CHAPTER 10 ALCOHOLS Structure of Alcohols The functional






- Slides: 80

CHAPTER 10 ALCOHOLS

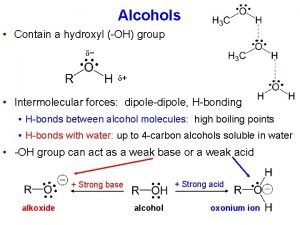
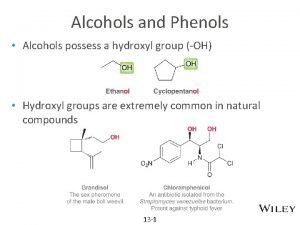
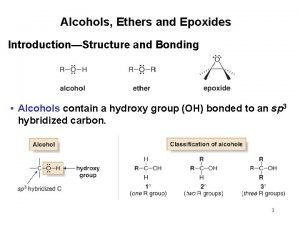
Structure of Alcohols • The functional group of an alcohol is an -OH group bonded to an sp 3 hybridized carbon. • Bond angles about the hydroxyl oxygen atom are approximately 109. 5° (Figure 10. 1). • Oxygen is sp 3 hybridized. • Two sp 3 hybrid orbitals form sigma bonds to a carbon and a hydrogen. • The remaining two sp 3 hybrid orbitals each contain an unshared pair of electrons. 2

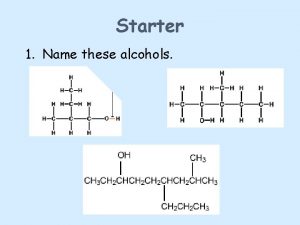
Nomenclature of Alcohols • IUPAC names • The parent chain is the longest carbon chain that contains the -OH group. • Number the parent chain to give the -OH group the lowest possible number. • Change the suffix -e to -ol • Common names • Name the alkyl group bonded to oxygen followed by the word alcohol 3

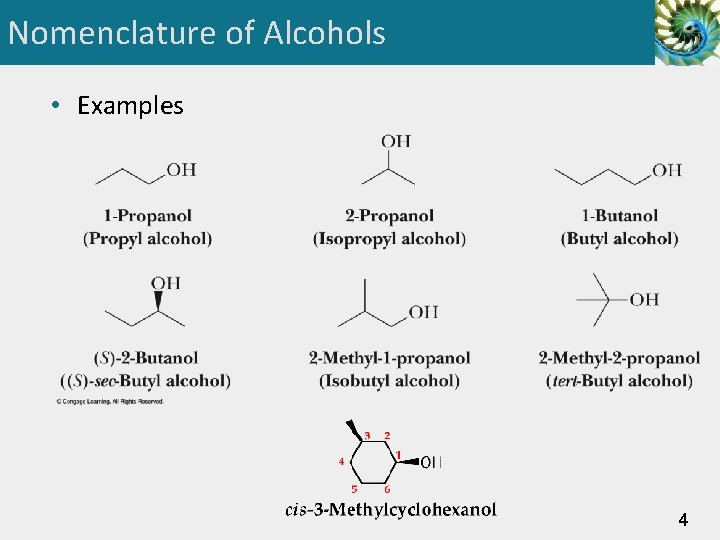
Nomenclature of Alcohols • Examples 4

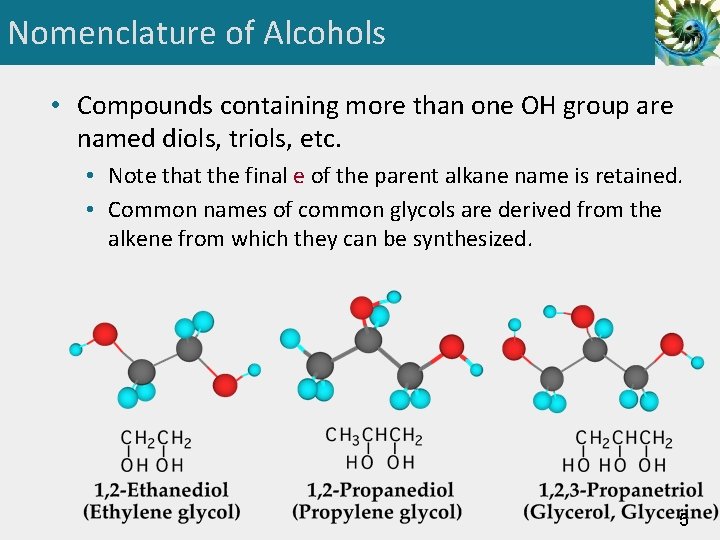
Nomenclature of Alcohols • Compounds containing more than one OH group are named diols, triols, etc. • Note that the final e of the parent alkane name is retained. • Common names of common glycols are derived from the alkene from which they can be synthesized. 5

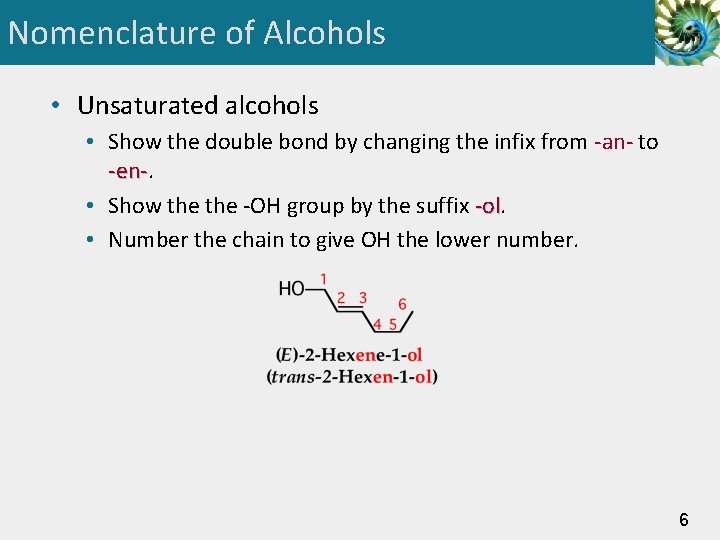
Nomenclature of Alcohols • Unsaturated alcohols • Show the double bond by changing the infix from -an- to -en-. -en • Show the -OH group by the suffix -ol • Number the chain to give OH the lower number. 6

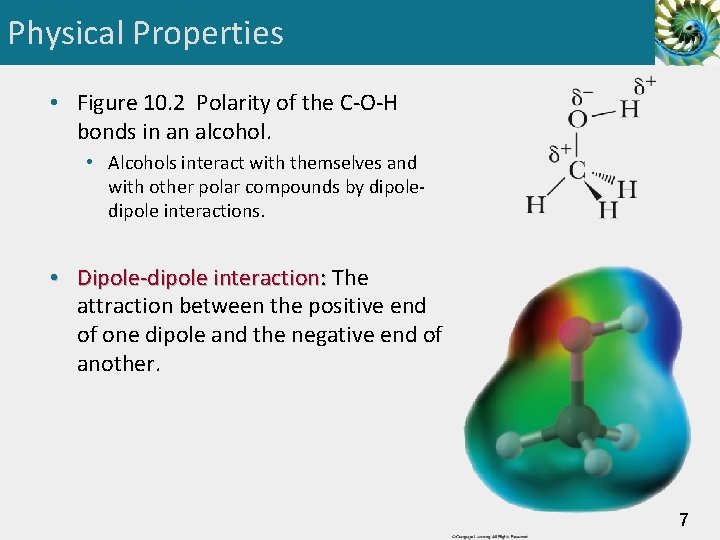
Physical Properties • Figure 10. 2 Polarity of the C-O-H bonds in an alcohol. • Alcohols interact with themselves and with other polar compounds by dipole interactions. • Dipole-dipole interaction: The attraction between the positive end of one dipole and the negative end of another. 7

Physical Properties • Hydrogen bonding: bonding When the positive end of one dipole is an H bonded to F, O, or N (atoms of high electronegativity) and the other end is F, O, or N. • The strength of hydrogen bonding in water is approximately 21 k. J (5 kcal)/mol. • Hydrogen bonds are considerably weaker than covalent bonds. • Nonetheless, they can have a significant effect on physical properties. 8

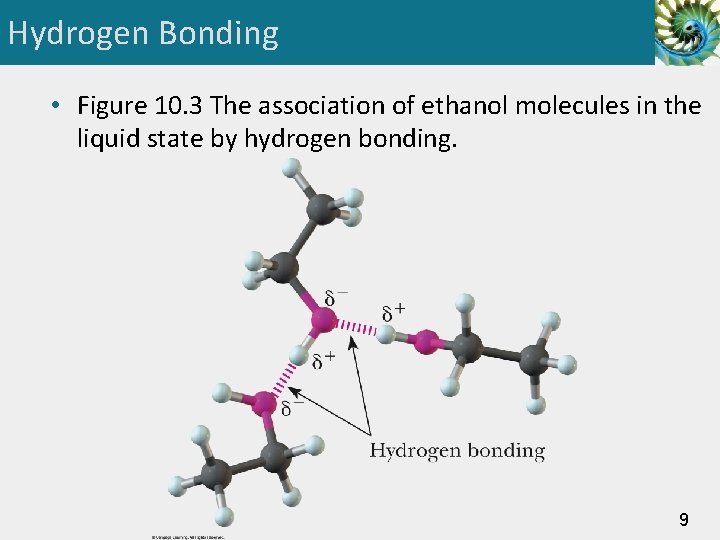
Hydrogen Bonding • Figure 10. 3 The association of ethanol molecules in the liquid state by hydrogen bonding. 9

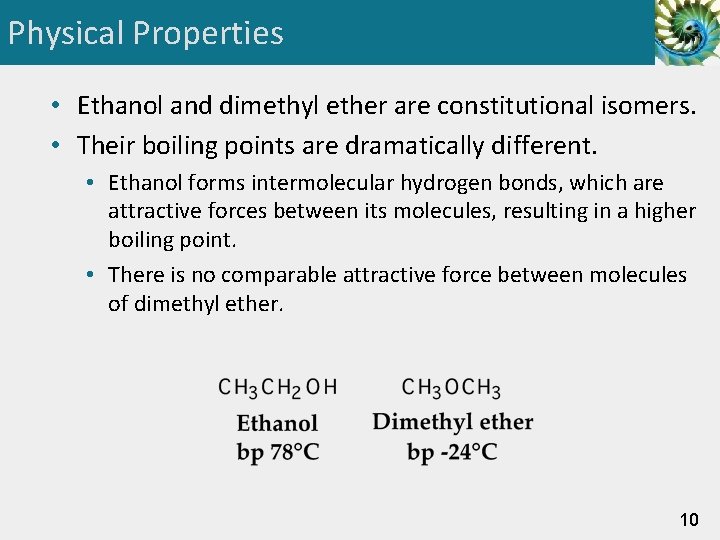
Physical Properties • Ethanol and dimethyl ether are constitutional isomers. • Their boiling points are dramatically different. • Ethanol forms intermolecular hydrogen bonds, which are attractive forces between its molecules, resulting in a higher boiling point. • There is no comparable attractive force between molecules of dimethyl ether. 10

Physical Properties • In relation to alkanes of comparable size and molecular weight, alcohols • have higher boiling points. • are more soluble in water. • The presence of additional -OH groups in a molecule further increases solubility in water and boiling point. 11

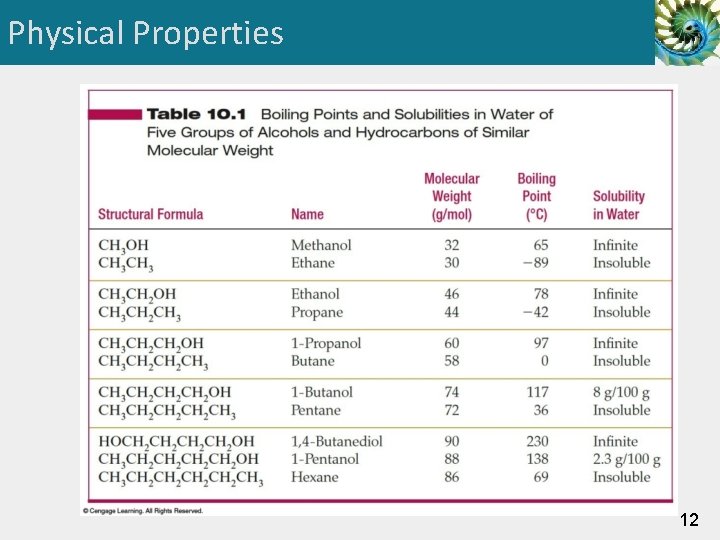
Physical Properties 12

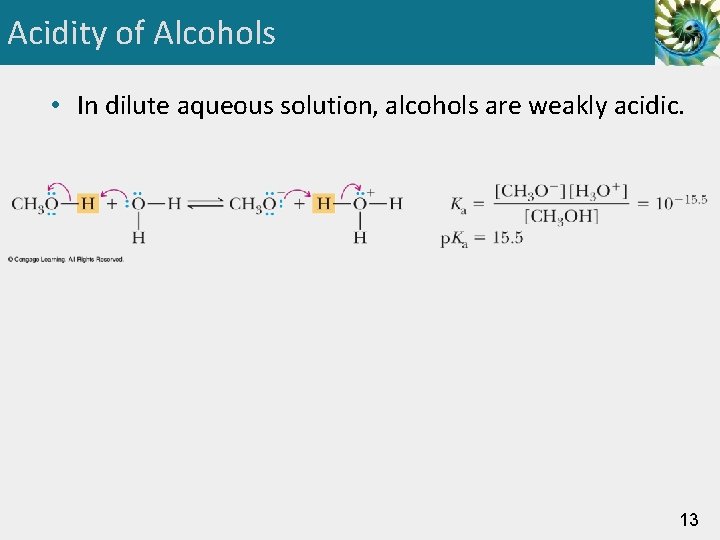
Acidity of Alcohols • In dilute aqueous solution, alcohols are weakly acidic. 13

