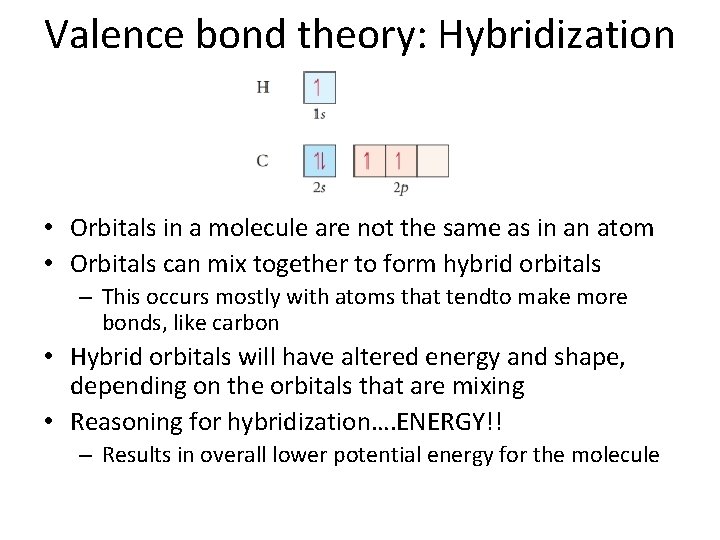
Valence bond theory: Hybridization • Orbitals in a molecule are not the same as in an atom • Orbitals can mix together to form hybrid orbitals – This occurs mostly with atoms that tendto make more bonds, like carbon • Hybrid orbitals will have altered energy and shape, depending on the orbitals that are mixing • Reasoning for hybridization…. ENERGY!! – Results in overall lower potential energy for the molecule
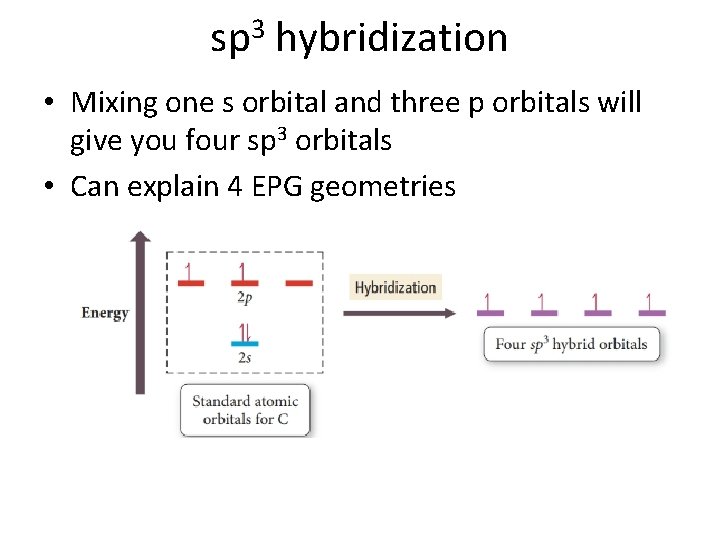
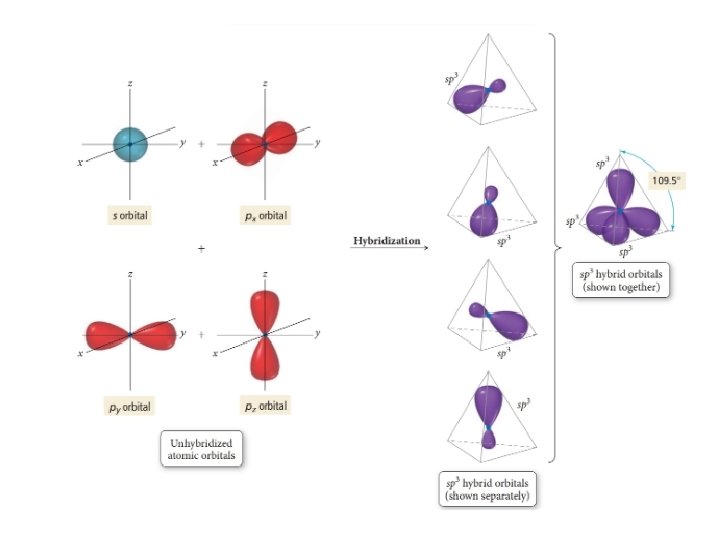
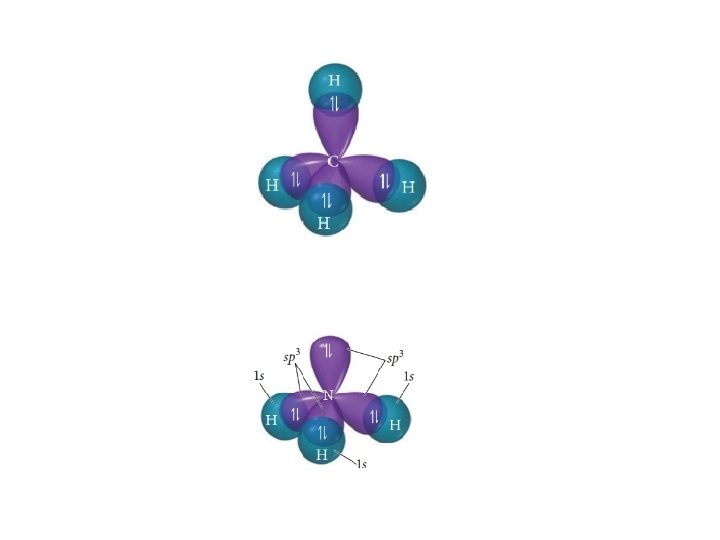
