Chapter 1 Structure and Bonding Acids and Bases
Chapter 1 Structure and Bonding Acids and Bases Acids & Bases/ Organic Chemistry Dr. Ron Rusay Fall 2009
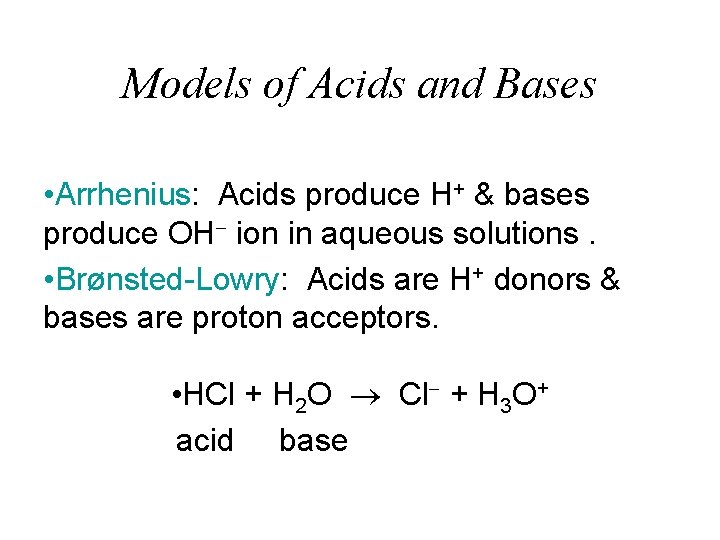
Models of Acids and Bases • Arrhenius: Acids produce H+ & bases produce OH ion in aqueous solutions. • Brønsted-Lowry: Acids are H+ donors & bases are proton acceptors. • HCl + H 2 O Cl + H 3 O+ acid base
Comprehensive Tutorial Acid & Base Principles in Organic Chemistry Highly recommended viewing
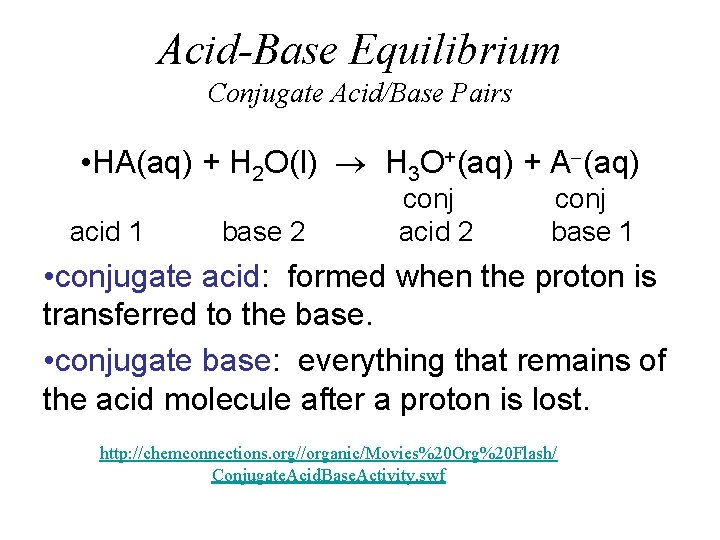
Acid-Base Equilibrium Conjugate Acid/Base Pairs • HA(aq) + H 2 O(l) H 3 O+(aq) + A (aq) acid 1 base 2 conj acid 2 conj base 1 • conjugate acid: formed when the proton is transferred to the base. • conjugate base: everything that remains of the acid molecule after a proton is lost. http: //chemconnections. org//organic/Movies%20 Org%20 Flash/ Conjugate. Acid. Base. Activity. swf
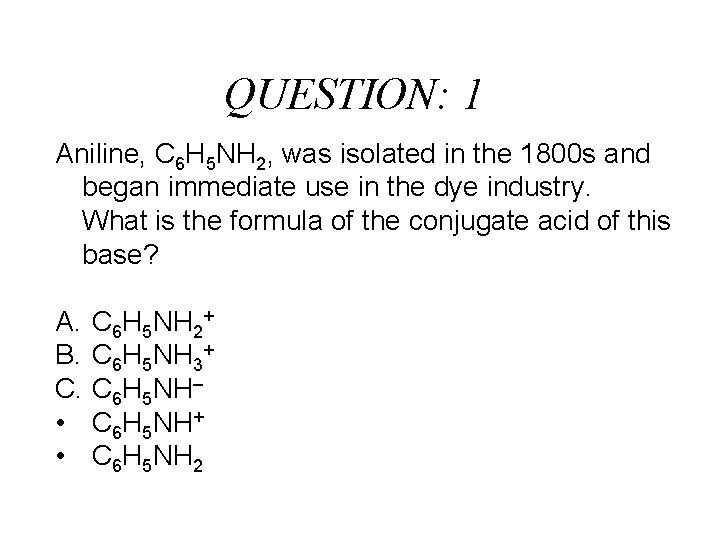
QUESTION: 1 Aniline, C 6 H 5 NH 2, was isolated in the 1800 s and began immediate use in the dye industry. What is the formula of the conjugate acid of this base? A. B. C. • • C 6 H 5 NH 2+ C 6 H 5 NH 3+ C 6 H 5 NH– C 6 H 5 NH+ C 6 H 5 NH 2
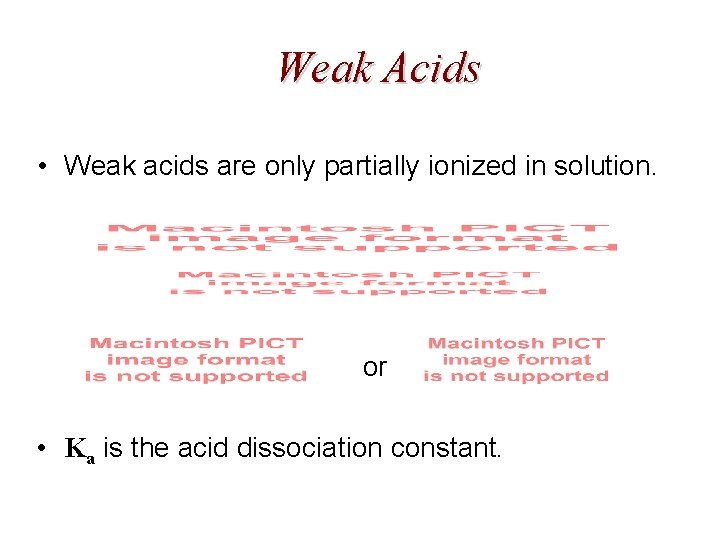
Weak Acids • Weak acids are only partially ionized in solution. or • Ka is the acid dissociation constant.