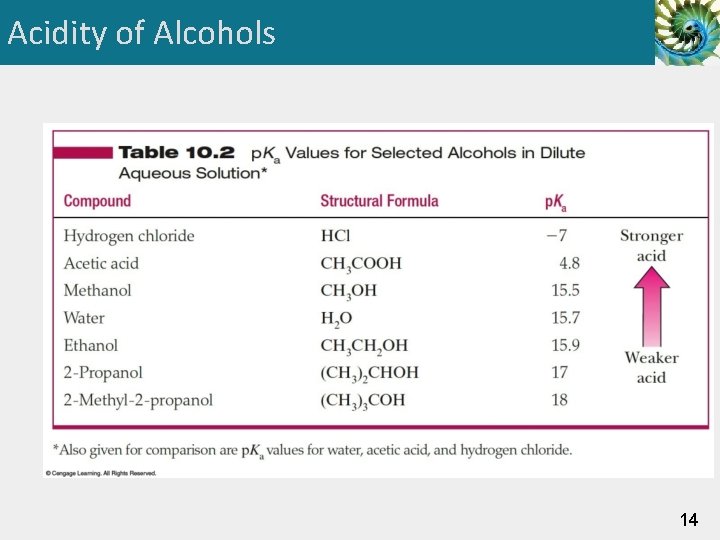
Acidity of Alcohols 14

Acidity of Alcohols • Acidity depends primarily on the degree of stabilization and solvation of the alkoxide ion. • The negatively charged oxygens of methoxide and ethoxide are about as accessible as the oxygen of hydroxide ion for solvation. These alcohol are about as acidic as water. • As the bulk of the alkyl group increases, the ability of water to solvate the alkoxide decreases, the acidity of the alcohol decreases, and the basicity of the alkoxide ion increases. 15

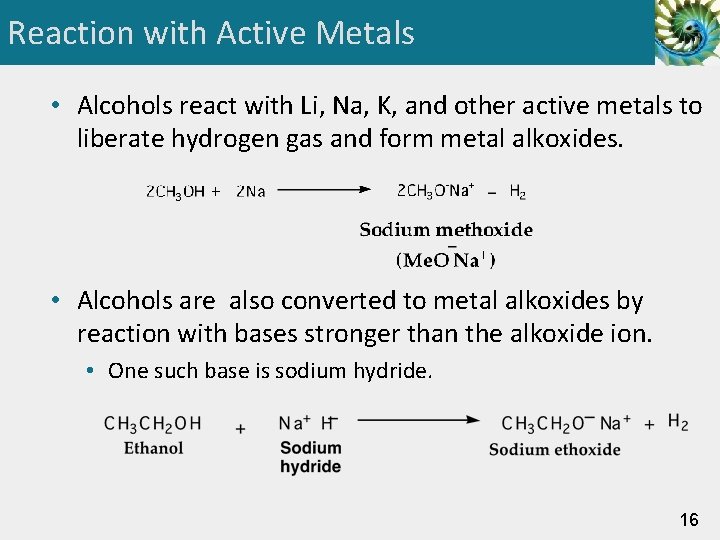
Reaction with Active Metals • Alcohols react with Li, Na, K, and other active metals to liberate hydrogen gas and form metal alkoxides. • Alcohols are also converted to metal alkoxides by reaction with bases stronger than the alkoxide ion. • One such base is sodium hydride. 16

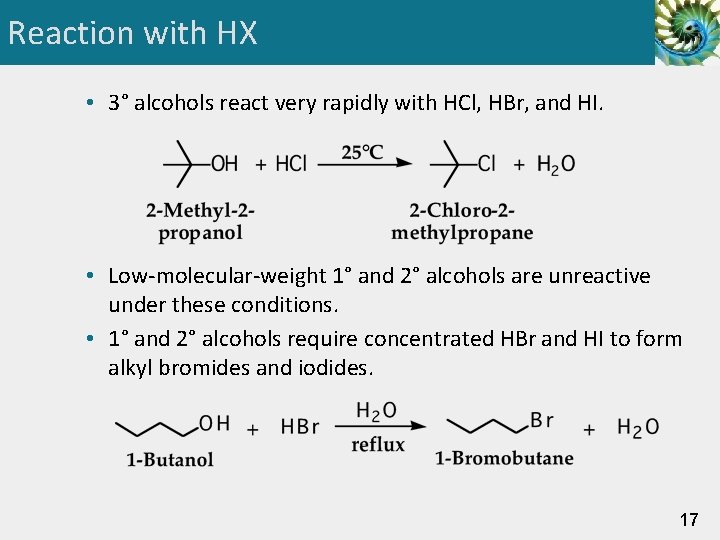
Reaction with HX • 3° alcohols react very rapidly with HCl, HBr, and HI. • Low-molecular-weight 1° and 2° alcohols are unreactive under these conditions. • 1° and 2° alcohols require concentrated HBr and HI to form alkyl bromides and iodides. 17

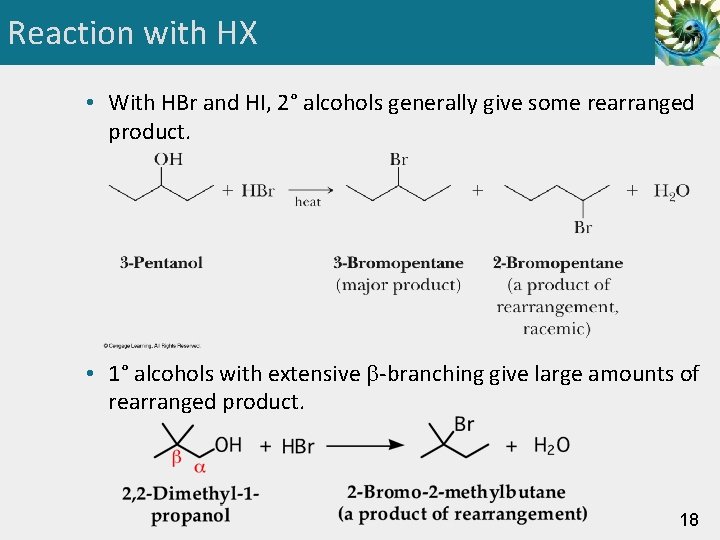
Reaction with HX • With HBr and HI, 2° alcohols generally give some rearranged product. • 1° alcohols with extensive -branching give large amounts of rearranged product. 18

Reaction with HX • Based on • the relative ease of reaction of alcohols with HX (3° > 2° > 1°) and • the occurrence of rearrangements, • Chemists propose that reaction of 2° and 3° alcohols with HX • Occurs by an SN 1 mechanism, and involves formation of a carbocation intermediate. 19

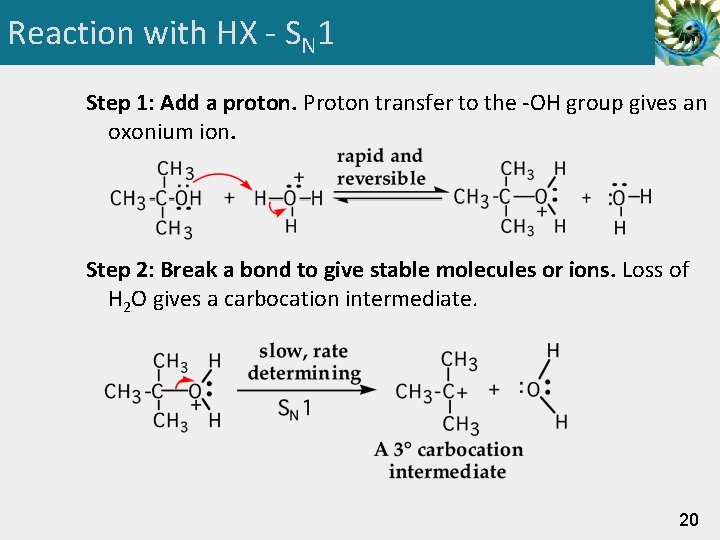
Reaction with HX - SN 1 Step 1: Add a proton. Proton transfer to the -OH group gives an oxonium ion. Step 2: Break a bond to give stable molecules or ions. Loss of H 2 O gives a carbocation intermediate. 20

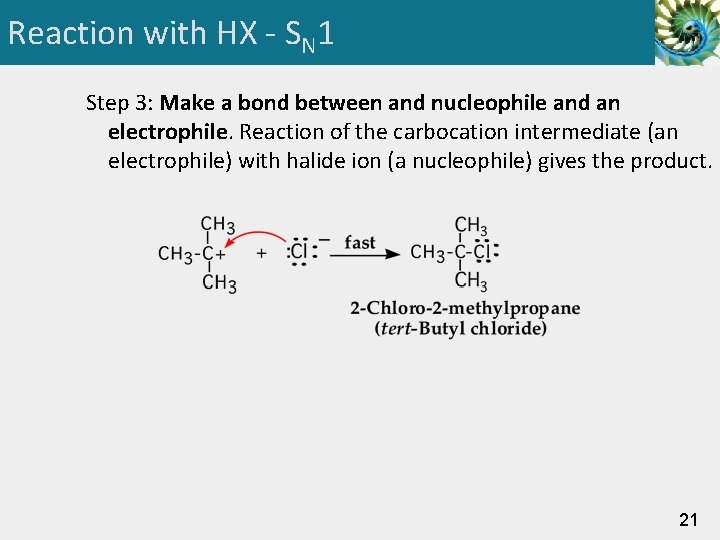
Reaction with HX - SN 1 Step 3: Make a bond between and nucleophile and an electrophile. Reaction of the carbocation intermediate (an electrophile) with halide ion (a nucleophile) gives the product. 21

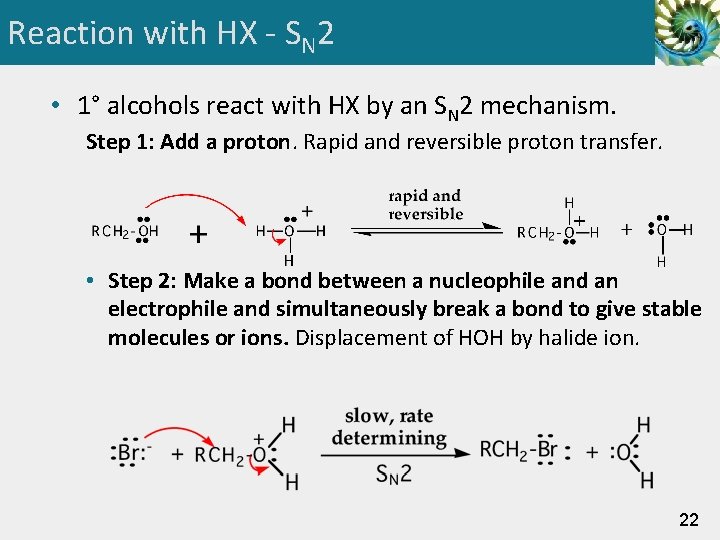
Reaction with HX - SN 2 • 1° alcohols react with HX by an SN 2 mechanism. Step 1: Add a proton. Rapid and reversible proton transfer. • Step 2: Make a bond between a nucleophile and an electrophile and simultaneously break a bond to give stable molecules or ions. Displacement of HOH by halide ion. 22

Reaction with HX • For 1° alcohols with extensive -branching • SN 1 is not possible because this pathway would require a 1° carbocation. • SN 2 is not possible because of steric hindrance created by the -branching. • These alcohols react by a concerted loss of HOH and migration of an alkyl group. 23

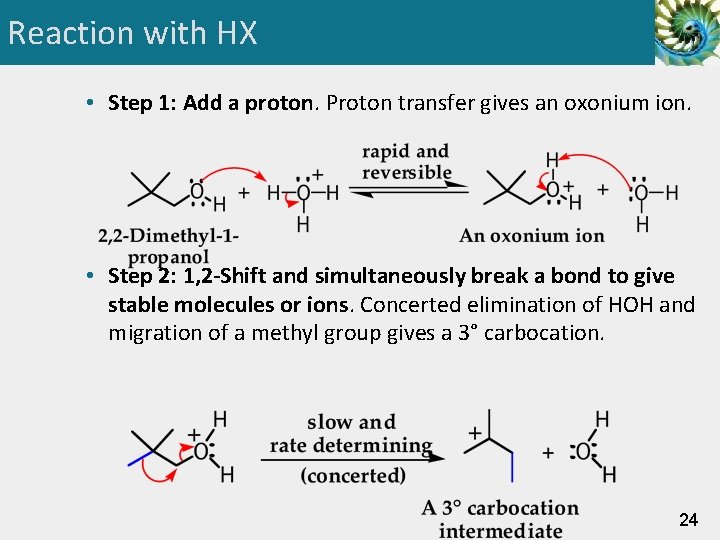
Reaction with HX • Step 1: Add a proton. Proton transfer gives an oxonium ion. • Step 2: 1, 2 -Shift and simultaneously break a bond to give stable molecules or ions. Concerted elimination of HOH and migration of a methyl group gives a 3° carbocation. 24