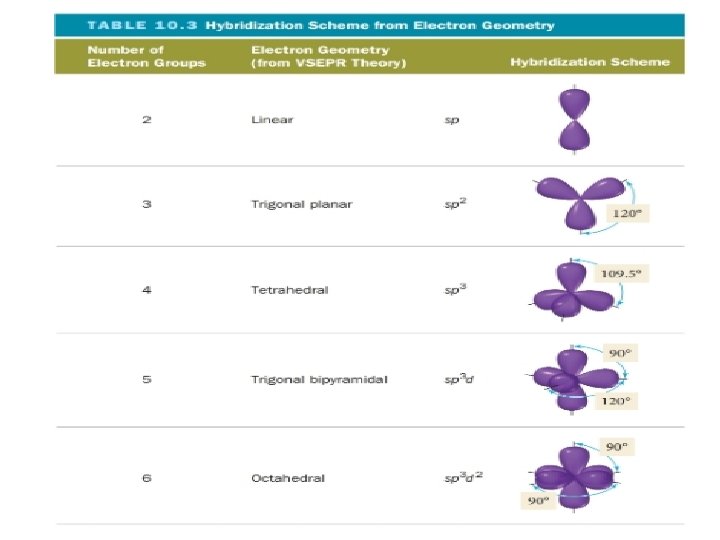
sp 3 hybridization • Mixing one s orbital and three p orbitals will give you four sp 3 orbitals • Can explain 4 EPG geometries
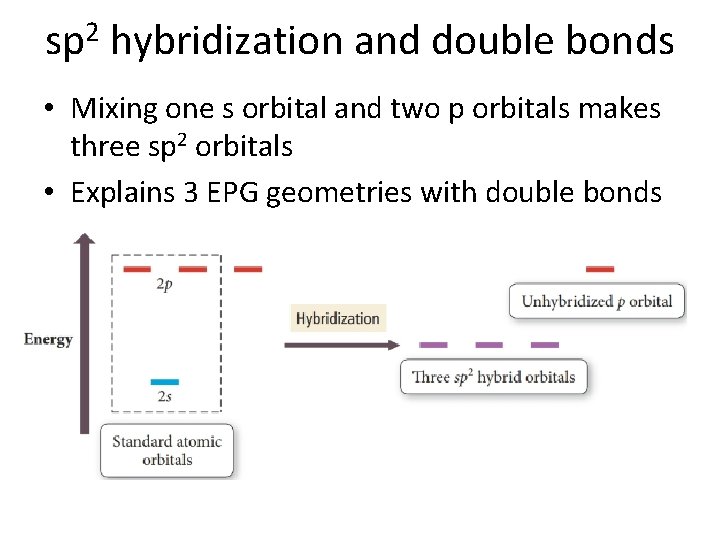
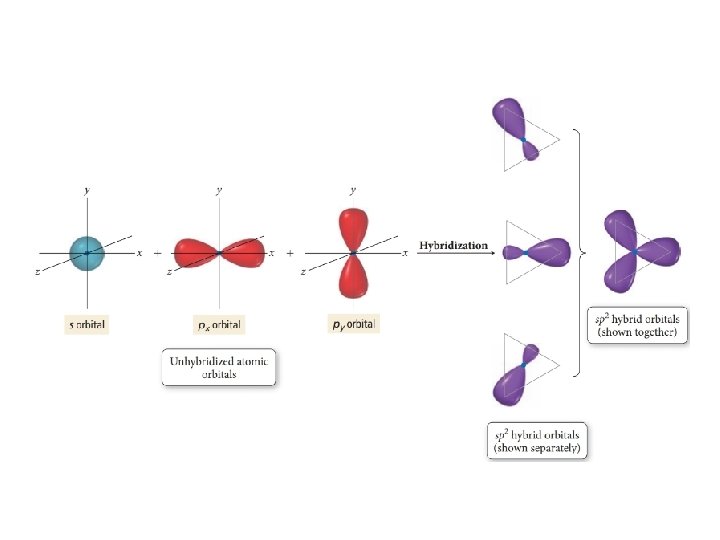
sp 2 hybridization and double bonds • Mixing one s orbital and two p orbitals makes three sp 2 orbitals • Explains 3 EPG geometries with double bonds
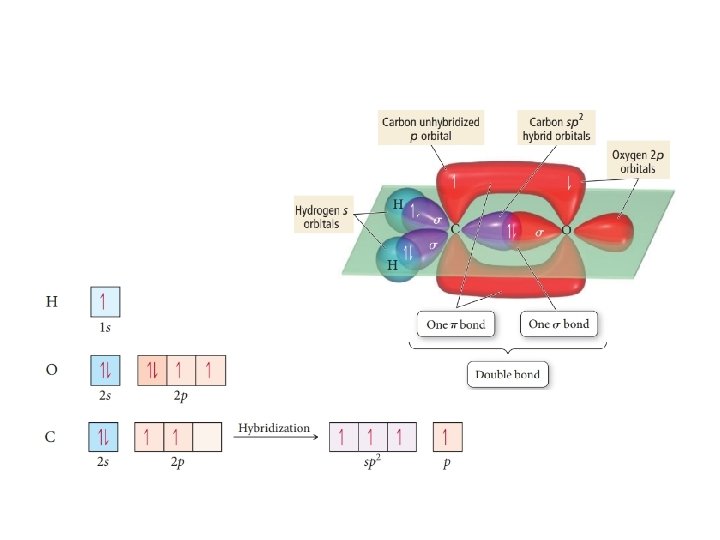
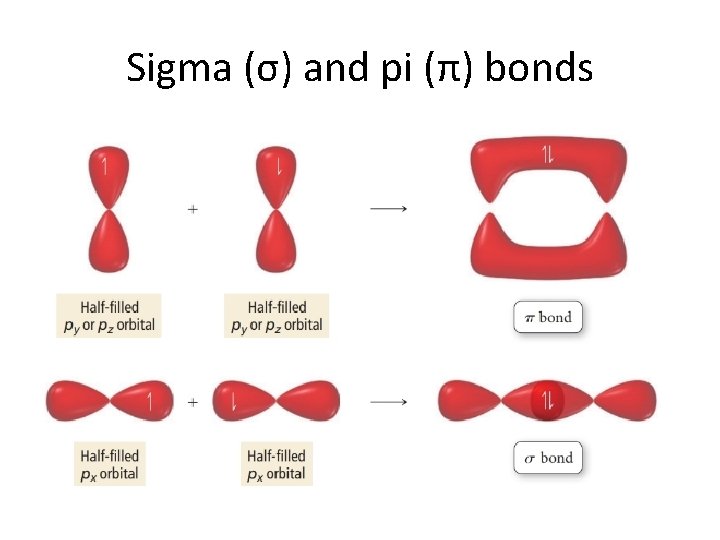
Sigma (σ) and pi (π) bonds
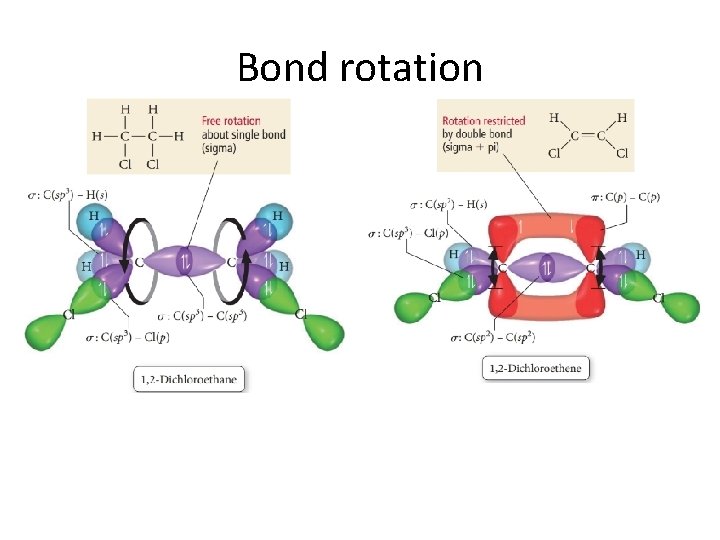
Bond rotation