Organic Acids & Bases • Organic acids are weak acids, eg. Acetic acid. • However, there can be substantial differences in their relative strengths. What could you use to compare relative acidities? • Organic bases are weak bases and relate to ammonia. • However, there can be substantial differences in their relative strengths. What could you use to compare relative basicity?
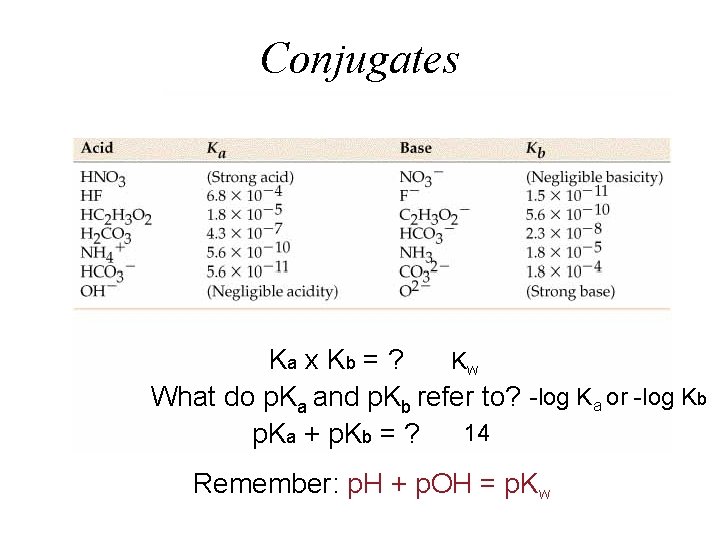
Conjugates Ka x K b = ? Kw What do p. Ka and p. Kb refer to? -log Ka or -log Kb 14 p. Ka + p. Kb = ? Remember: p. H + p. OH = p. Kw
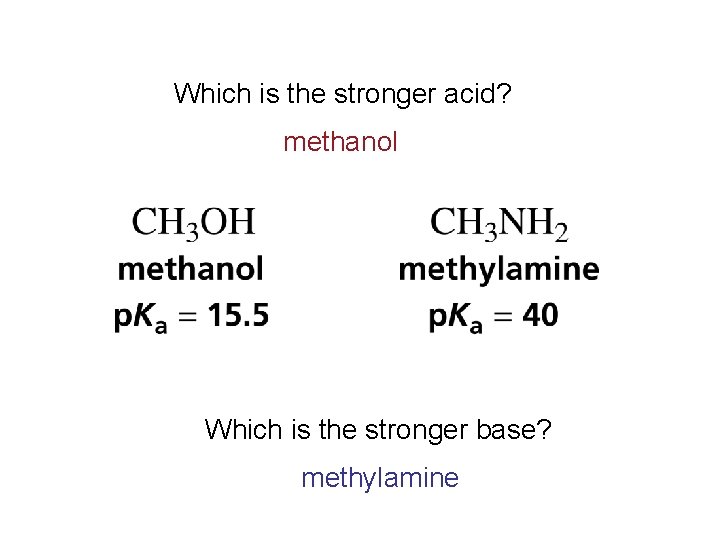
Which is the stronger acid? methanol Which is the stronger base? methylamine
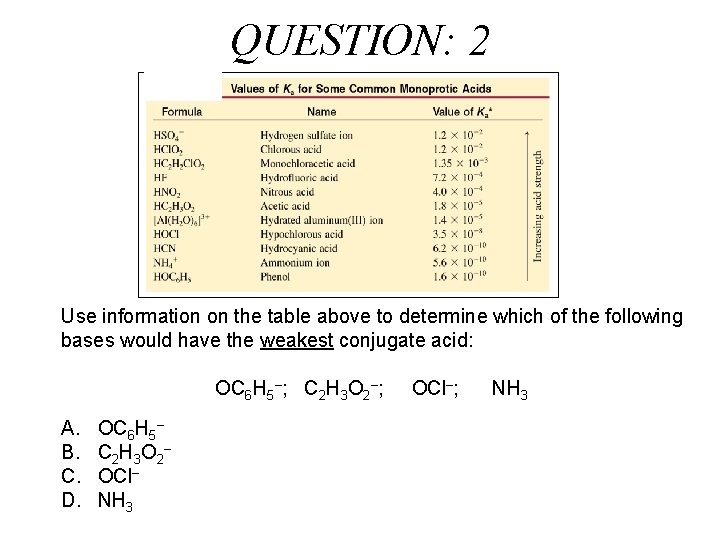
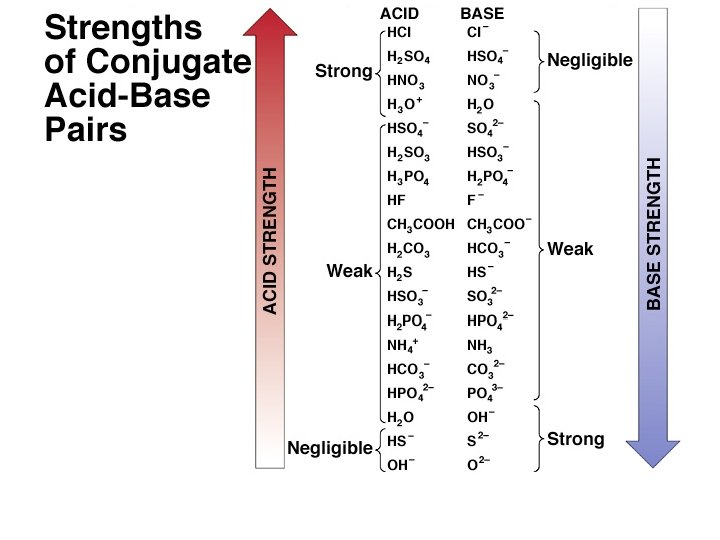
QUESTION: 2 Use information on the table above to determine which of the following bases would have the weakest conjugate acid: OC 6 H 5–; C 2 H 3 O 2–; A. B. C. D. OC 6 H 5– C 2 H 3 O 2 – OCl– NH 3 OCl–; NH 3
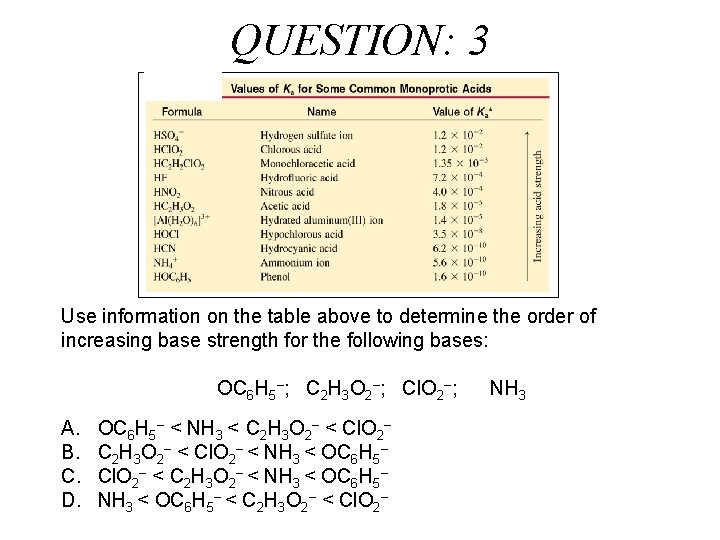