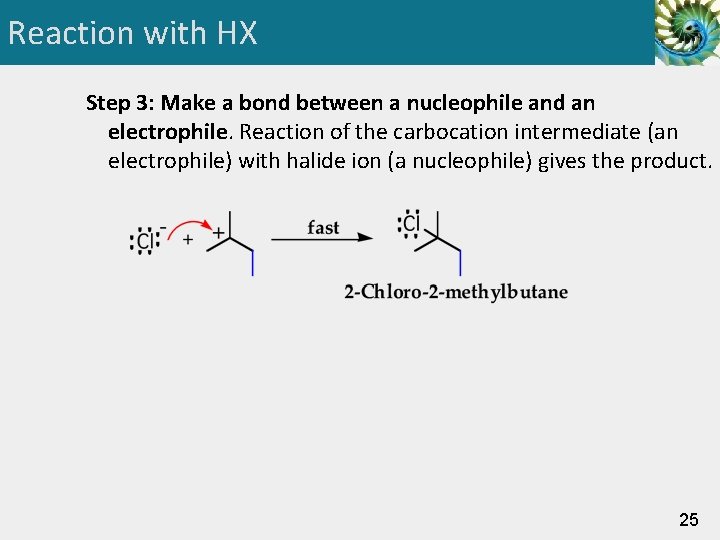
Reaction with HX Step 3: Make a bond between a nucleophile and an electrophile. Reaction of the carbocation intermediate (an electrophile) with halide ion (a nucleophile) gives the product. 25

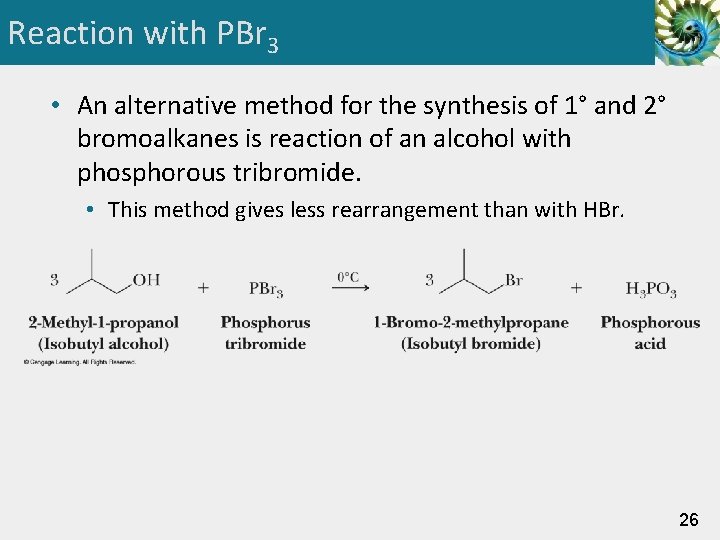
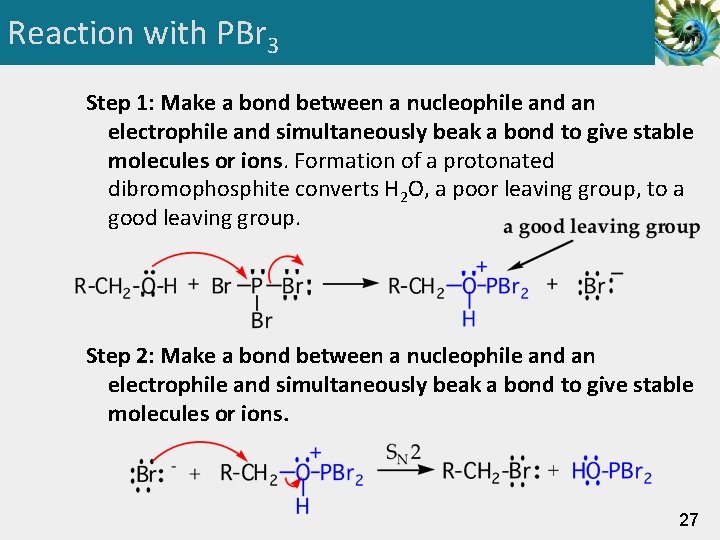
Reaction with PBr 3 • An alternative method for the synthesis of 1° and 2° bromoalkanes is reaction of an alcohol with phosphorous tribromide. • This method gives less rearrangement than with HBr. 26

Reaction with PBr 3 Step 1: Make a bond between a nucleophile and an electrophile and simultaneously beak a bond to give stable molecules or ions. Formation of a protonated dibromophosphite converts H 2 O, a poor leaving group, to a good leaving group. Step 2: Make a bond between a nucleophile and an electrophile and simultaneously beak a bond to give stable molecules or ions. 27

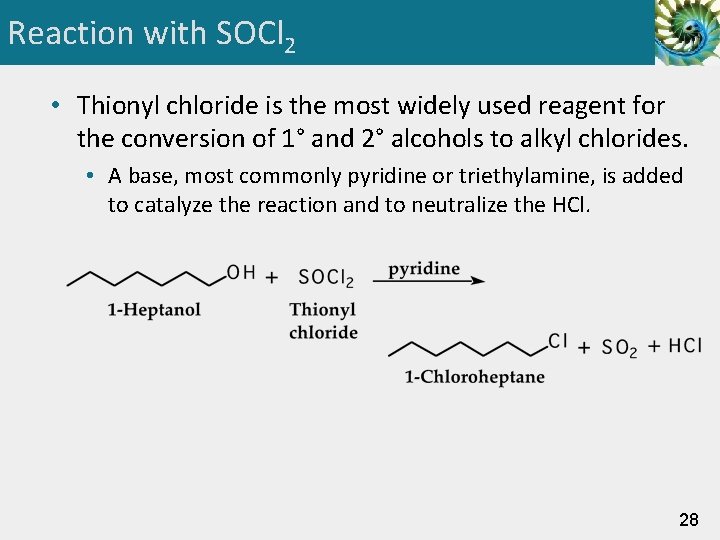
Reaction with SOCl 2 • Thionyl chloride is the most widely used reagent for the conversion of 1° and 2° alcohols to alkyl chlorides. • A base, most commonly pyridine or triethylamine, is added to catalyze the reaction and to neutralize the HCl. 28

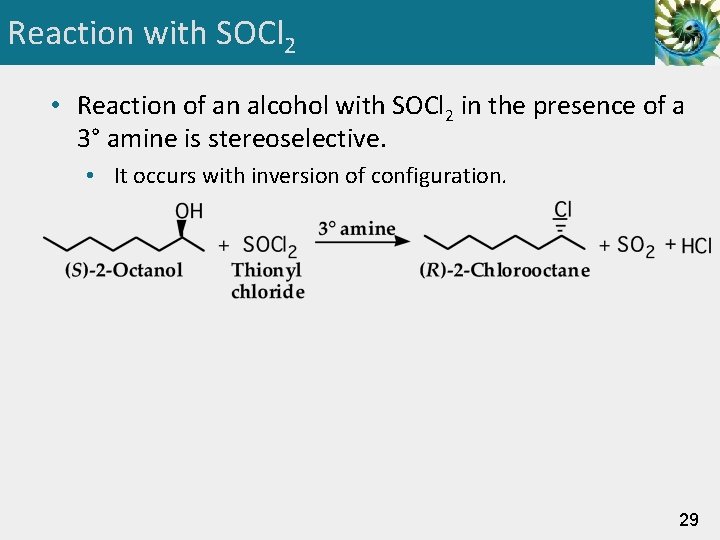
Reaction with SOCl 2 • Reaction of an alcohol with SOCl 2 in the presence of a 3° amine is stereoselective. • It occurs with inversion of configuration. 29

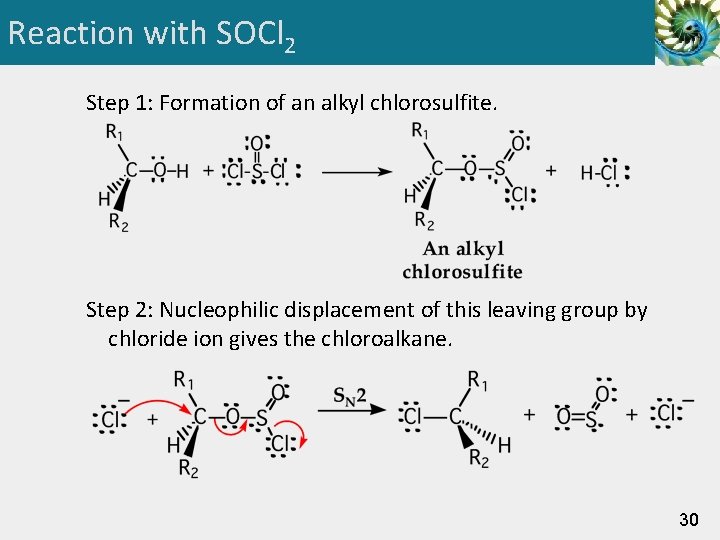
Reaction with SOCl 2 Step 1: Formation of an alkyl chlorosulfite. Step 2: Nucleophilic displacement of this leaving group by chloride ion gives the chloroalkane. 30

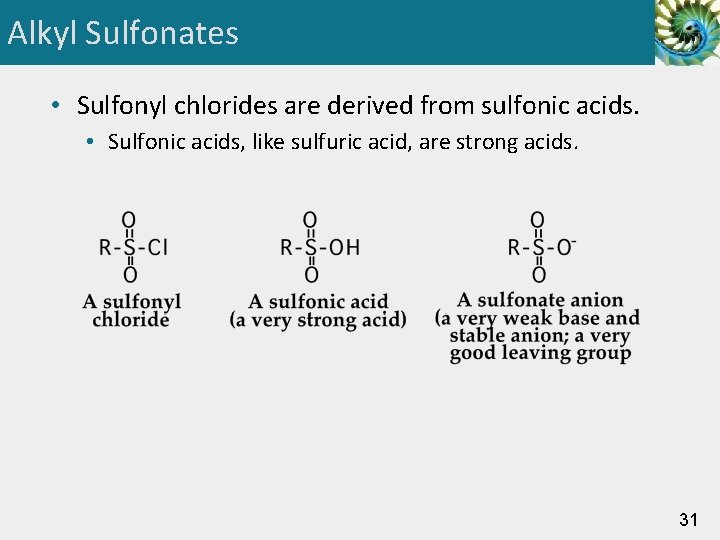
Alkyl Sulfonates • Sulfonyl chlorides are derived from sulfonic acids. • Sulfonic acids, like sulfuric acid, are strong acids. 31

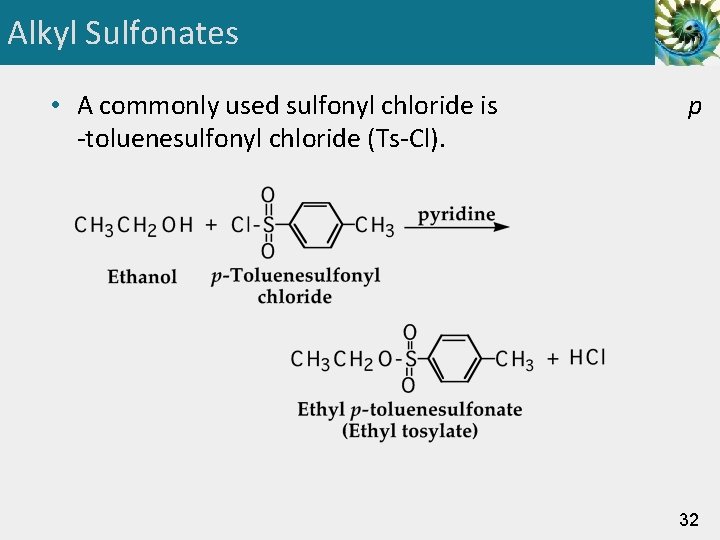
Alkyl Sulfonates • A commonly used sulfonyl chloride is -toluenesulfonyl chloride (Ts-Cl). p 32

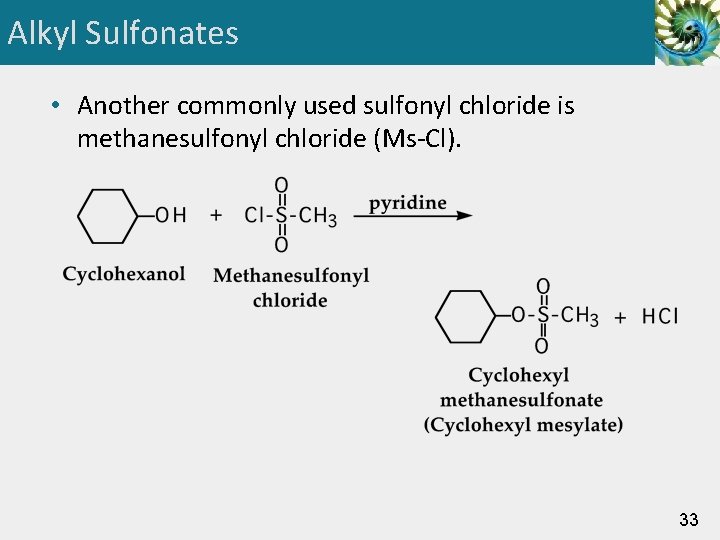
Alkyl Sulfonates • Another commonly used sulfonyl chloride is methanesulfonyl chloride (Ms-Cl). 33

Alkyl Sulfonates • Sulfonate anions are very weak bases (the conjugate bases of strong acids) and are very good leaving groups for SN 2 reactions. • Conversion of an alcohol to a sulfonate ester converts HOH, a very poor leaving group, into a sulfonic ester, a very good leaving group. 34