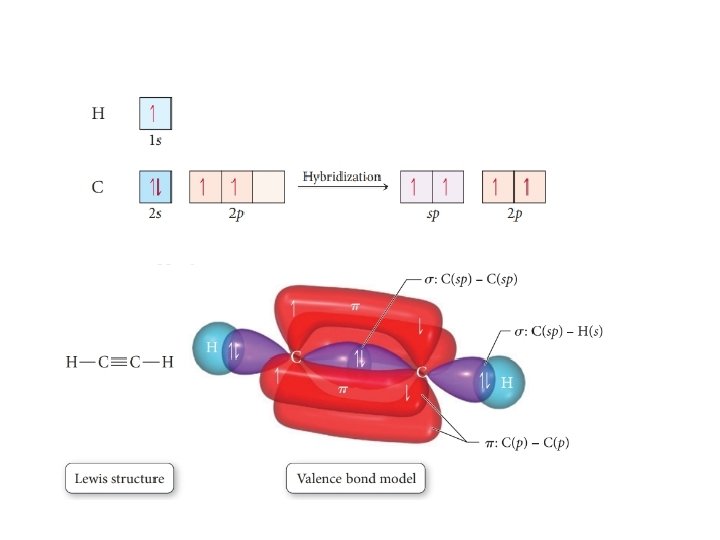
sp hybridization • Mixing one s orbital and one p orbital makes 2 sp orbitals • Explains triple bonds


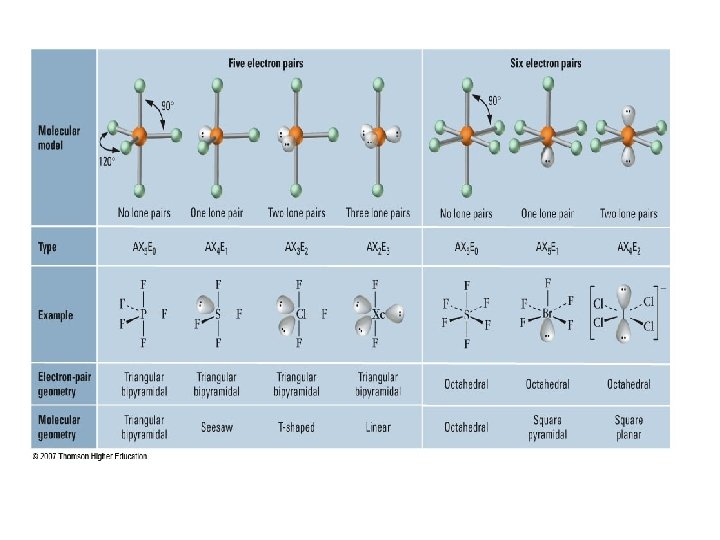
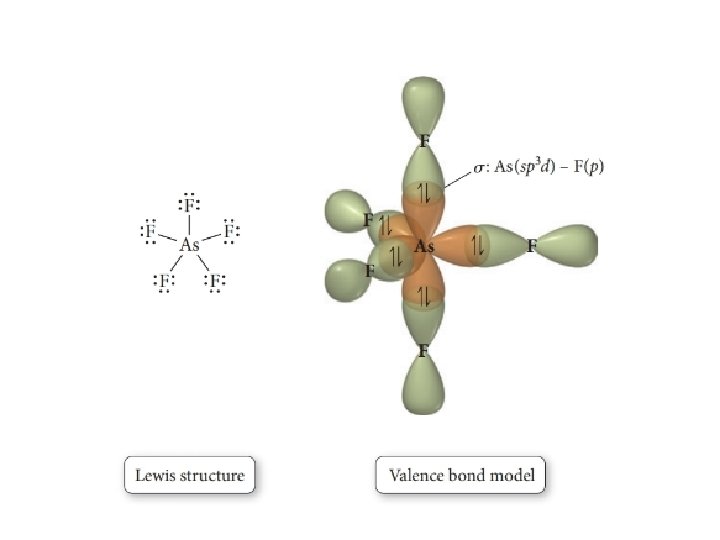
sp 3 d hybridization • Mixing one s, three p, and one d orbital produce five sp 3 d orbitals • This can explain 5 EPG geometries
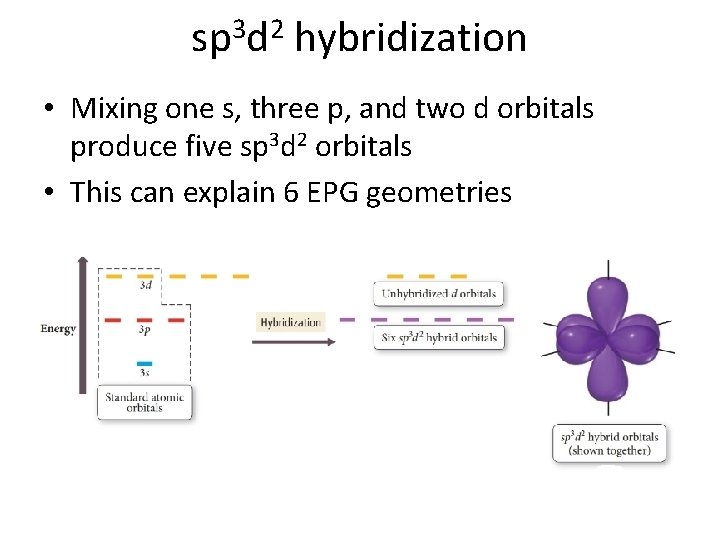
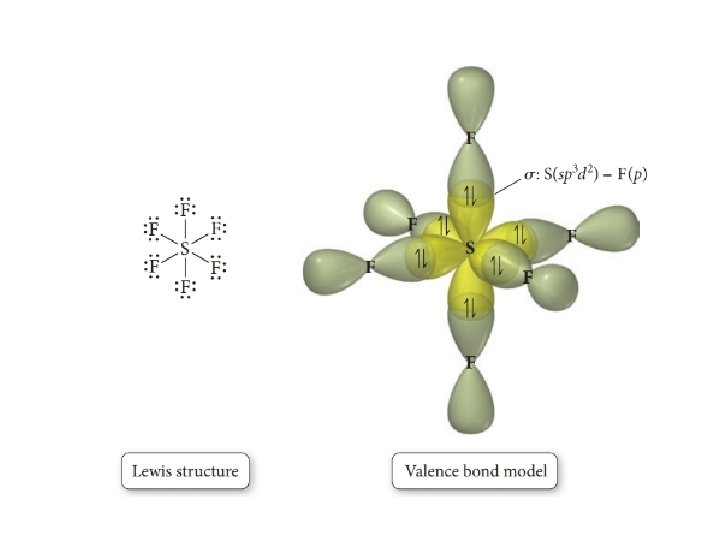
sp 3 d 2 hybridization • Mixing one s, three p, and two d orbitals produce five sp 3 d 2 orbitals • This can explain 6 EPG geometries