QUESTION: 3 Use information on the table above to determine the order of increasing base strength for the following bases: OC 6 H 5–; C 2 H 3 O 2–; Cl. O 2–; A. B. C. D. OC 6 H 5– < NH 3 < C 2 H 3 O 2– < Cl. O 2– < NH 3 < OC 6 H 5– Cl. O 2– < C 2 H 3 O 2– < NH 3 < OC 6 H 5– < C 2 H 3 O 2– < Cl. O 2– NH 3
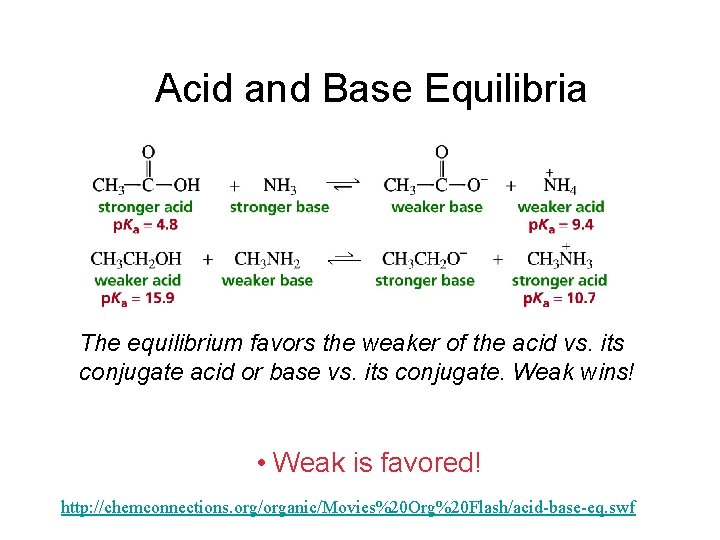
Acid and Base Equilibria The equilibrium favors the weaker of the acid vs. its conjugate acid or base vs. its conjugate. Weak wins! • Weak is favored! http: //chemconnections. org/organic/Movies%20 Org%20 Flash/acid-base-eq. swf
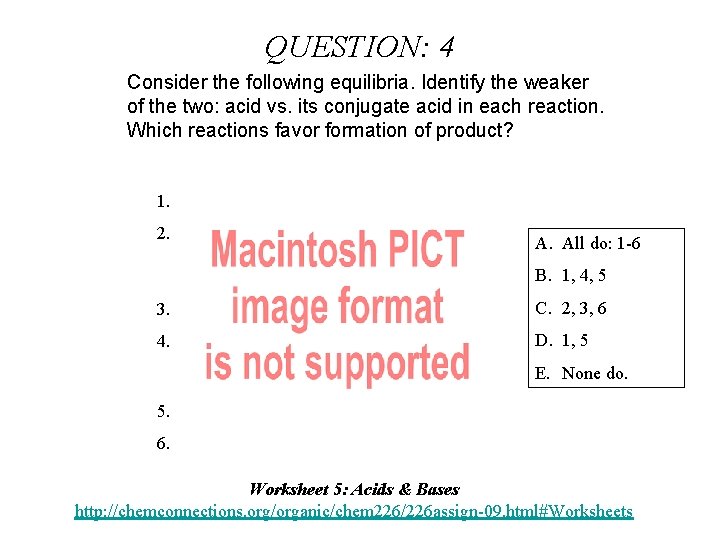
QUESTION: 4 Consider the following equilibria. Identify the weaker of the two: acid vs. its conjugate acid in each reaction. Which reactions favor formation of product? 1. 2. A. All do: 1 -6 B. 1, 4, 5 3. C. 2, 3, 6 4. D. 1, 5 E. None do. 5. 6. Worksheet 5: Acids & Bases http: //chemconnections. org/organic/chem 226/226 assign-09. html#Worksheets