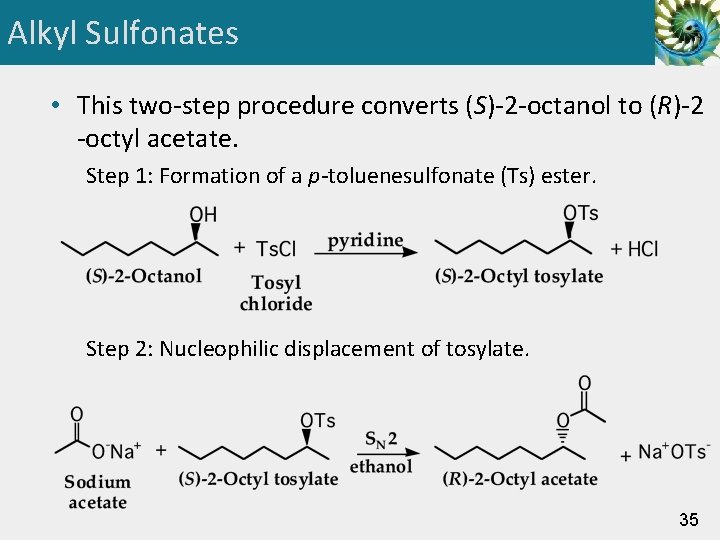
Alkyl Sulfonates • This two-step procedure converts (S)-2 -octanol to (R)-2 -octyl acetate. Step 1: Formation of a p-toluenesulfonate (Ts) ester. Step 2: Nucleophilic displacement of tosylate. 35

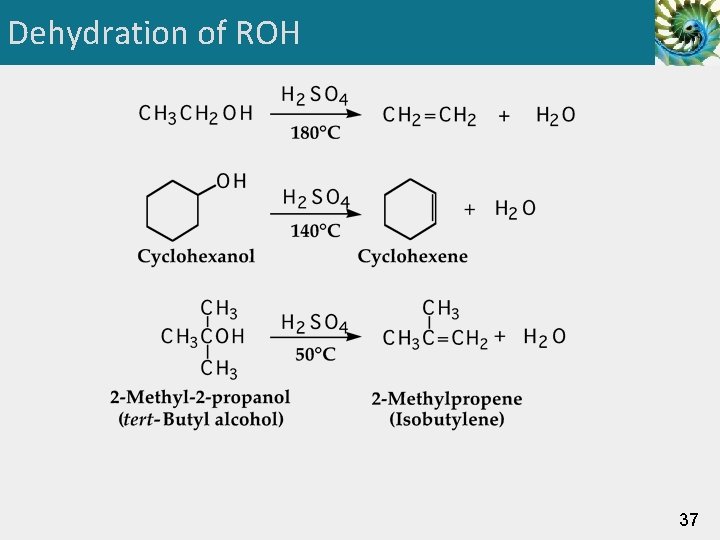
Dehydration of ROH • An alcohol can be converted to an alkene by acidcatalyzed dehydration (a type of -elimination). • 1° alcohols must be heated at high temperature in the presence of an acid catalyst, such as H 2 SO 4 or H 3 PO 4. 2° alcohols undergo dehydration at somewhat lower temperatures. • 3° alcohols often require temperatures at or only slightly above room temperature. 36

Dehydration of ROH 37

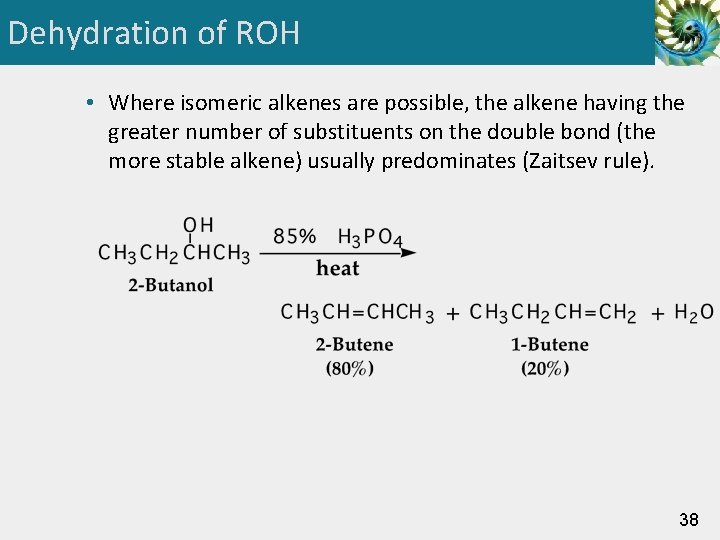
Dehydration of ROH • Where isomeric alkenes are possible, the alkene having the greater number of substituents on the double bond (the more stable alkene) usually predominates (Zaitsev rule). 38

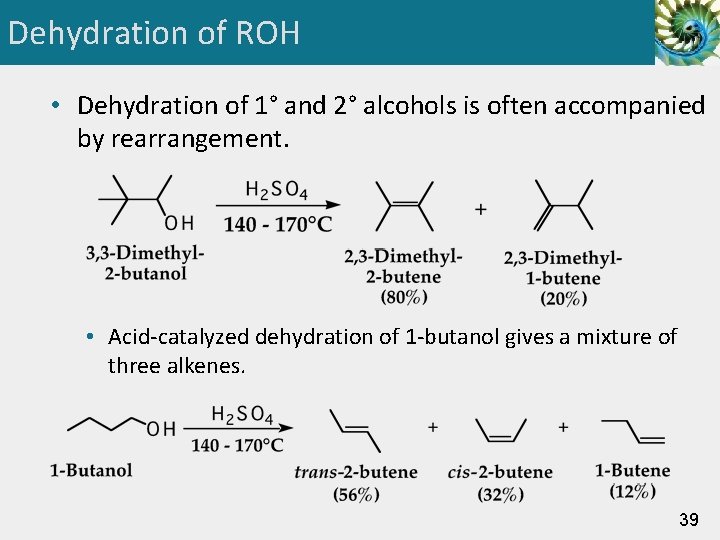
Dehydration of ROH • Dehydration of 1° and 2° alcohols is often accompanied by rearrangement. • Acid-catalyzed dehydration of 1 -butanol gives a mixture of three alkenes. 39

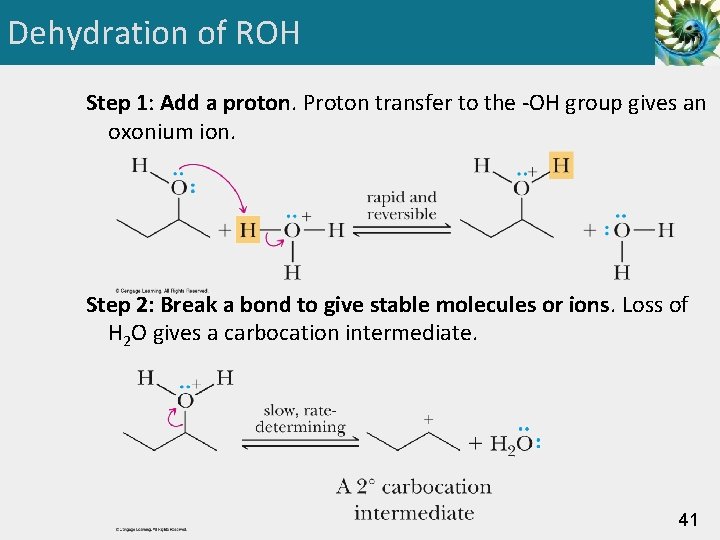
Dehydration of ROH • Based on evidence of • ease of dehydration (3° > 2° > 1°) and the prevalence of rearrangements • Chemists propose a three-step mechanism for the dehydration of 1° and 2° alcohols. • Because this mechanism involves formation of a carbocation intermediate in the rate-determining step, it is classified as E 1. 40

Dehydration of ROH Step 1: Add a proton. Proton transfer to the -OH group gives an oxonium ion. Step 2: Break a bond to give stable molecules or ions. Loss of H 2 O gives a carbocation intermediate. 41

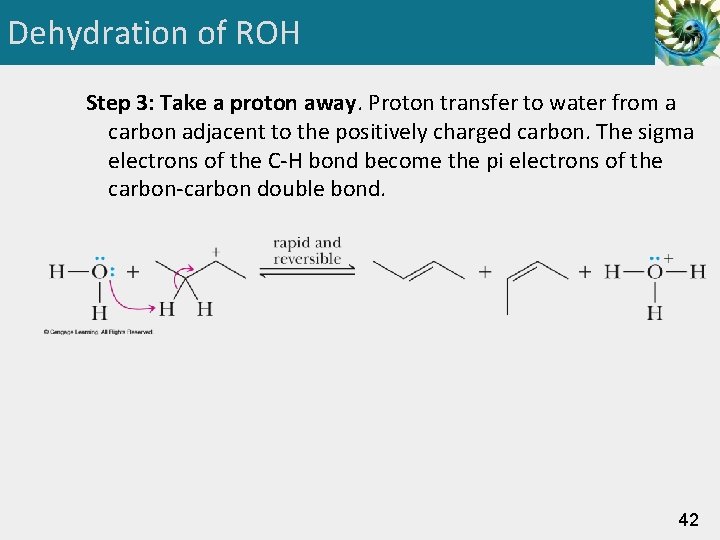
Dehydration of ROH Step 3: Take a proton away. Proton transfer to water from a carbon adjacent to the positively charged carbon. The sigma electrons of the C-H bond become the pi electrons of the carbon-carbon double bond. 42

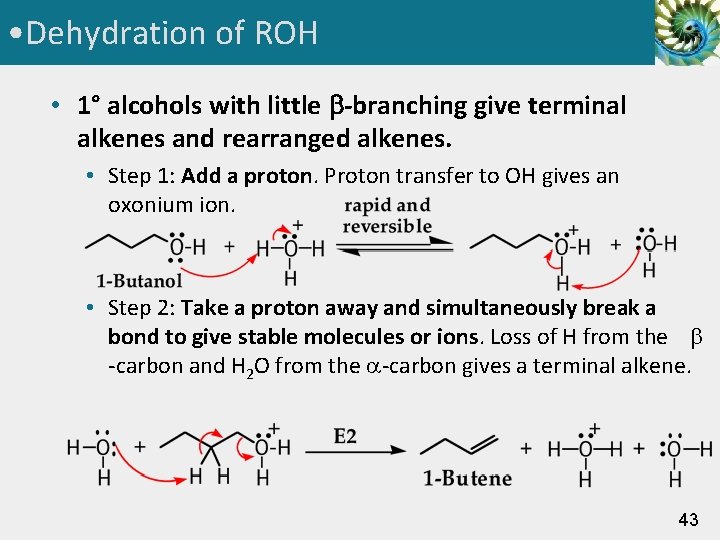
• Dehydration of ROH • 1° alcohols with little -branching give terminal alkenes and rearranged alkenes. • Step 1: Add a proton. Proton transfer to OH gives an oxonium ion. • Step 2: Take a proton away and simultaneously break a bond to give stable molecules or ions. Loss of H from the -carbon and H 2 O from the -carbon gives a terminal alkene. 43

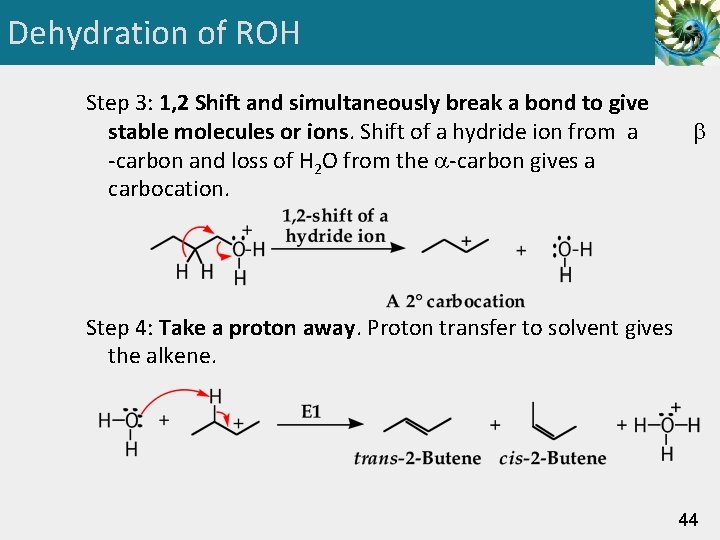
Dehydration of ROH Step 3: 1, 2 Shift and simultaneously break a bond to give stable molecules or ions. Shift of a hydride ion from a -carbon and loss of H 2 O from the -carbon gives a carbocation. Step 4: Take a proton away. Proton transfer to solvent gives the alkene. 44

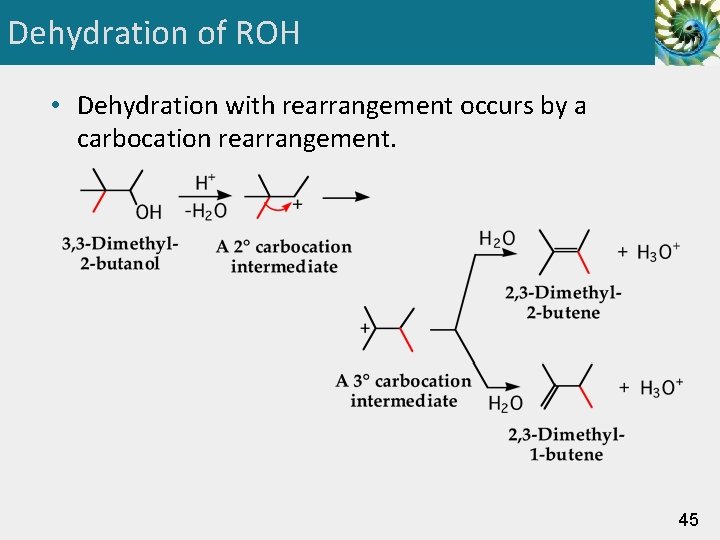
Dehydration of ROH • Dehydration with rearrangement occurs by a carbocation rearrangement. 45