Molecular orbital (MO) theory!!! • Solving the Schrodinger equation allowed for the calculation of the atomic orbitals (1 s, 2 p, 3 d, etc. ) • A similar calculation can be applied for molecular orbitals, this results in a process similar to hybridization • Linear combination of atomic orbitals (LCAOs) – a weighted linear sum of the valence atomic orbitals – a mixing of the atomic orbitals to produce molecular orbitals
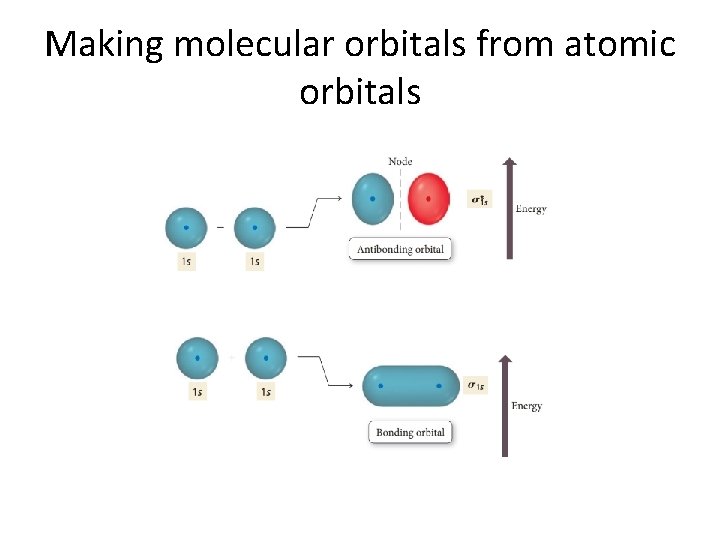
Making molecular orbitals from atomic orbitals
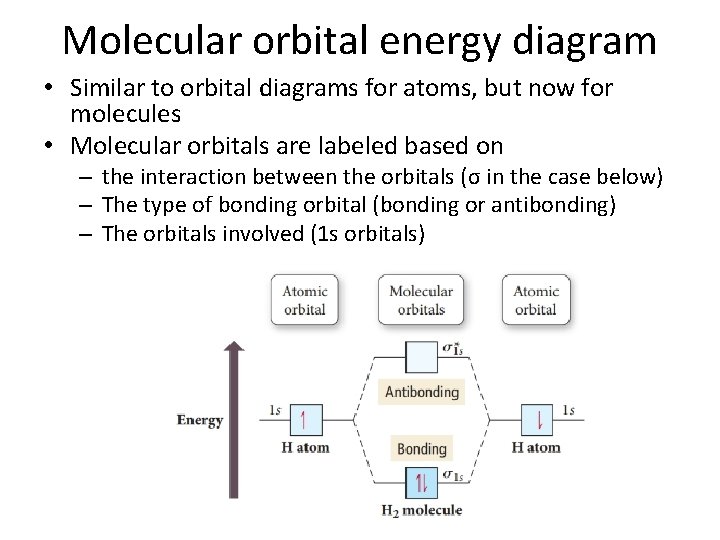
