Chapter 1 Problem 19 Calculations Unit Conversion How

Chapter 1, Problem 19 Calculations & Unit Conversion How many grams of perchloric acid, HCl. O 4, are contained in 31. 3 g of 72. 7 wt% aqueous perchloric acid? How many grams of water are in the same solution? What’s the definition of weight % (wt%)? If you’re unsure you should go back and read the chapter Oregon State University Chemistry 324 Chapter 1 Problem 19 1

Why Are We Interested? • When we’re running experiments we need to know that we have enough reactants • Usually that means being able to compare compounds based on moles • We’re often given concentrations in different units There an almost infinite number of ways of expressing concentration Some are specific to particular areas of science Engineers have a complete different set of units Oregon State University Chemistry 324 Chapter 1 Problem 19 2
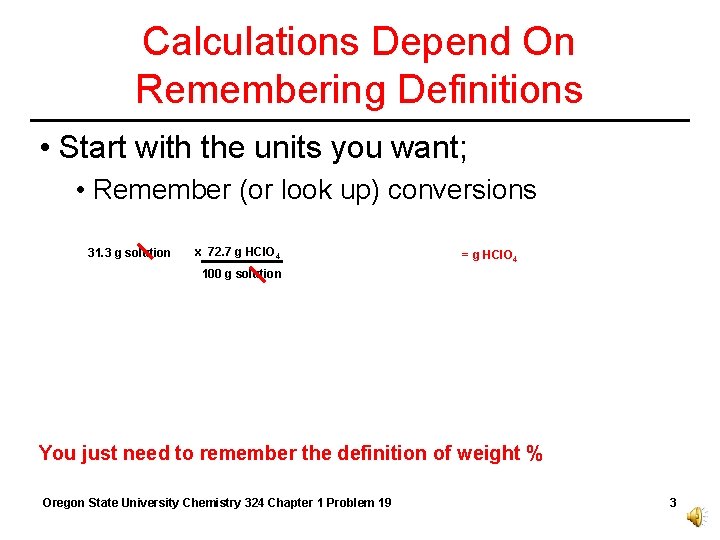
Calculations Depend On Remembering Definitions • Start with the units you want; • Remember (or look up) conversions 31. 3 g solution x 72. 7 g HCl. O 4 = g HCl. O 4 100 g solution You just need to remember the definition of weight % Oregon State University Chemistry 324 Chapter 1 Problem 19 3
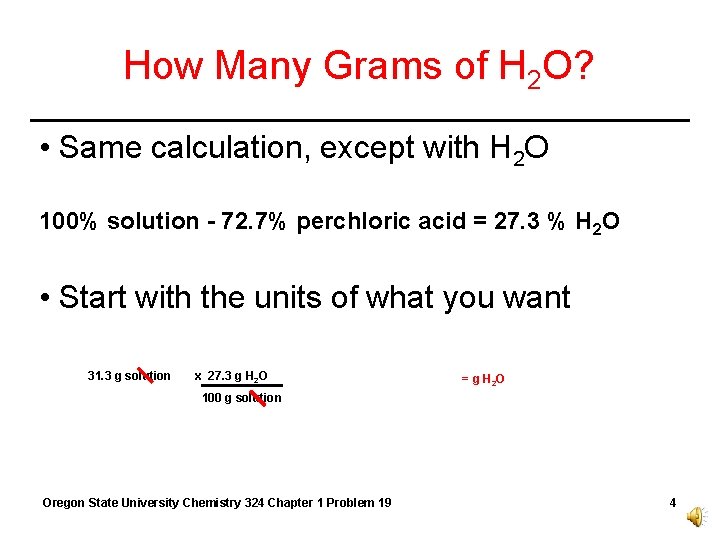
How Many Grams of H 2 O? • Same calculation, except with H 2 O 100% solution - 72. 7% perchloric acid = 27. 3 % H 2 O • Start with the units of what you want 31. 3 g solution x 27. 3 g H 2 O = g H 2 O 100 g solution Oregon State University Chemistry 324 Chapter 1 Problem 19 4
- Slides: 4