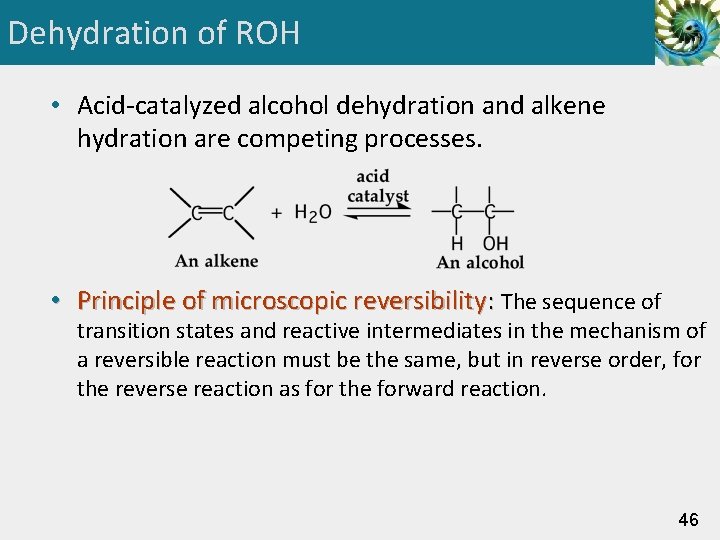
Dehydration of ROH • Acid-catalyzed alcohol dehydration and alkene hydration are competing processes. • Principle of microscopic reversibility: The sequence of transition states and reactive intermediates in the mechanism of a reversible reaction must be the same, but in reverse order, for the reverse reaction as for the forward reaction. 46

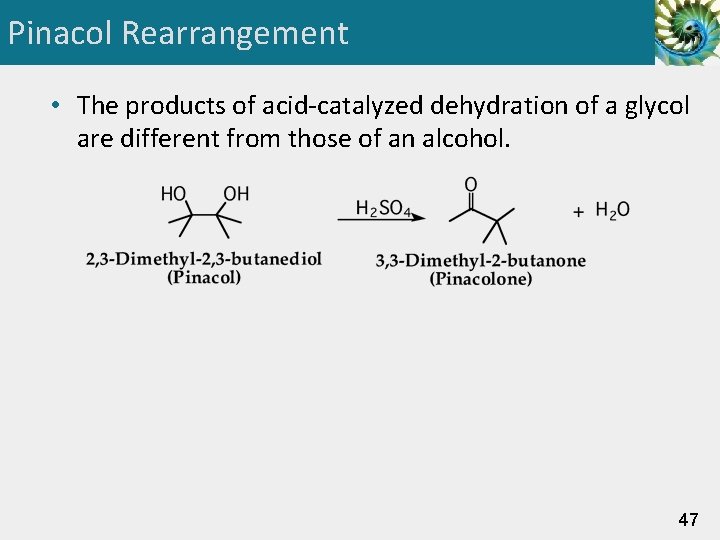
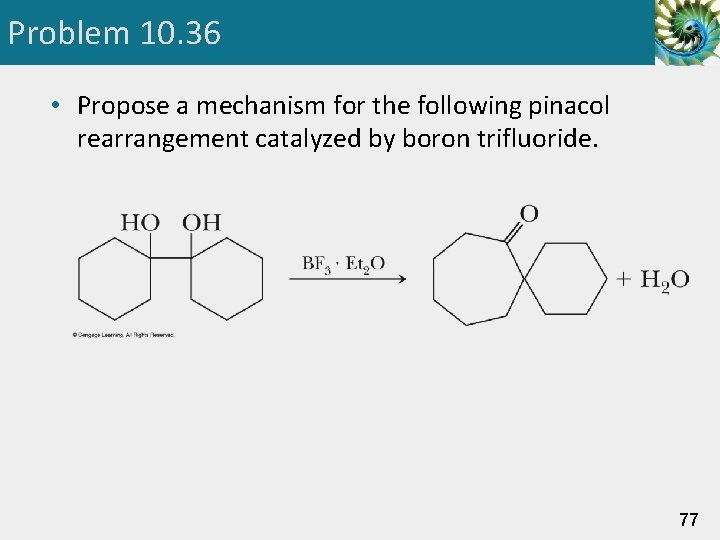
Pinacol Rearrangement • The products of acid-catalyzed dehydration of a glycol are different from those of an alcohol. 47

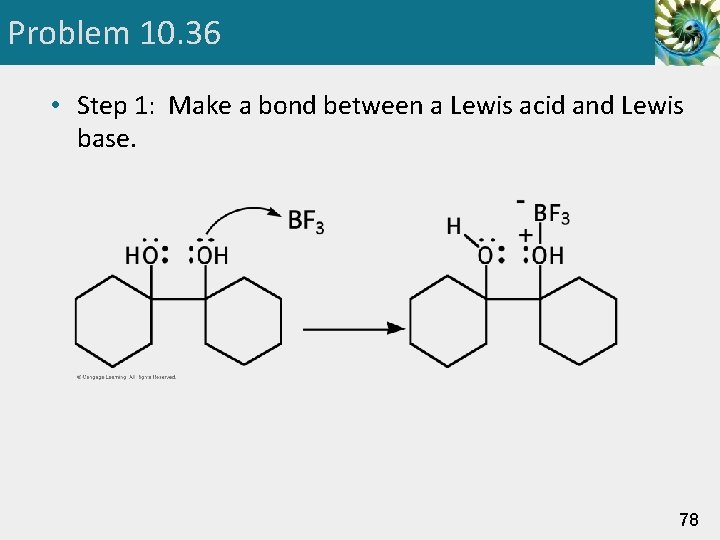
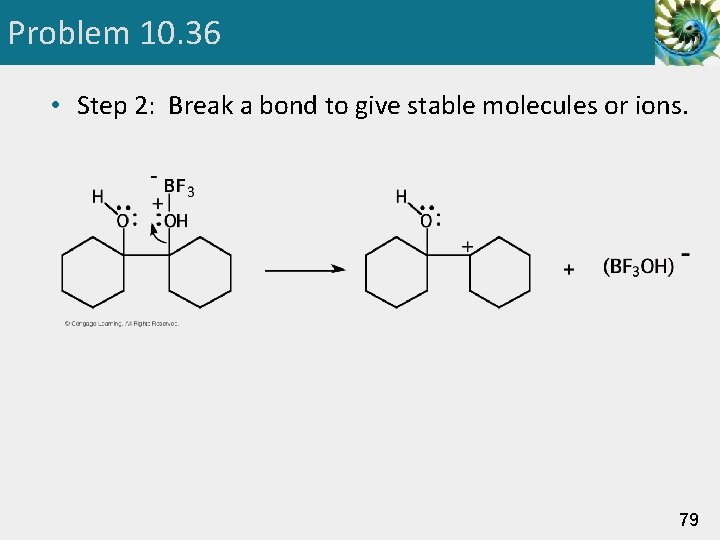
Pinacol Rearrangement Step 1: Add a proton. Proton transfer to -OH gives an oxonium ion. Step 2: Break a bond to give stable molecules or ions. Loss of water gives a carbocation intermediate. 48

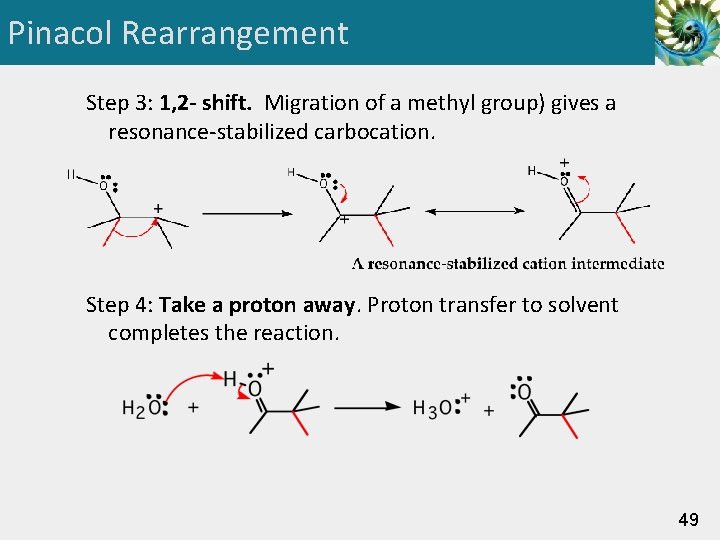
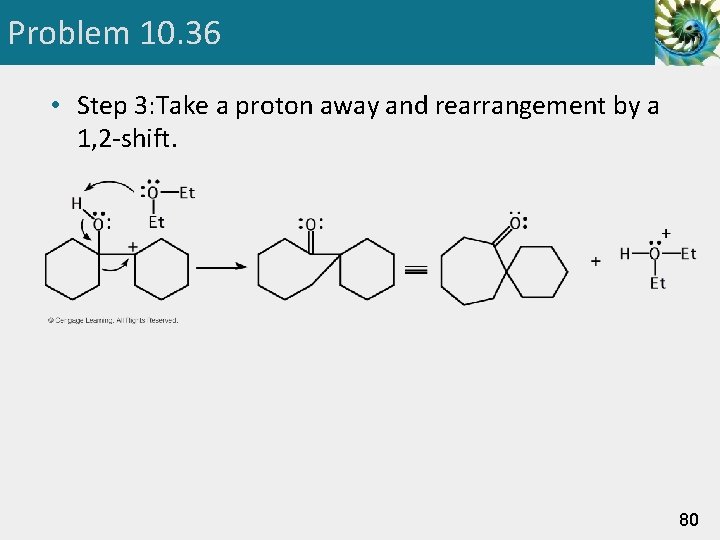
Pinacol Rearrangement Step 3: 1, 2 - shift. Migration of a methyl group) gives a resonance-stabilized carbocation. Step 4: Take a proton away. Proton transfer to solvent completes the reaction. 49

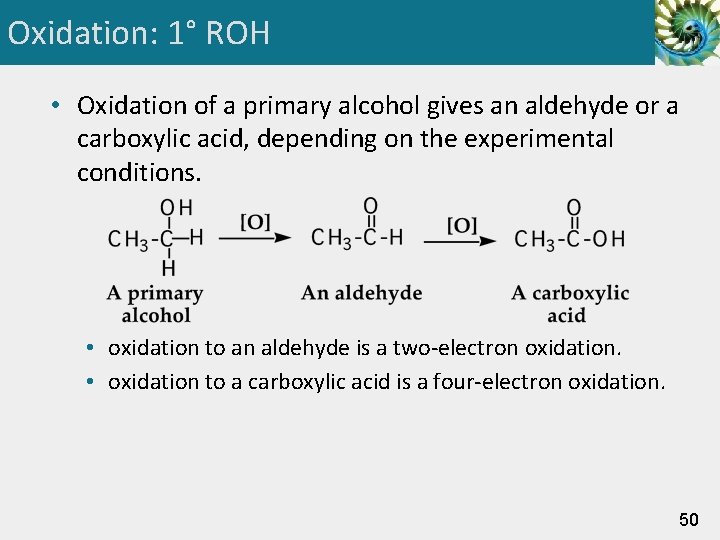
Oxidation: 1° ROH • Oxidation of a primary alcohol gives an aldehyde or a carboxylic acid, depending on the experimental conditions. • oxidation to an aldehyde is a two-electron oxidation. • oxidation to a carboxylic acid is a four-electron oxidation. 50

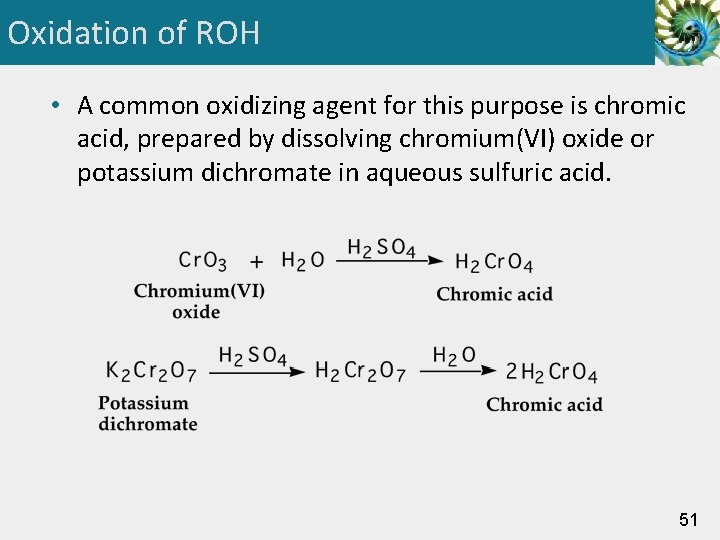
Oxidation of ROH • A common oxidizing agent for this purpose is chromic acid, prepared by dissolving chromium(VI) oxide or potassium dichromate in aqueous sulfuric acid. 51

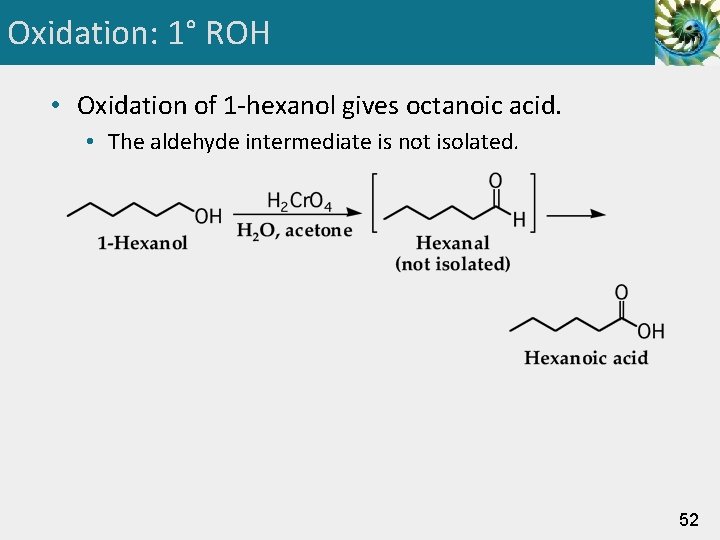
Oxidation: 1° ROH • Oxidation of 1 -hexanol gives octanoic acid. • The aldehyde intermediate is not isolated. 52