Organic Acids & Bases • Organic molecules in context can be considered as behaving relatively as weak acids or weak bases. • Formal Charge is important in considering which. • Knowing the Formal Charge allows a prediction. • (+) positive atoms behave acid-like, (-) negative atoms behave base-like. • This can be used in predicting how molecules will react--- or don’t react, and the products of reactions.
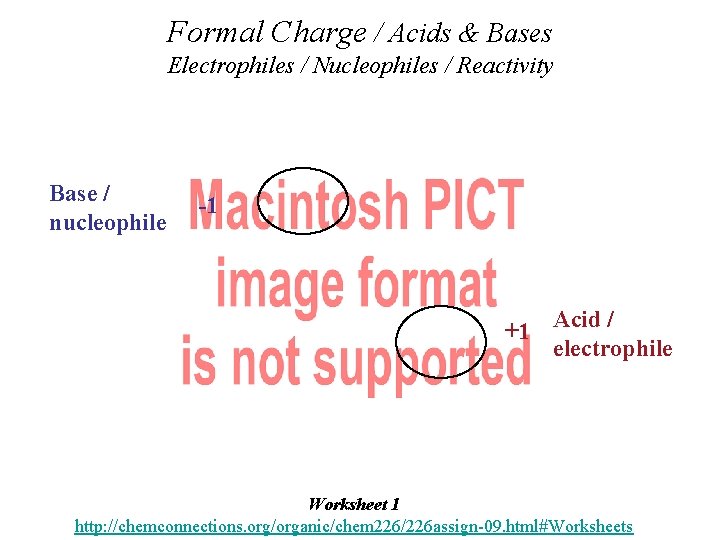
Formal Charge / Acids & Bases Electrophiles / Nucleophiles / Reactivity Base / nucleophile -1 +1 Acid / electrophile Worksheet 1 http: //chemconnections. org/organic/chem 226/226 assign-09. html#Worksheets
Structure and Acid-Base Properties Important factors that effect acidity in binary compounds, eg HCl (having only two elements): • Bond Length (shorter = stronger bonds; lower acidity) • Bond Strength (longer = weaker bonds; higher acidity: more dissociation, more protons [hydronium ions] in solution) • Bond Polarity (smaller e. n. differences favor higher acidities) Select & explain which is the stronger acid: HBr or HF.
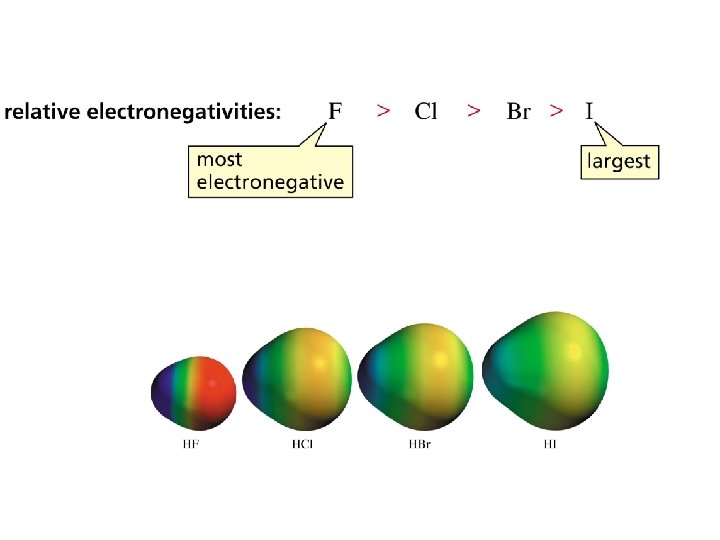
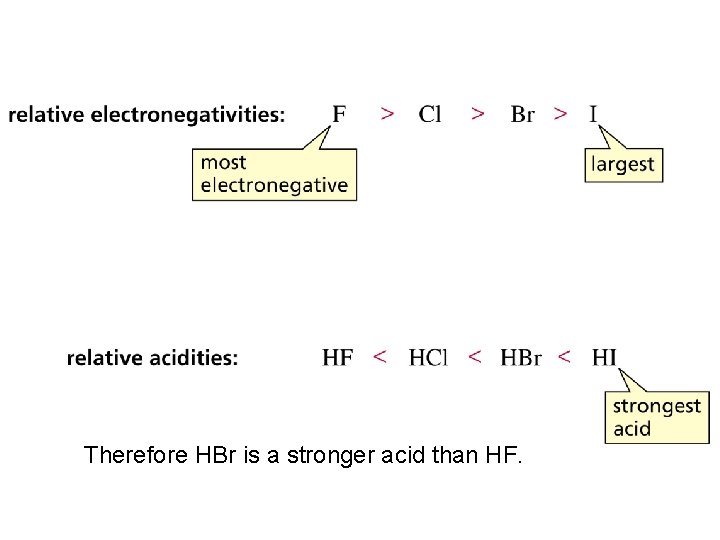
Therefore HBr is a stronger acid than HF.