Molecular orbital energy diagram • Similar to orbital diagrams for atoms, but now for molecules • Molecular orbitals are labeled based on – the interaction between the orbitals (σ in the case below) – The type of bonding orbital (bonding or antibonding) – The orbitals involved (1 s orbitals)
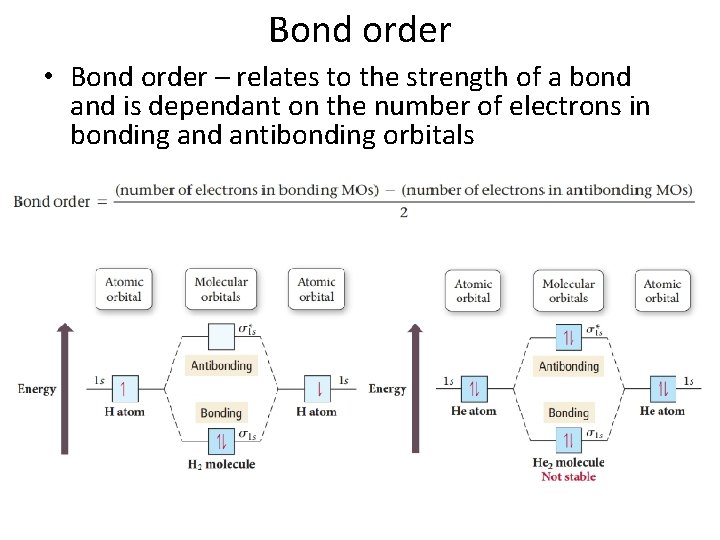
Bond order • Bond order – relates to the strength of a bond and is dependant on the number of electrons in bonding and antibonding orbitals
Summary of LCAO-MO Theory • We can represent molecular orbitals (MOs) as a linear combination of atomic orbitals (AOs), where the