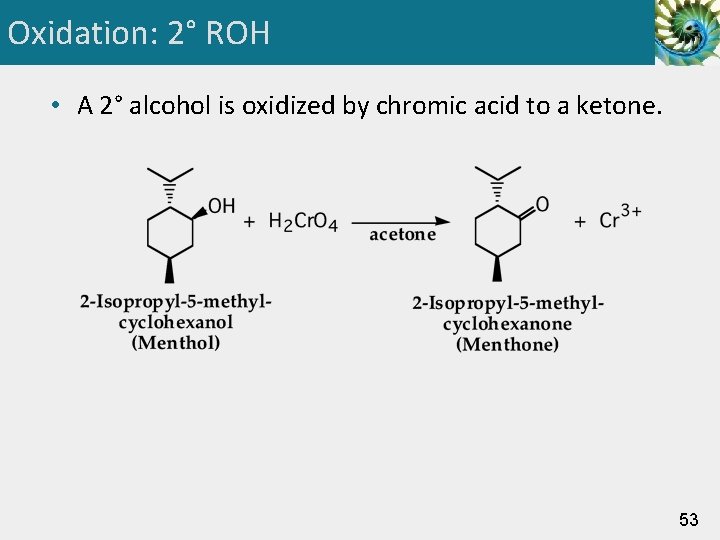
Oxidation: 2° ROH • A 2° alcohol is oxidized by chromic acid to a ketone. 53

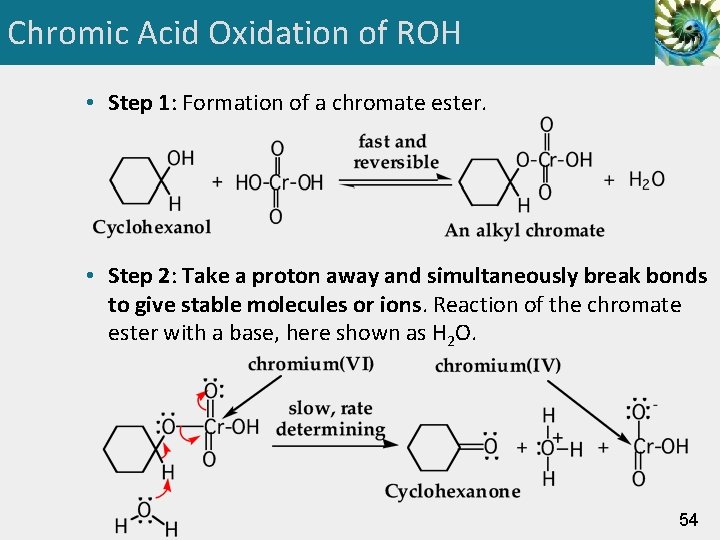
Chromic Acid Oxidation of ROH • Step 1: Formation of a chromate ester. • Step 2: Take a proton away and simultaneously break bonds to give stable molecules or ions. Reaction of the chromate ester with a base, here shown as H 2 O. 54

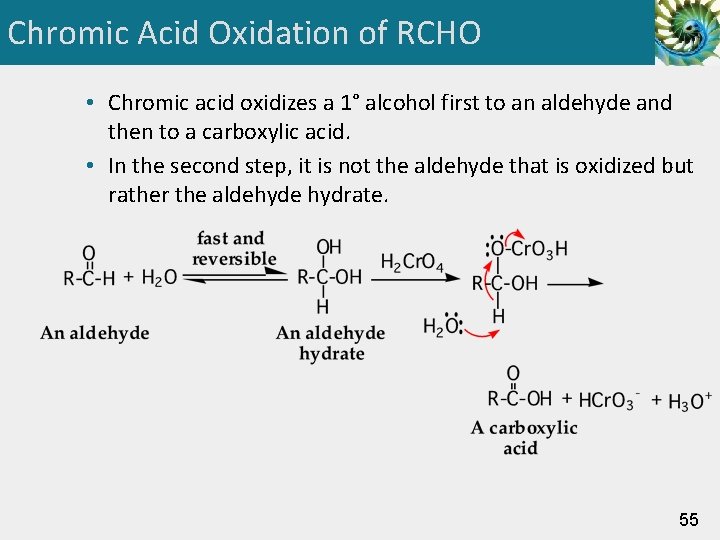
Chromic Acid Oxidation of RCHO • Chromic acid oxidizes a 1° alcohol first to an aldehyde and then to a carboxylic acid. • In the second step, it is not the aldehyde that is oxidized but rather the aldehyde hydrate. 55

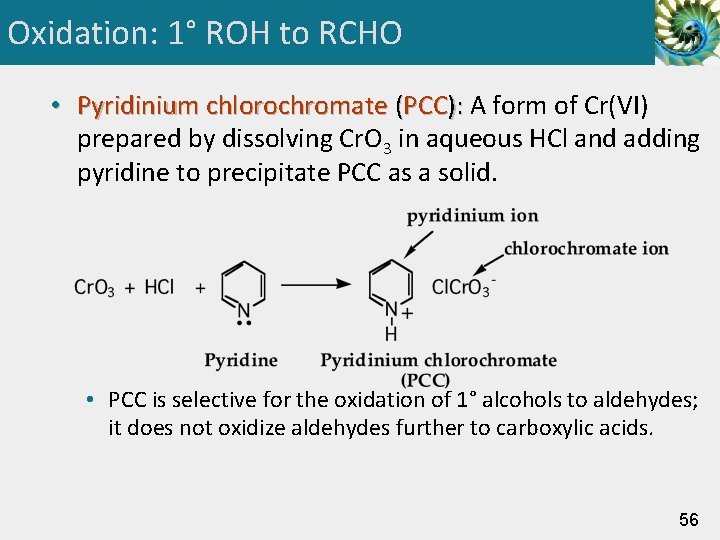
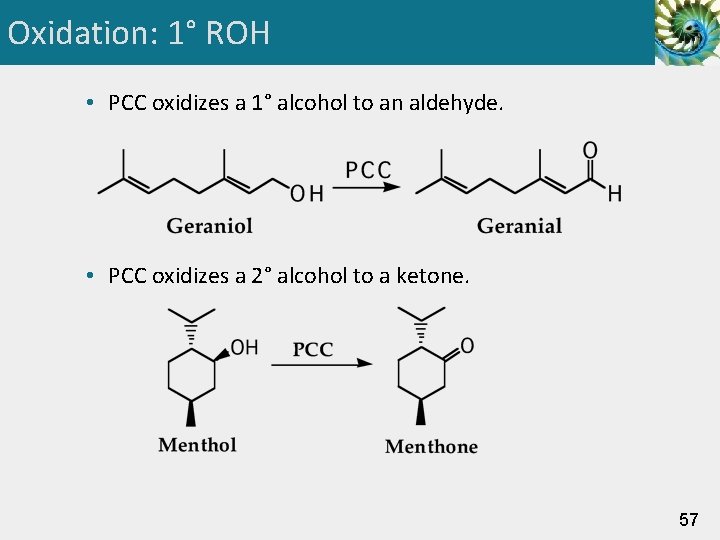
Oxidation: 1° ROH to RCHO • Pyridinium chlorochromate (PCC): A form of Cr(VI) prepared by dissolving Cr. O 3 in aqueous HCl and adding pyridine to precipitate PCC as a solid. • PCC is selective for the oxidation of 1° alcohols to aldehydes; it does not oxidize aldehydes further to carboxylic acids. 56

Oxidation: 1° ROH • PCC oxidizes a 1° alcohol to an aldehyde. • PCC oxidizes a 2° alcohol to a ketone. 57

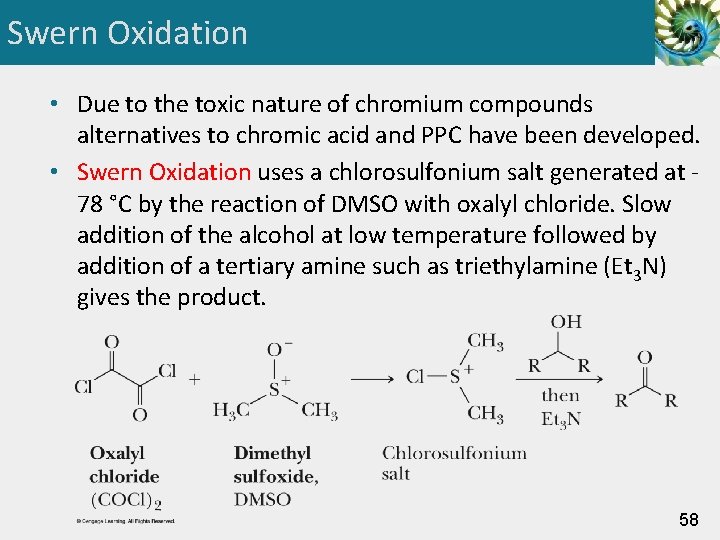
Swern Oxidation • Due to the toxic nature of chromium compounds alternatives to chromic acid and PPC have been developed. • Swern Oxidation uses a chlorosulfonium salt generated at 78 °C by the reaction of DMSO with oxalyl chloride. Slow addition of the alcohol at low temperature followed by addition of a tertiary amine such as triethylamine (Et 3 N) gives the product. 58

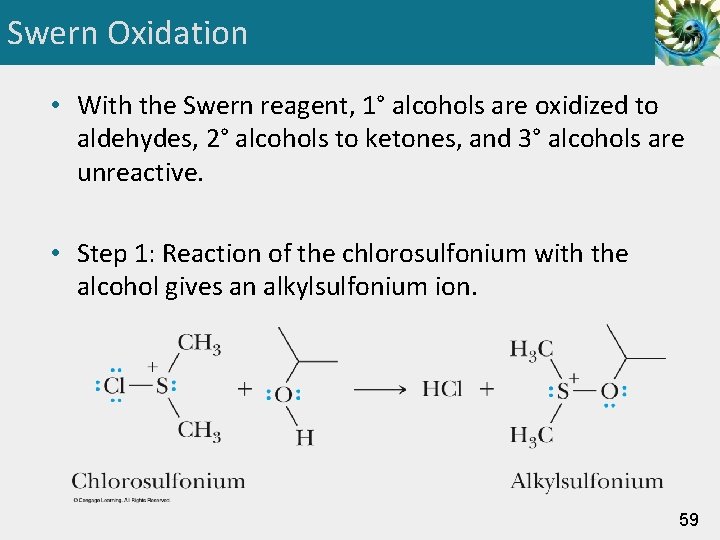
Swern Oxidation • With the Swern reagent, 1° alcohols are oxidized to aldehydes, 2° alcohols to ketones, and 3° alcohols are unreactive. • Step 1: Reaction of the chlorosulfonium with the alcohol gives an alkylsulfonium ion. 59

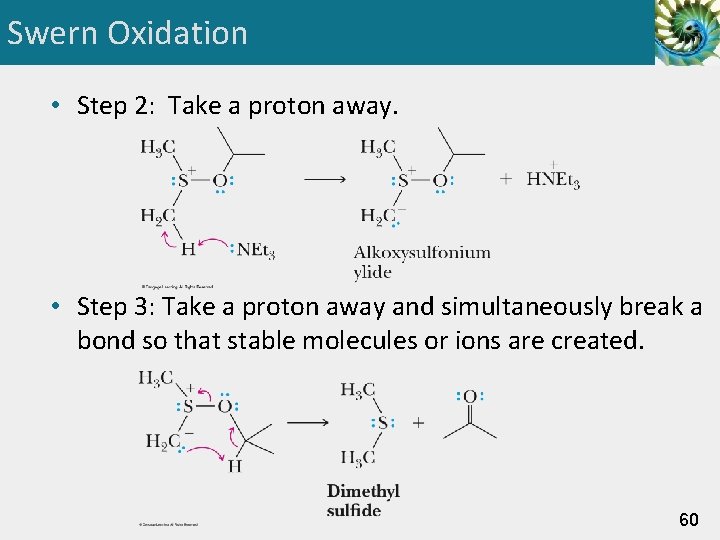
Swern Oxidation • Step 2: Take a proton away. • Step 3: Take a proton away and simultaneously break a bond so that stable molecules or ions are created. 60

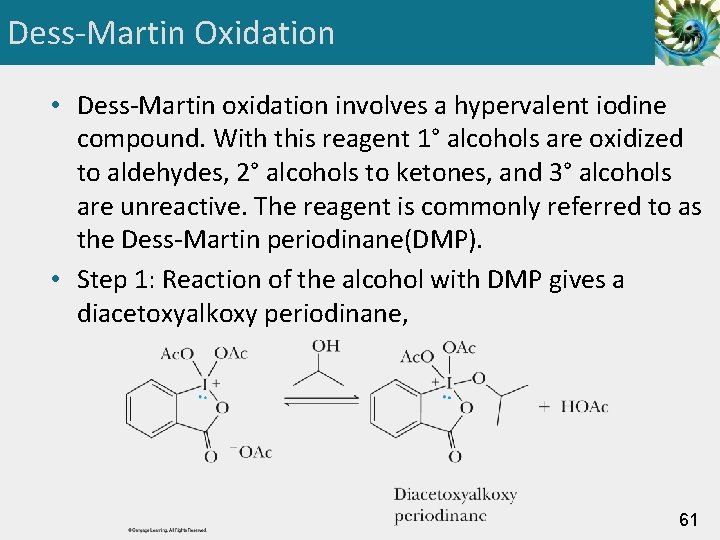
Dess-Martin Oxidation • Dess-Martin oxidation involves a hypervalent iodine compound. With this reagent 1° alcohols are oxidized to aldehydes, 2° alcohols to ketones, and 3° alcohols are unreactive. The reagent is commonly referred to as the Dess-Martin periodinane(DMP). • Step 1: Reaction of the alcohol with DMP gives a diacetoxyalkoxy periodinane, 61