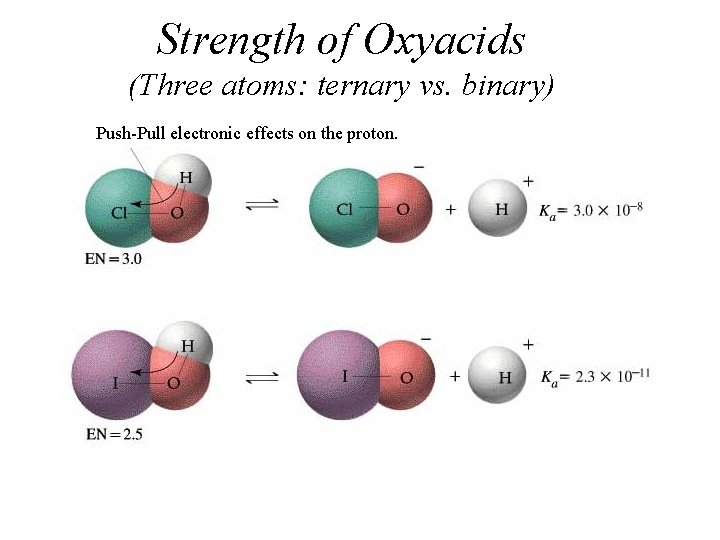
Strength of Oxyacids (Three atoms: ternary vs. binary) Push-Pull electronic effects on the proton.
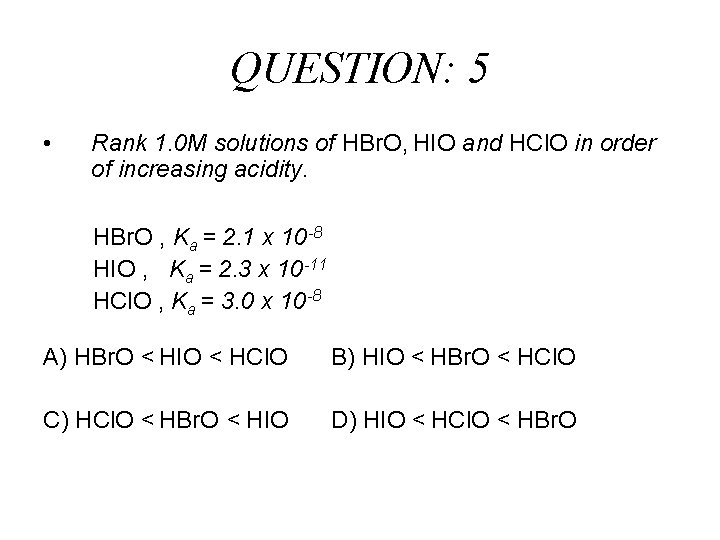
QUESTION: 5 • Rank 1. 0 M solutions of HBr. O, HIO and HCl. O in order of increasing acidity. HBr. O , Ka = 2. 1 x 10 -8 HIO , Ka = 2. 3 x 10 -11 HCl. O , Ka = 3. 0 x 10 -8 A) HBr. O < HIO < HCl. O B) HIO < HBr. O < HCl. O C) HCl. O < HBr. O < HIO D) HIO < HCl. O < HBr. O
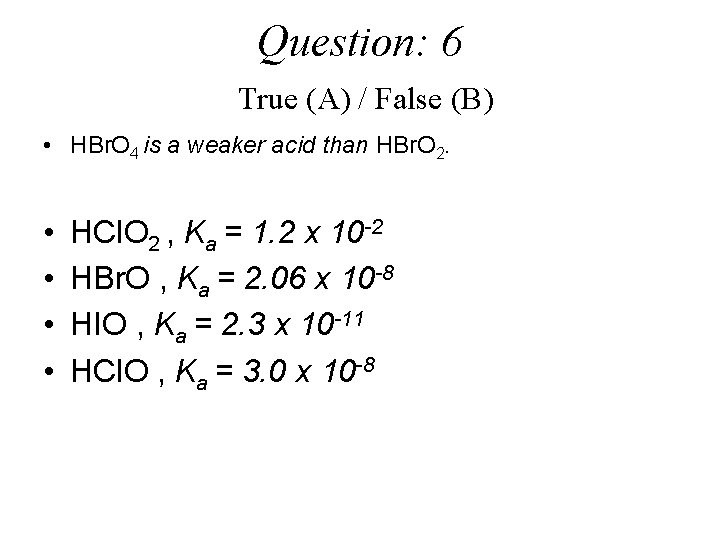
Question: 6 True (A) / False (B) • HBr. O 4 is a weaker acid than HBr. O 2. • • HCl. O 2 , Ka = 1. 2 x 10 -2 HBr. O , Ka = 2. 06 x 10 -8 HIO , Ka = 2. 3 x 10 -11 HCl. O , Ka = 3. 0 x 10 -8
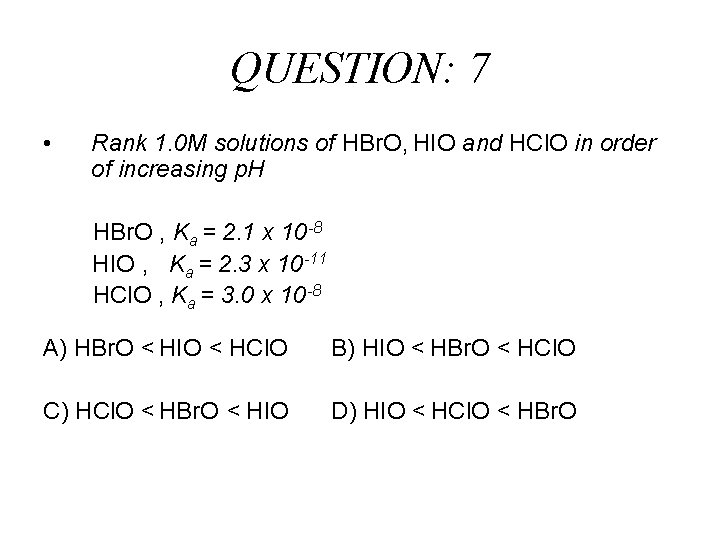
QUESTION: 7 • Rank 1. 0 M solutions of HBr. O, HIO and HCl. O in order of increasing p. H HBr. O , Ka = 2. 1 x 10 -8 HIO , Ka = 2. 3 x 10 -11 HCl. O , Ka = 3. 0 x 10 -8 A) HBr. O < HIO < HCl. O B) HIO < HBr. O < HCl. O C) HCl. O < HBr. O < HIO D) HIO < HCl. O < HBr. O
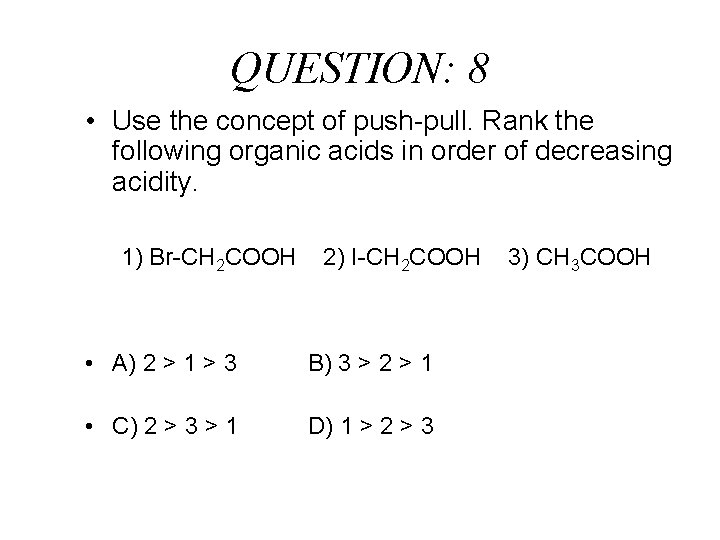