total number of atomic orbitals will equal the total number of produced MOs • When two AOs mix, a lower energy bonding MO is produced, and a higher energy antibonding MO is produced • Filling in the molecular orbital energy diagram with the total number of electrons from the atoms, following general electron configuration rules • Bond order can be determined from the bond order formula
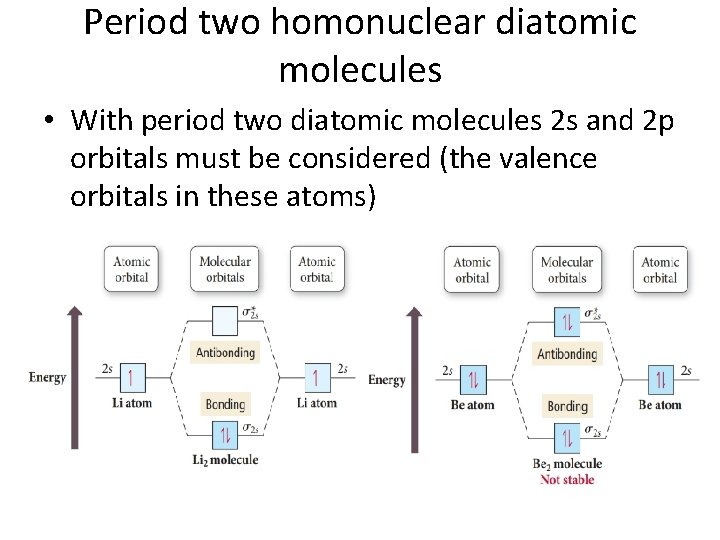
Period two homonuclear diatomic molecules • With period two diatomic molecules 2 s and 2 p orbitals must be considered (the valence orbitals in these atoms)