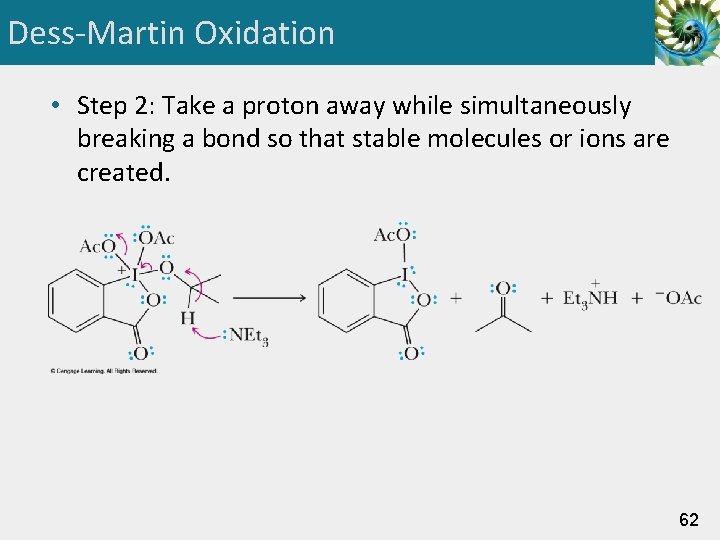
Dess-Martin Oxidation • Step 2: Take a proton away while simultaneously breaking a bond so that stable molecules or ions are created. 62

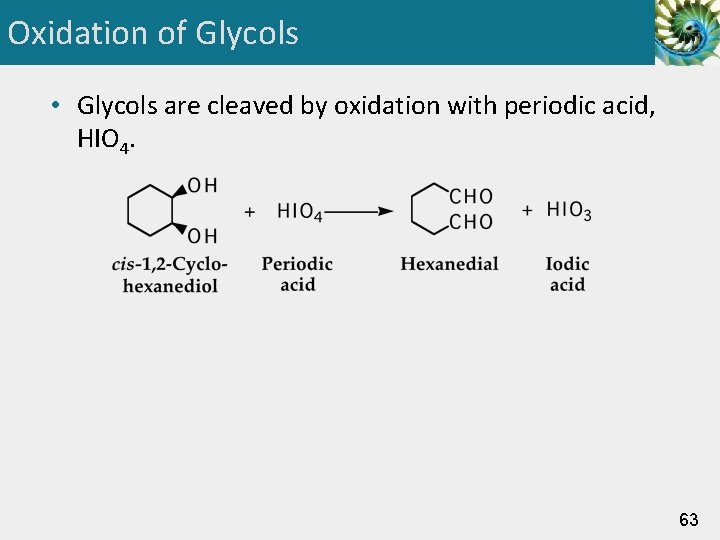
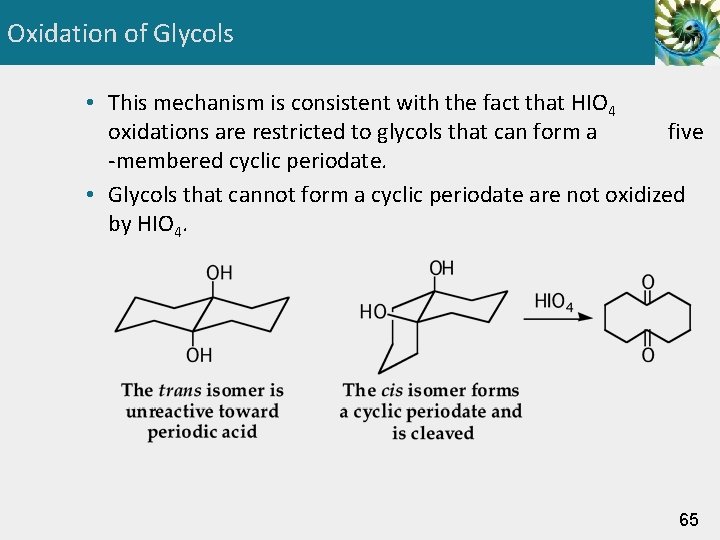
Oxidation of Glycols • Glycols are cleaved by oxidation with periodic acid, HIO 4. 63

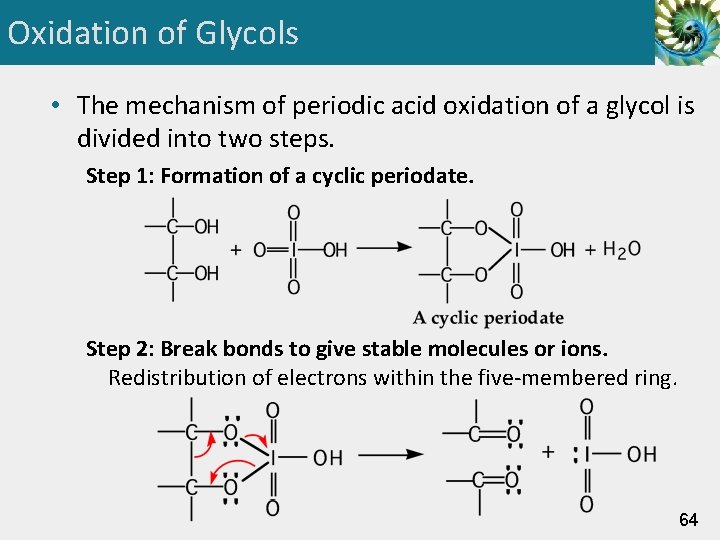
Oxidation of Glycols • The mechanism of periodic acid oxidation of a glycol is divided into two steps. Step 1: Formation of a cyclic periodate. Step 2: Break bonds to give stable molecules or ions. Redistribution of electrons within the five-membered ring. 64

Oxidation of Glycols • This mechanism is consistent with the fact that HIO 4 oxidations are restricted to glycols that can form a five -membered cyclic periodate. • Glycols that cannot form a cyclic periodate are not oxidized by HIO 4. 65

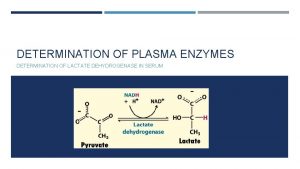
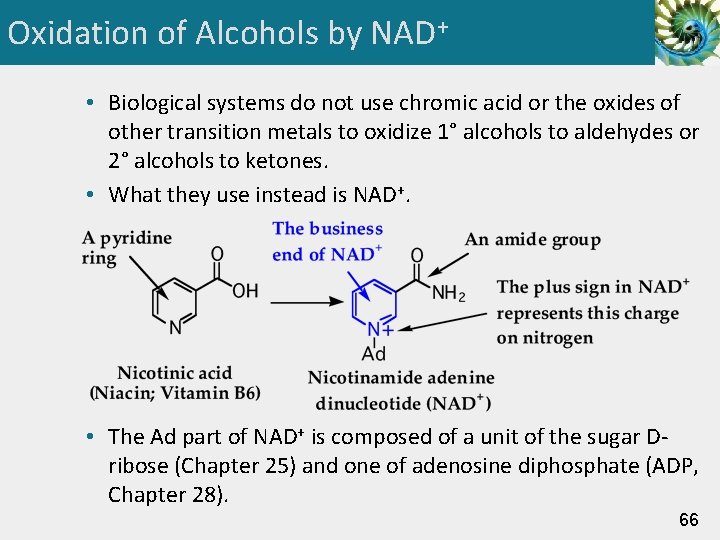
Oxidation of Alcohols by NAD+ • Biological systems do not use chromic acid or the oxides of other transition metals to oxidize 1° alcohols to aldehydes or 2° alcohols to ketones. • What they use instead is NAD+. • The Ad part of NAD+ is composed of a unit of the sugar Dribose (Chapter 25) and one of adenosine diphosphate (ADP, Chapter 28). 66

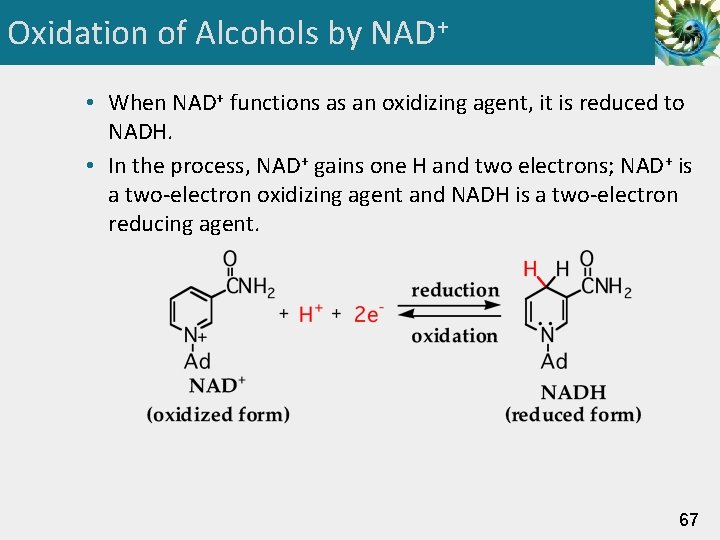
Oxidation of Alcohols by NAD+ • When NAD+ functions as an oxidizing agent, it is reduced to NADH. • In the process, NAD+ gains one H and two electrons; NAD+ is a two-electron oxidizing agent and NADH is a two-electron reducing agent. 67

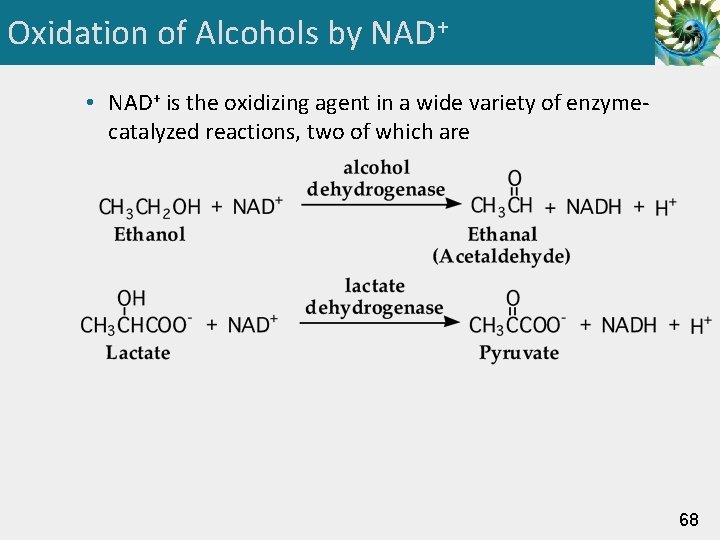
Oxidation of Alcohols by NAD+ • NAD+ is the oxidizing agent in a wide variety of enzymecatalyzed reactions, two of which are 68

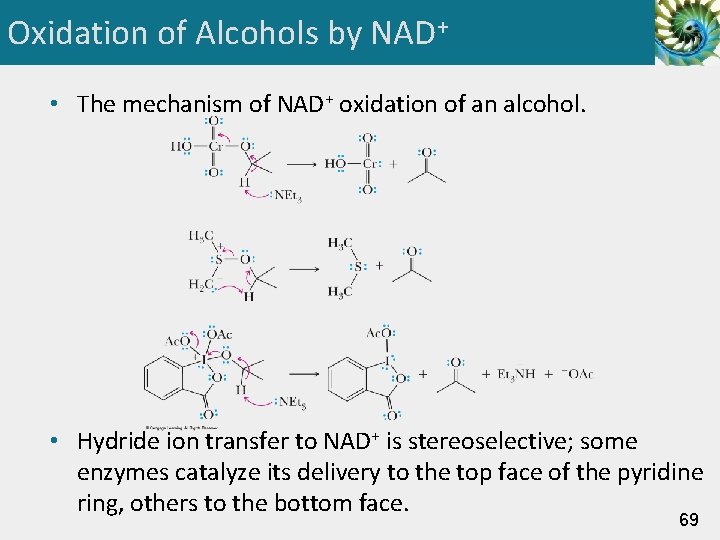
Oxidation of Alcohols by NAD+ • The mechanism of NAD+ oxidation of an alcohol. • Hydride ion transfer to NAD+ is stereoselective; some enzymes catalyze its delivery to the top face of the pyridine ring, others to the bottom face. 69

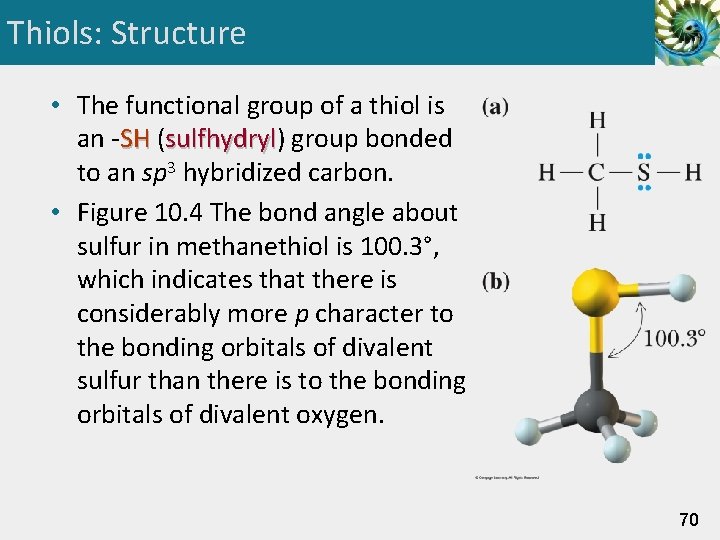
Thiols: Structure • The functional group of a thiol is an -SH (sulfhydryl) sulfhydryl group bonded to an sp 3 hybridized carbon. • Figure 10. 4 The bond angle about sulfur in methanethiol is 100. 3°, which indicates that there is considerably more p character to the bonding orbitals of divalent sulfur than there is to the bonding orbitals of divalent oxygen. 70