QUESTION: 8 • Use the concept of push-pull. Rank the following organic acids in order of decreasing acidity. 1) Br-CH 2 COOH 2) I-CH 2 COOH • A) 2 > 1 > 3 B) 3 > 2 > 1 • C) 2 > 3 > 1 D) 1 > 2 > 3 3) CH 3 COOH

QUESTION: 9 Rank the following acids in order of decreasing acidity. A) Cl. CH 2 COOH C) Cl 3 CCOOH 1) A > B > C > D 2) D > C > B > A B) Cl 2 CHCOOH D) CH 3 COOH 2) C > B > A > D 3) B > C > A > D

Question: 10 True (A) / False (B) Trichloroacetic acid, Cl 3 CCOOH, is more acidic than trifluoroacetic acid, F 3 CCOOH.

An Organic Base in Context Erythroxylon spp. • It is very valuable. The leaves are chewed by indigenous tribes in the Andes to boost their energy. • It has been used as a psycho-therapeutic, an opthalmic anesthetic and was purportedly used in a popular beverage that is at the heart of a $20 billion corporation. • However, both its base and conjugate acid are currently controlled substances under U. S. Federal Regulations: Title 21 secs. 329. 1 & 1308. 12 (1987). • Can you name the beverage and the base?

The beverage reportedly produced using the extract of leaves of Erythroxylon coca: The compound: cocaine, is an organic base: Merck Index, #2450, 11 th ed. : Caution: May be habit forming….