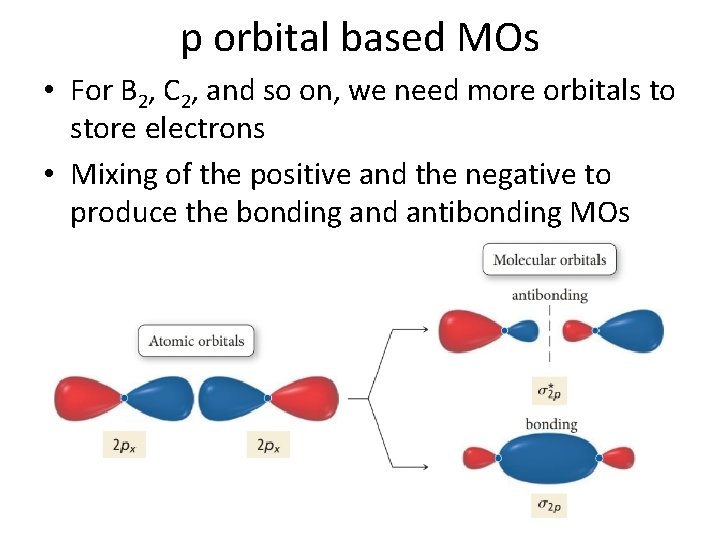
p orbital based MOs • For B 2, C 2, and so on, we need more orbitals to store electrons • Mixing of the positive and the negative to produce the bonding and antibonding MOs
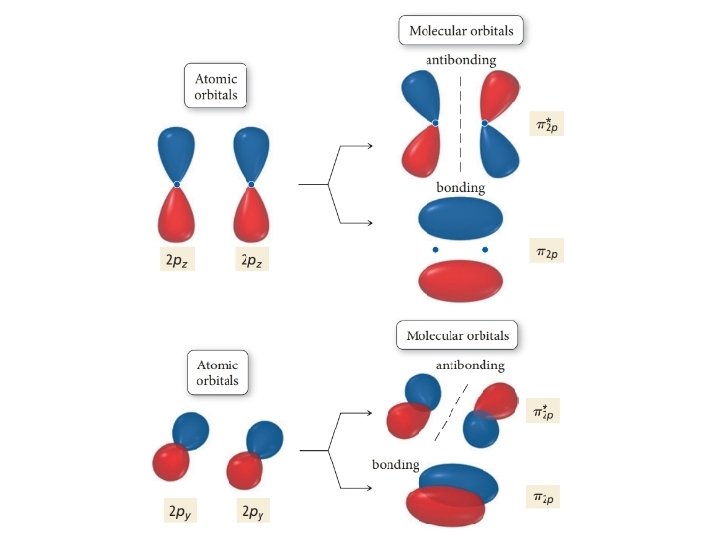
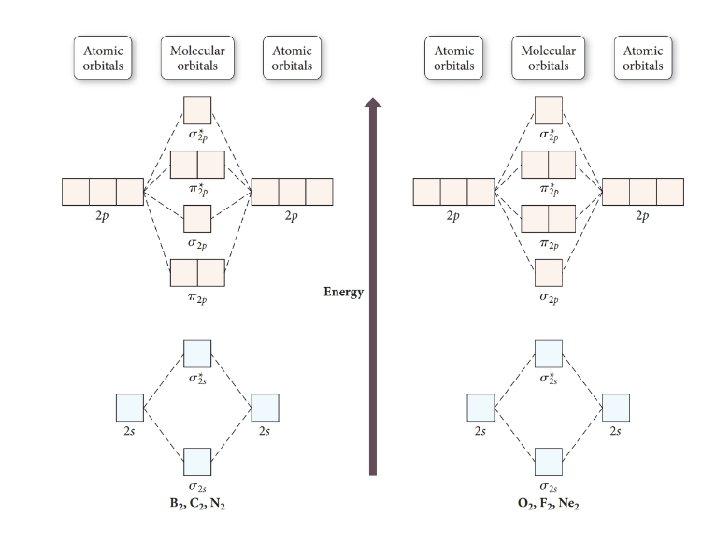
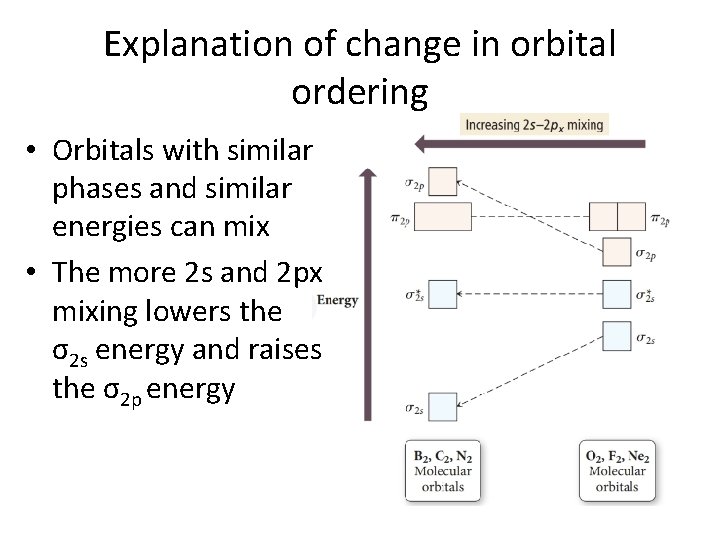
Explanation of change in orbital ordering • Orbitals with similar phases and similar energies can mix • The more 2 s and 2 px mixing lowers the σ2 s energy and raises the σ2 p energy
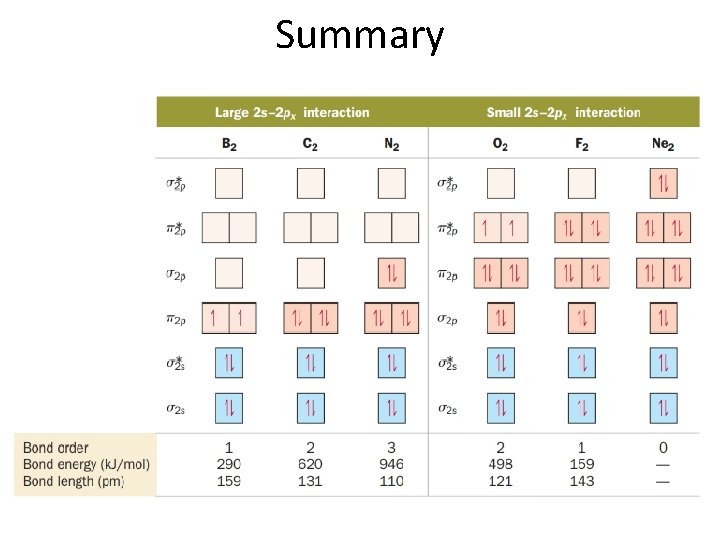