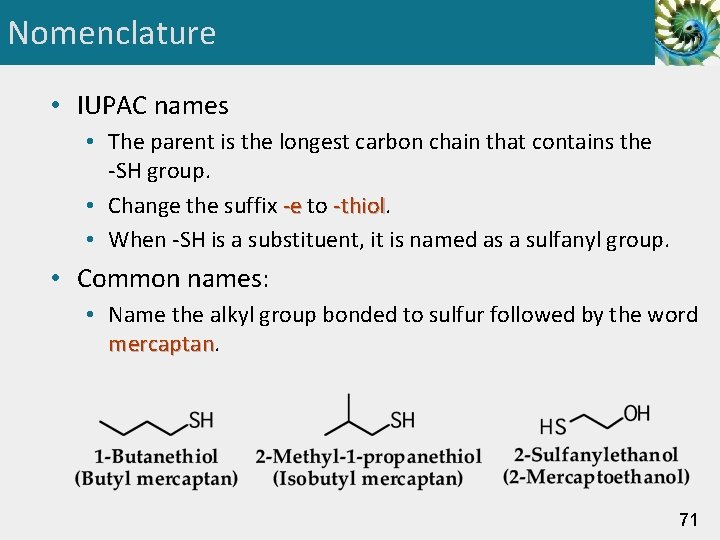
Nomenclature • IUPAC names • The parent is the longest carbon chain that contains the -SH group. • Change the suffix -e to -thiol • When -SH is a substituent, it is named as a sulfanyl group. • Common names: • Name the alkyl group bonded to sulfur followed by the word mercaptan 71

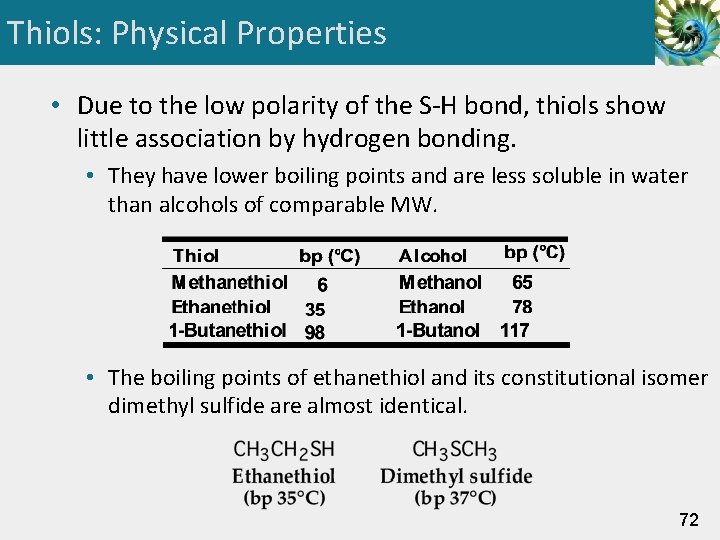
Thiols: Physical Properties • Due to the low polarity of the S-H bond, thiols show little association by hydrogen bonding. • They have lower boiling points and are less soluble in water than alcohols of comparable MW. • The boiling points of ethanethiol and its constitutional isomer dimethyl sulfide are almost identical. 72

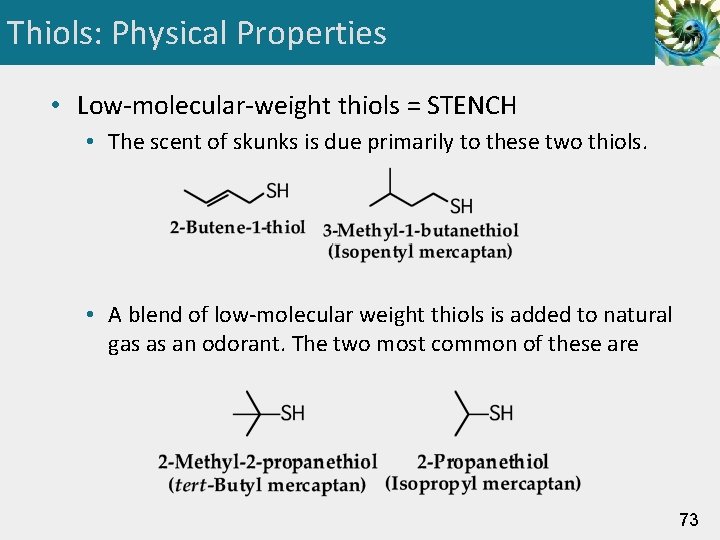
Thiols: Physical Properties • Low-molecular-weight thiols = STENCH • The scent of skunks is due primarily to these two thiols. • A blend of low-molecular weight thiols is added to natural gas as an odorant. The two most common of these are 73

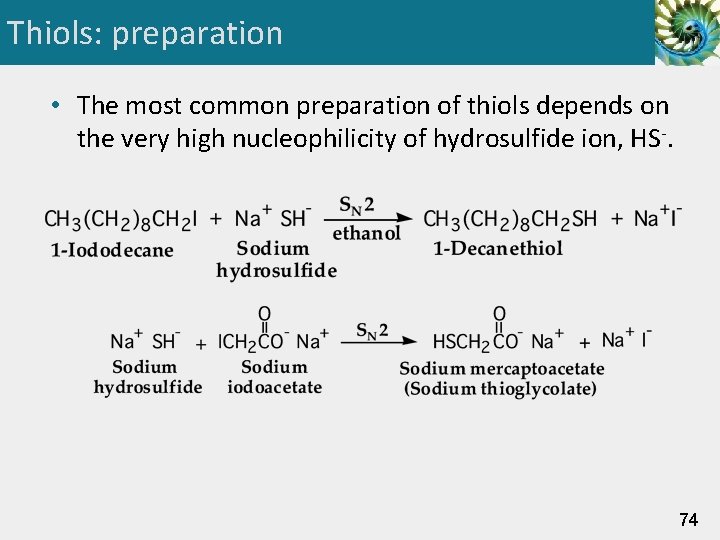
Thiols: preparation • The most common preparation of thiols depends on the very high nucleophilicity of hydrosulfide ion, HS-. 74

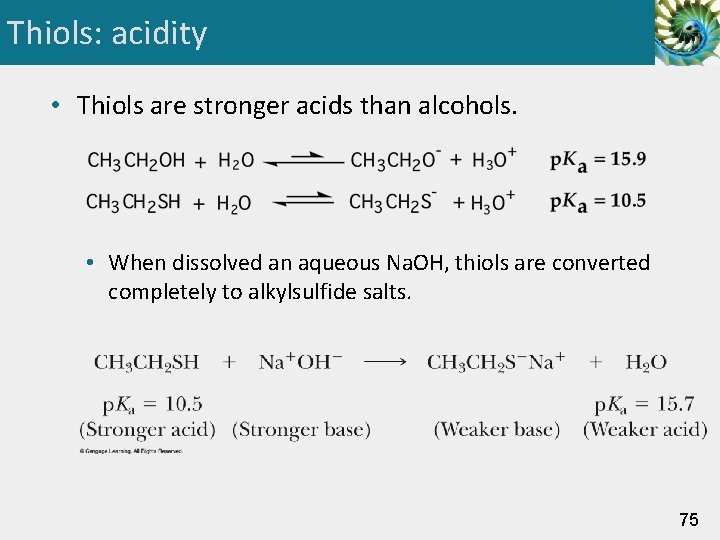
Thiols: acidity • Thiols are stronger acids than alcohols. • When dissolved an aqueous Na. OH, thiols are converted completely to alkylsulfide salts. 75

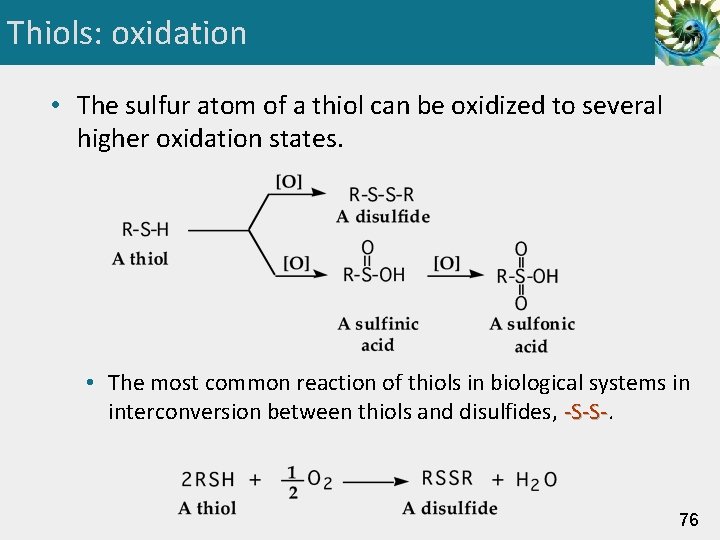
Thiols: oxidation • The sulfur atom of a thiol can be oxidized to several higher oxidation states. • The most common reaction of thiols in biological systems in interconversion between thiols and disulfides, -S-S- 76

Problem 10. 36 • Propose a mechanism for the following pinacol rearrangement catalyzed by boron trifluoride. 77

Problem 10. 36 • Step 1: Make a bond between a Lewis acid and Lewis base. 78

Problem 10. 36 • Step 2: Break a bond to give stable molecules or ions. 79

Problem 10. 36 • Step 3: Take a proton away and rearrangement by a 1, 2 -shift. 80
 Band and loop space maintainer advantages
Band and loop space maintainer advantages Non functional plasma enzyme
Non functional plasma enzyme Plasma enzymes
Plasma enzymes Functional and non functional
Functional and non functional Oxidation of primary alcohol to aldehyde
Oxidation of primary alcohol to aldehyde These are alcohols containing cppp nucleus
These are alcohols containing cppp nucleus What is secondary alcohol
What is secondary alcohol Primary alcohol secondary alcohol
Primary alcohol secondary alcohol Naocl reaction with alcohol
Naocl reaction with alcohol Lucas test
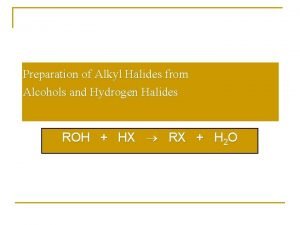
Lucas test Preparation of alkyl halides from alcohols
Preparation of alkyl halides from alcohols Chemsheets reactions of alcohols 1 answers
Chemsheets reactions of alcohols 1 answers Acidity of alcohols
Acidity of alcohols Sp
Sp Names of sugar alcohols
Names of sugar alcohols Alcohols nomenclature
Alcohols nomenclature Alcohols nomenclature
Alcohols nomenclature Ethers naming
Ethers naming Butanone isomers
Butanone isomers Diol oxidation
Diol oxidation Reduction of alcohol to alkane
Reduction of alcohol to alkane Alcohols phenols thiols and ethers
Alcohols phenols thiols and ethers Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Lp html
Lp html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Thang điểm glasgow
Thang điểm glasgow Hát lên người ơi
Hát lên người ơi Các môn thể thao bắt đầu bằng tiếng chạy
Các môn thể thao bắt đầu bằng tiếng chạy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Cong thức tính động năng
Cong thức tính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Phép trừ bù
Phép trừ bù Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Bàn tay mà dây bẩn
Bàn tay mà dây bẩn Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Nguyên nhân của sự mỏi cơ sinh 8
Nguyên nhân của sự mỏi cơ sinh 8 đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ V. c c
V. c c Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Tia chieu sa te
Tia chieu sa te Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Dot
Dot Các số nguyên tố
Các số nguyên tố Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Hổ sinh sản vào mùa nào
Hổ sinh sản vào mùa nào Sự nuôi và dạy con của hổ
Sự nuôi và dạy con của hổ Sơ đồ cơ thể người
Sơ đồ cơ thể người Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Integrated cost leadership/differentiation strategy
Integrated cost leadership/differentiation strategy Basis of strategy
Basis of strategy Fundamen
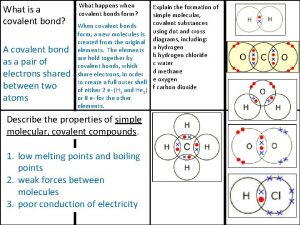
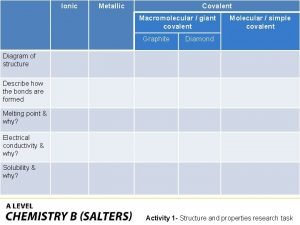
Fundamen Covalently bonded substances
Covalently bonded substances Union myunion structure my structure integer m
Union myunion structure my structure integer m Ionic covalent metallic
Ionic covalent metallic Structural ambiguity exercises
Structural ambiguity exercises Deep surface structure
Deep surface structure S s' 's grammar
S s' 's grammar Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Static data structure
Static data structure Yang merupakan pengertian dari rekaman data adalah ?..
Yang merupakan pengertian dari rekaman data adalah ?.. Deep surface structure
Deep surface structure Workwell functional capacity evaluation
Workwell functional capacity evaluation Why are geographers concerned with scale and connectedness
Why are geographers concerned with scale and connectedness Srmvcas
Srmvcas Iot design methodology examples
Iot design methodology examples