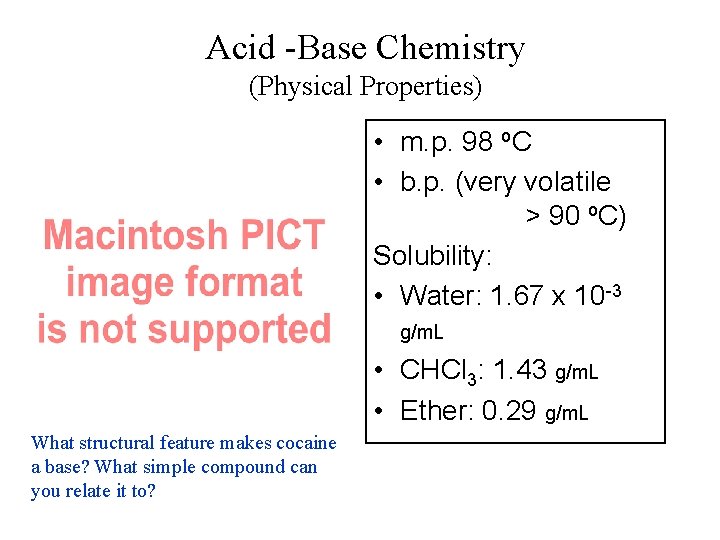
Acid -Base Chemistry (Physical Properties) • m. p. 98 o. C • b. p. (very volatile > 90 o. C) Solubility: • Water: 1. 67 x 10 -3 g/m. L • CHCl 3: 1. 43 g/m. L • Ether: 0. 29 g/m. L What structural feature makes cocaine a base? What simple compound can you relate it to?
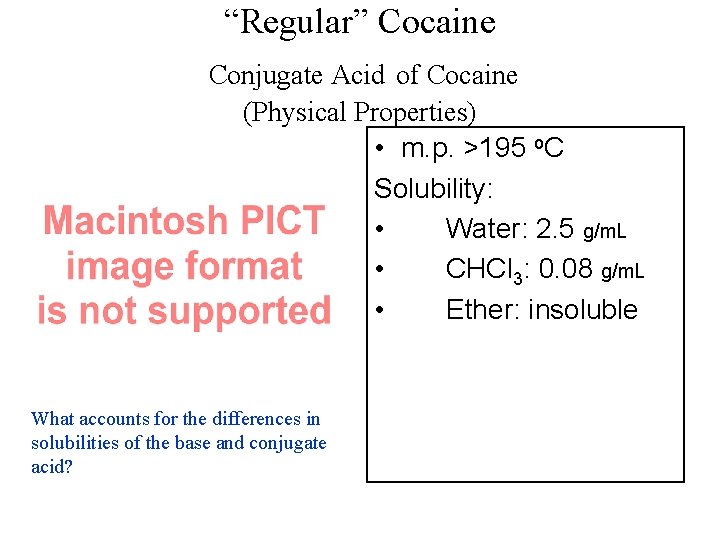
“Regular” Cocaine Conjugate Acid of Cocaine (Physical Properties) • m. p. >195 o. C Solubility: • Water: 2. 5 g/m. L • CHCl 3: 0. 08 g/m. L • Ether: insoluble What accounts for the differences in solubilities of the base and conjugate acid?
Acid -Base Reactions
Acid Base Reactions

Which form, Acid or its Conjugate Base? The p. H of a solution (its surroundings) determines which and is related by the following equation. • A compound will exist primarily in its acidic form if the p. H of the solution is < than its p. Ka • A compound will exist primarily in its basic form if the p. H of the solution is > than its p. Ka • NOTE: A buffer solution maintains a nearly constant p. H within certain parameters.
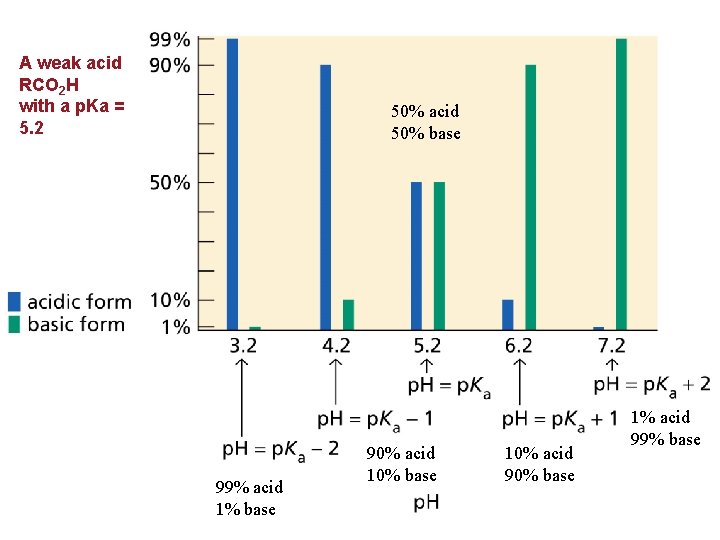
A weak acid RCO 2 H with a p. Ka = 5. 2 50% acid 50% base 99% acid 1% base 90% acid 10% base 10% acid 90% base 1% acid 99% base

QUESTIONS from Worksheet: The p. Ka of a general anesthetic, sodium pentothal, is 7. 4. If a patient is given sodium pentothal orally instead of iv, will it put the patient to sleep? What information is needed to answer this fundamental anesthesiology (“gas passer”) question? A drug has a p. Ka of 7. 8 and is a known teratogen. If given iv to a pregnant woman whose blood p. H is within normal levels, will this drug cross the placenta and affect the baby? What information is needed to answer this anesthesiology (“gas passer”) question?