Summary
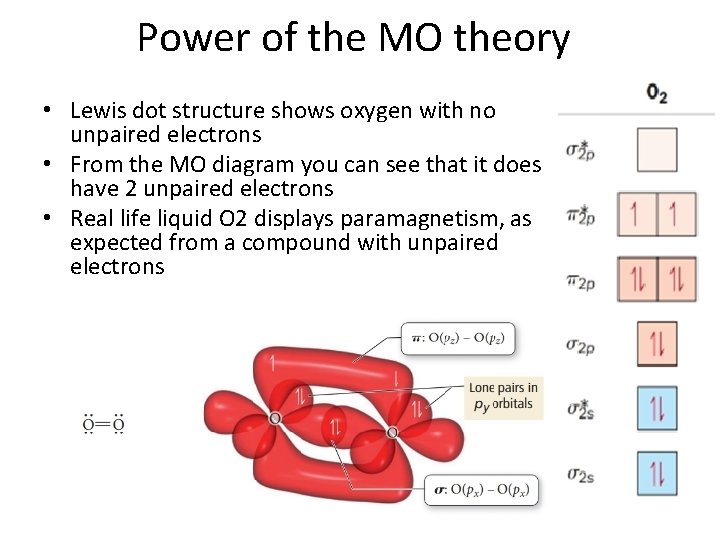
Power of the MO theory • Lewis dot structure shows oxygen with no unpaired electrons • From the MO diagram you can see that it does have 2 unpaired electrons • Real life liquid O 2 displays paramagnetism, as expected from a compound with unpaired electrons
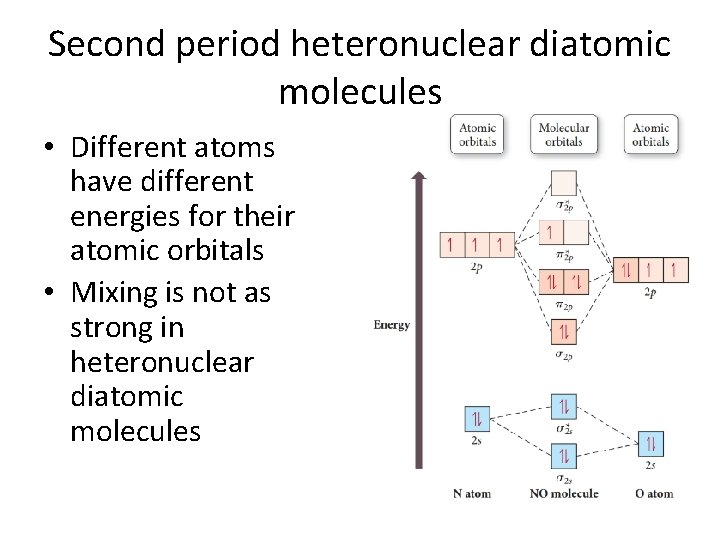
Second period heteronuclear diatomic molecules • Different atoms have different energies for their atomic orbitals • Mixing is not as strong in heteronuclear diatomic molecules

Polyatomic molecules

No MO Chapter 10
- Slides: 51