Lewis Acids and Bases • Lewis Acid: electron pair acceptor • Lewis Base: electron pair donor • Example:
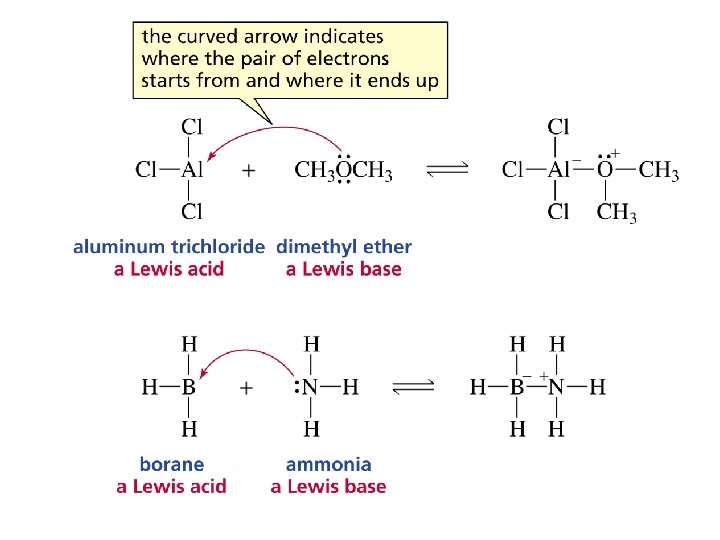
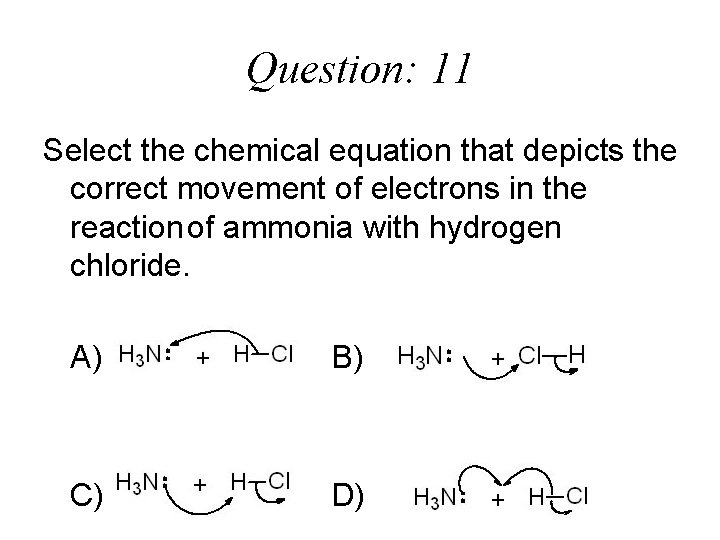
Question: 11 Select the chemical equation that depicts the correct movement of electrons in the reaction of ammonia with hydrogen chloride. A) B) C) D)
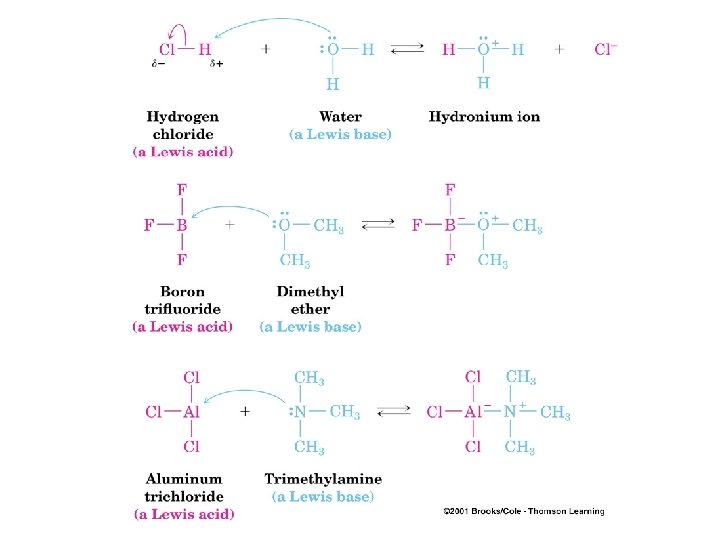
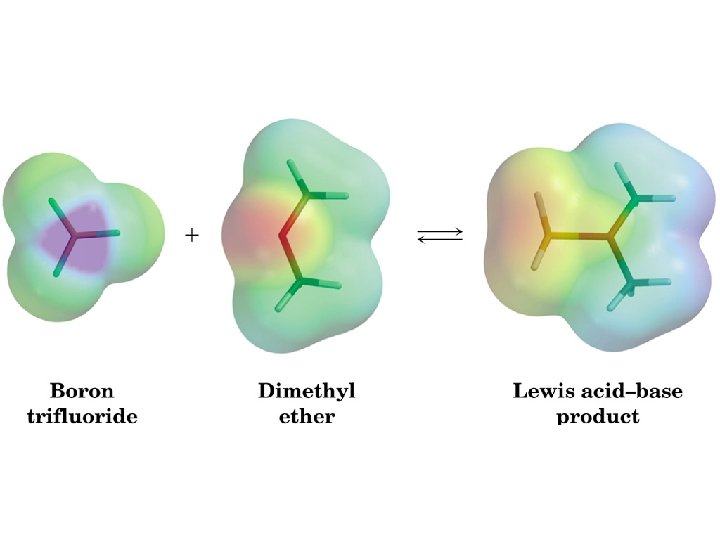

Acid-Base Reactions Showing a reaction with arrows Worksheet 5: Acids & Bases http: //chemconnections. org/organic/chem 226/226 assign-09. html#Worksheets
Acid-Base Reactions Predicting Products Worksheet 5: Acids & Bases http: //chemconnections. org/organic/chem 226/226 assign-09. html#Worksheets

Predicting if reactions occur and the products if they do Worksheet 5: Acids & Bases http: //chemconnections. org/organic/chem 226/226 assign-09. html#Worksheets
- Slides: 43